Abstract
Free full text

Matrix metalloproteinases, the pros and cons, in liver fibrosis
Abstract
Residing in the space of Disse within loose extracellular matrix (ECM) resembling that in basement membranes, the hepatic stellate cells (HSC) remain in quiescence as vitamin A storage cells. In response to liver injury HSC undergo morphologic and functional trans-differentiation, converting from vitamin A-storing, star-like cells into contractile myofibroblastic cells, a process called activation. Accompanying cellular activation, the ECM components in the space of Disse switch from matrices rich in type-IV collagen and laminin, into condensed interstitial ECM, indicating that proteolytic degradation may occur to change the microenvironment in sinusoids as well as the fate of HSC. Indeed, matrix metalloproteinases (MMP), a family of ECM degradative enzymes, are promptly expressed by HSC in response to diverse hepatic toxins. In vitro experiments also demonstrated the role of MMP in activation of HSC cultured in 3-D ECM. Conversely, MMP may also contribute to regression of liver fibrosis through cleavage of the fibrillar ECM and promotion of apoptosis among the activated HSC. Thus, MMP play dual roles both bad and good in liver fibrosis, depending on the timing.
Matrix metalloproteinases
If cells were seeds then the extracellular matrix (ECM) would be the soil. The mutual interaction between the cells and ECM determines cell fate, tissue specificity, and homeostasis. Controlled and limited proteolytic degradation of the ECM is essential for normal development, and maintenance of tissue homeostasis. Disregulation of matrix metalloproteinase (MMP) is associated with many degenerative diseases.1 The dynamics of the ECM in tissues are balanced between matrix breakdown, mediated by specific proteinases, and matrix protein synthesis as well as deposition and reorganization processes.2 Matrix degradation is carried out by a fine balance between activities of proteinases and their inhibitors. The MMP are a family of zinc metallo-endopeptidases and are responsible for the turnover of the ECM.3 Distinguishing from other proteinases, all MMP have two conserved motifs, namely pro-peptide and catalytic domains.4 Located at the N-terminus, the pro-domain of most MMP has approximately 80 amino residues, containing a consensus sequence of PRCXXPD with a critical cysteine. The catalytic domain, approximately 140–200 amino acid residues, holds a zinc ion, which interacts with three conserved histidines in the sequence HEXXHXXGXXHS and a methionine in the motif of TXXXXXXM. Most MMP are secreted into the extracellular milieu while a few are membrane anchored (termed ‘MT-MMP’). Many MMP have a proline-rich hinge region followed by the catalytic domain, and a hemopexin-like C-terminal domain. Substrate specificity is largely determined by the domains located at the C-terminus. For instance, two gelatinases (MMP-2 and MMP-9) have gelatin-binding domains similar to those found in fibronectin, through which the proteinases can bind denatured type-I collagen or native type-IV collagen.5
Many secreted MMP are absent in healthy and resting tissues. In contrast, as a general rule MMP are expressed in active tissues during embryonic development, injury (chemical or mechanical), and disease development as well as cells cultured in vitro. In such conditions the cells undergo activation, proliferation, migration and apoptosis. The MMP-mediated breakdown of ECM seems to be indispensable to breach the resting state to permit the cellular functions. Matrix metalloproteinase activities are under extremely restrained control at such multiple levels as transcriptional, translational regulation and auto-inhibition by the pro-domain as well as by the tissue inhibitors of matrix metalloproteinases (TIMP). Furthermore, the proMMP or zymogen is subjected to another hierarchal control, converting of the zymogen through proteolytic cleavage of the pro-domains by upstream activators. Many MMP, during tissue injury and repair, are orchestrated by inflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1). A single cytokine may stimulate a certain MMP in one cell type but inhibit the same MMP in another cell type.6 Besides cytokines, defined ECM components as well as mechanical stress also determine the expression of MMP.7–11 In addition to digestion of connective fibrillar and non-fibrillar ECM, MMP can also selectively cleave non-ECM proteins. For instance, MMP-1, -3, and -7, among others, can either directly convert cytokine precursors or release the cytokines, growth factors from ECM or carrier proteins.12,13 Many MMP knockout mice are viable, indicating possible redundancy in development, which may, at least in part, be due to the overlapped substrate specificities. In contrast, excessive amounts of MMP are always associated with degenerative diseases such as cancer, arthritis, chronic wounds and liver injury.
MMP play bad roles in liver injury and fibrosis
Liver fibrosis represents chronic wound repair following diverse insults.14 The ultimate outcome of liver fibrosis is the formation of nodules encapsulated by fibrillar scar matrix.15 Located within the space of Disse between hepatocytes and sinusoidal endothelial cells, hepatic stellate cells (HSC) play a key role in liver fibrogenesis.16 In liver fibrogenesis, HSC undergo dramatic morphological and functional changes, a process called ‘activation’, during which the star-shaped HSC are converted to myofibroblastic cells with increased expression of α-smooth muscle actin and decreased retinoid storage. In fibrogenesis, the normal ECM in the space of Disse rich in basement membrane-like matrices is switched to fibrillar, contractile ECM.17–20 Thus, it is reasonable to speculate that a proteolytic degradation of the normal ECM may occur at the onset of liver fibrogenesis.
Compelling evidence has documented the association of MMP with liver fibrosis. First, MMP are expressed prior to the onset of HSC activation in liver fibrogenesis. For instance, elevation of MMP and their tissue inhibitors occurs quickly after CCl4-induced liver injury in rats, and the hepatic expression of MMP-3 (stromelysin-1) is detectable as early as 6 h after CCl4 administration in rats and peaks at 48–72 h.21 A comprehensive study was conducted by Ramadori’s group, which measured MMP and TIMP expression in liver injury and fibrosis. After a single dose of CCl4, MMP-13, MMP-2, MMP-9, MT1-MMP, MMP-3 and MMP-10 were all increased, with peak expression coinciding with induction of inflammatory cytokines.22 This indicates the role of MMP in acute wound healing. Additionally, immunostaining of the liver revealed localization of MMP-9, a major MMP in basement membrane-like ECM remodeling. Matrix metalloproteinase-9 was found in the scar areas of active fibrogenesis, indicating that HSC may be an important source of MMP-9 in addition to macrophages.23 In rat hepatic fibrosis induced by bile duct ligation (BDL), activities of MMP-2 and MMP-9 increased 2 days after ligation, reached maximal levels at day 10, and remained high throughout the study period, suggesting that sustained tissue damage and inflammation due to chronic cholestasis would induce MMP.24 Expression of MMP is also regarded as an early signal of tissue regeneration. For example, following partial hepatectomy there is rapid induction of urokinase-type plasminogen activator (uPA), as well as MMP-2 and MMP-9.25 The elevations of MMP are detectable as early as 15–30 min after partial hepatectomy, with peak increases by 3–12 h, including the presence of active form of MMP. This indicates that MMP may be involved in mobilization, migration and proliferation of cells during repair and regeneration. In summary, the expression of MMP is an early event in wound healing, involving mobilization of cells through ECM degradation.
Potential role of MMP in HSC activation in liver fibrogenesis
Hepatic stellate cells are the principal cell types to produce ECM in normal liver.26,27 Isolated HSC undergo spontaneous activation when plated on plastic. Such in vitro activation is also accelerated by addition of Kupffer cell-conditioned medium or soluble factors identified to be pivotal in stimulating HSC; of note, transforming growth factor-β and platelet-derived growth factor are the two most potent mediators.28–30 However, most in vitro studies have cultured HSC either on plastic or matrix-coated dishes, which do not reflect the ECM environment in vivo. Future studies require culture models to mimic the trans-differentiation of HSC in 3-D ECM. In this regard, little is known about the mechanistic link between ECM remodeling and the activation of HSC in 3D ECM. Particularly, it is not clear how MMP participate in the activation of HSC. To investigate this we created a model in which the freshly isolated HSC were embedded in 3-D ECM. This model, as initially described by Imai and Senoo, and Wake et al., reproduces the true quiescent state of the HSC.31,32 Using this model, we tested a panel of inflammatory cytokines for their roles in activation of HSC and induction of MMP.33 To our surprise, 3-D type I collagen produced a marked synergism with IL-1 as a potent cytokine agonist in inducing HSC activation in ECM. Accompanied by the IL-1-induced cellular activation, MMP-9, a type-IV collagenase, is most impressively induced and converted into its active form. Matrix metalloproteinase-13 is also synergistically induced, resulting in the degradation of 3-D matrix. Thus we hypothesized that MMP and their mediated degradation of ECM in the space of Disse were essential for fibrotic activation of HSC in ECM and in liver fibrogenesis (Fig. 1). In line with the clinical observations and experimental model, the fully activated HSC placed in the culture system, lost their ability to produce MMP and to degrade type-I collagen. This hypothesis is currently under scrutiny in animal models with knockout MMP. The decision of when and which MMP are produced by HSC may be vital to the cellular plasticity of quiescent state versus fibrogenesis. At the early stage of the HSC activation, MMP-3, interstitial collagenase (MMP-13) but not TIMP are detected.34 With prolonged culture, interstitial collagenases are downregulated while expression of TIMP is increased, resulting in the net inhibition of degradation and favoring the deposition of ECM. These findings are important because they mirror the pattern of changes in MMP and TIMP expression profile in liver fibrosis.
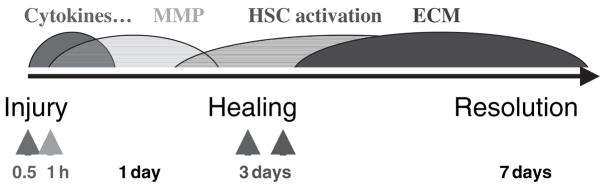
Timeline of events during activation of hepatic stellate cells (HSC) and fibrogenesis in an animal model. In response to injury pro-inflammatory cytokines are promptly increased in wound areas, which induce matrix metalloproteinase (MMP) expression by hepatic cells including HSC. The MMP secreted by HSC degrade the normal extracellular matrix (ECM) in the space of Disse. This cytokine-induced ECM degradation leads to activation of HSC. Consequently, a population of the HSC undergo apoptosis while others trans-differentiate into myofibroblasts that produce fibrillar ECM.
Matrix metalloproteinases as ‘good guys’ in resolving liver fibrosis
Scarring is controlled in two ways: the deposition and the removal of ECM. There may now be hope for the reversal of cirrhosis, previously regarded as the end-point of liver disease, largely based on the evidence from clinical and animal studies showing that withdrawal of the sources of injury (viral or chemical) can lead to the disappearance of fibrotic tissues.35 For instance, adenoviral-mediated delivery of MMP-1 promotes resolution of fibrotic tissues.36 Conversely, mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis.37 The MMP-mediated resolution of tissue fibrosis may act through ECM degradation as well as by induction of HSC apoptosis. Inhibition of apoptosis of activated HSC by tissue inhibitor of metalloproteinase-1 is mediated via effects on MMP activity: implications for reversibility of liver fibrosis.38 Despite these preliminary studies, it is totally unknown how the fibrotic tissues are removed in vivo. A particularly difficult question is how MMP, which are usually downregulated in established fibrotic tissues, are expressed again during the resolution process. However, it is certainly a dynamic area to address the interaction between the EMC and cells in disease development.
Biography

Han Y-P
References
Full text links
Read article at publisher's site: https://doi.org/10.1111/j.1440-1746.2006.04586.x
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2646497?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/j.1440-1746.2006.04586.x
Article citations
S-nitrosylation of EMMPRIN influences the migration of HSCs and MMP activity in liver fibrosis.
Acta Biochim Biophys Sin (Shanghai), 55(10):1640-1649, 01 Oct 2023
Cited by: 0 articles | PMID: 37700592 | PMCID: PMC10577453
Characterization of Hepatic Dysfunction in Subjects Diagnosed With Chronic GVHD by NIH Consensus Criteria.
Transplant Cell Ther, 28(11):747.e1-747.e10, 22 Jul 2022
Cited by: 2 articles | PMID: 35878742 | PMCID: PMC10707451
Role of hepatic stellate cells in liver ischemia-reperfusion injury.
Front Immunol, 13:891868, 28 Jul 2022
Cited by: 6 articles | PMID: 35967364 | PMCID: PMC9366147
Review Free full text in Europe PMC
Role of Vitamin D in Liver Disease and Complications of Advanced Chronic Liver Disease.
Int J Mol Sci, 23(16):9016, 12 Aug 2022
Cited by: 11 articles | PMID: 36012285 | PMCID: PMC9409132
Review Free full text in Europe PMC
Switching to Regular Diet Partially Resolves Liver Fibrosis Induced by High-Fat, High-Cholesterol Diet in Mice.
Nutrients, 14(2):386, 17 Jan 2022
Cited by: 4 articles | PMID: 35057565 | PMCID: PMC8778944
Go to all (85) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Essential role of matrix metalloproteinases in interleukin-1-induced myofibroblastic activation of hepatic stellate cell in collagen.
J Biol Chem, 279(6):4820-4828, 14 Nov 2003
Cited by: 91 articles | PMID: 14617627 | PMCID: PMC2430939
Hepatocyte produced matrix metalloproteinases are regulated by CD147 in liver fibrogenesis.
PLoS One, 9(7):e90571, 30 Jul 2014
Cited by: 22 articles | PMID: 25076423 | PMCID: PMC4116334
A matrix metalloproteinase-9 activation cascade by hepatic stellate cells in trans-differentiation in the three-dimensional extracellular matrix.
J Biol Chem, 282(17):12928-12939, 23 Feb 2007
Cited by: 57 articles | PMID: 17322299 | PMCID: PMC2376818
Cooperation of liver cells in health and disease.
Adv Anat Embryol Cell Biol, 161:III-XIII, 1-151, 01 Jan 2001
Cited by: 210 articles | PMID: 11729749
Review
Funding
Funders who supported this work.
NIAMS NIH HHS (1)
Grant ID: R01 AR051558-02
NIDDK NIH HHS (1)
Grant ID: R01 DK069418-01





