Abstract
Free full text

Pseudotyping with human T-cell leukemia virus type I broadens the human immunodeficiency virus host range.
Abstract
Several epidemiologic and clinical studies suggest that patients coinfected with human immunodeficiency virus (HIV), the primary etiologic agent in AIDS, and other viruses, such as cytomegalovirus or human T-cell leukemia virus (HTLV), have a more severe clinical course than those infected with HIV alone. Cells infected with two viruses can, in some cases, give rise to phenotypically mixed virions with altered or broadened cell tropism and could therefore account for some of these findings. Such pseudotypes could alter the course of disease by infecting more tissues than are normally infected by HIV. We show here that HIV type 1 (HIV-1) efficiently incorporates the HTLV type I (HTLV-I) envelope glycoprotein and that both HIV-1 and HTLV-II accept other widely divergent envelope glycoproteins to form infectious pseudotype viruses whose cellular tropisms and relative abilities to be transmitted by cell-free virions or by cell contact are determined by the heterologous envelope. We also show that the mechanism by which virions incorporate heterologous envelope glycoproteins is independent of the presence of the homologous glycoprotein or heterologous gag proteins. These results may have important implications for the mechanism of HIV pathogenesis.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.6M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
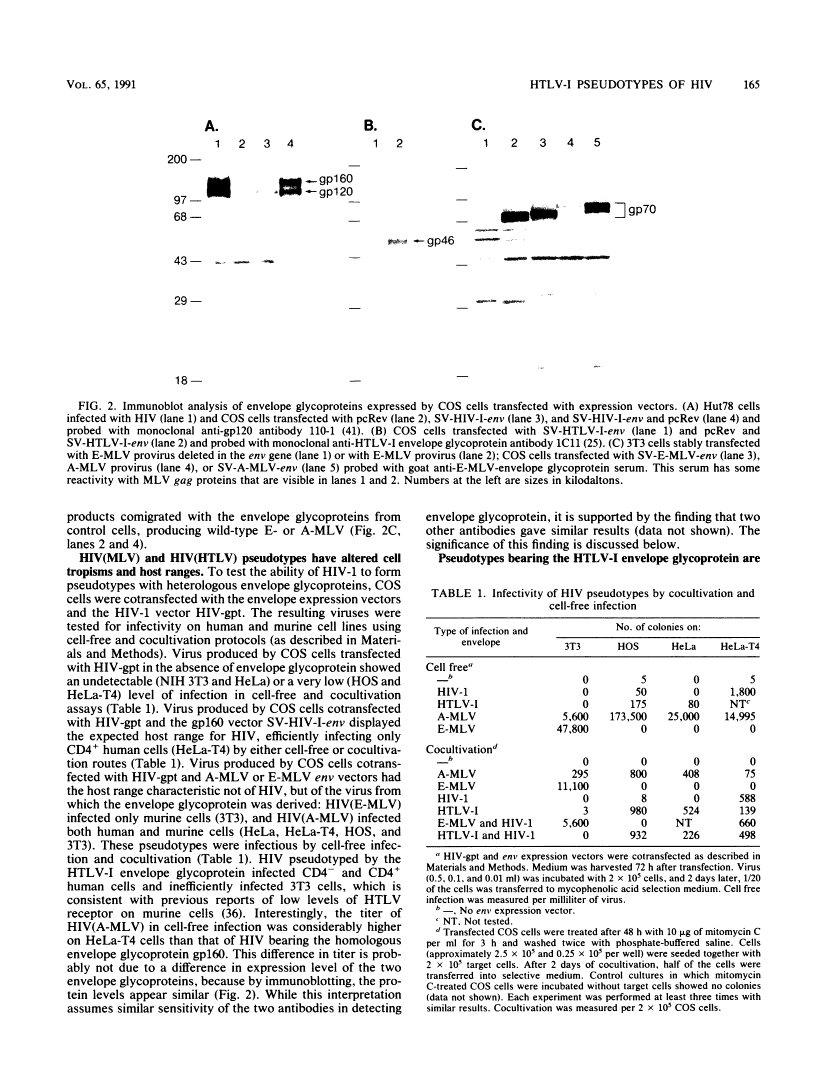
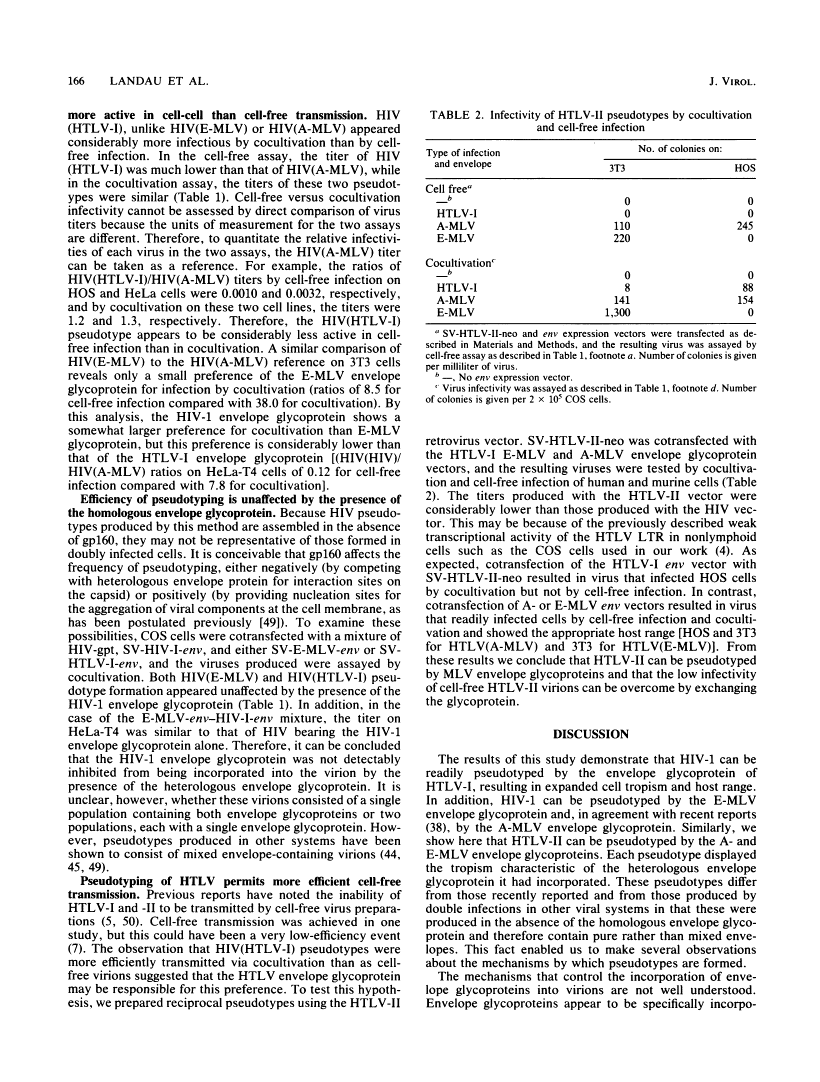
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boettiger D. Animal virus pseudotypes. Prog Med Virol. 1979;25:37–68. [Abstract] [Google Scholar]
- Bonetti A, Weber R, Vogt MW, Wunderli W, Siegenthaler W, Lüthy R. Co-infection with human immunodeficiency virus-type 1 (HIV-1) and cytomegalovirus in two intravenous drug users. Ann Intern Med. 1989 Aug 15;111(4):293–296. [Abstract] [Google Scholar]
- Calafat J, Janssen H, Démant P, Hilgers J, Závada J. Specific selection of host cell glycoproteins during assembly of murine leukaemia virus and vesicular stomatitis virus: presence of Thy-1 glycoprotein and absence of H-2, Pgp-1 and T-200 glycoproteins on the envelopes of these virus particles. J Gen Virol. 1983 Jun;64(Pt 6):1241–1253. [Abstract] [Google Scholar]
- Chen IS, McLaughlin J, Golde DW. Long terminal repeats of human T-cell leukaemia virus II genome determine target cell specificity. Nature. 1984 May 17;309(5965):276–279. [Abstract] [Google Scholar]
- Chen IS, Quan SG, Golde DW. Human T-cell leukemia virus type II transforms normal human lymphocytes. Proc Natl Acad Sci U S A. 1983 Nov;80(22):7006–7009. [Europe PMC free article] [Abstract] [Google Scholar]
- Chesebro B, Buller R, Portis J, Wehrly K. Failure of human immunodeficiency virus entry and infection in CD4-positive human brain and skin cells. J Virol. 1990 Jan;64(1):215–221. [Europe PMC free article] [Abstract] [Google Scholar]
- Clapham P, Nagy K, Cheingsong-Popov R, Exley M, Weiss RA. Productive infection and cell-free transmission of human T-cell leukemia virus in a nonlymphoid cell line. Science. 1983 Dec 9;222(4628):1125–1127. [Abstract] [Google Scholar]
- Fiala M, Cone LA, Chang CM, Mocarski ES. Cytomegalovirus viremia increases with progressive immune deficiency in patients infected with HTLV-III. AIDS Res. 1986 Summer;2(3):175–181. [Abstract] [Google Scholar]
- Gabuzda DH, Ho DD, de la Monte SM, Hirsch MS, Rota TR, Sobel RA. Immunohistochemical identification of HTLV-III antigen in brains of patients with AIDS. Ann Neurol. 1986 Sep;20(3):289–295. [Abstract] [Google Scholar]
- Gebhardt A, Bosch JV, Ziemiecki A, Friis RR. Rous sarcoma virus p19 and gp35 can be chemically crosslinked to high molecular weight complexes. An insight into virus assembly. J Mol Biol. 1984 Apr 5;174(2):297–317. [Abstract] [Google Scholar]
- Gendelman HE, Phelps W, Feigenbaum L, Ostrove JM, Adachi A, Howley PM, Khoury G, Ginsberg HS, Martin MA. Trans-activation of the human immunodeficiency virus long terminal repeat sequence by DNA viruses. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9759–9763. [Europe PMC free article] [Abstract] [Google Scholar]
- Harouse JM, Kunsch C, Hartle HT, Laughlin MA, Hoxie JA, Wigdahl B, Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. [Europe PMC free article] [Abstract] [Google Scholar]
- Hartley JW, Rowe WP. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. [Europe PMC free article] [Abstract] [Google Scholar]
- Hattori T, Koito A, Takatsuki K, Ikematsu S, Matsuda J, Mori H, Fukui M, Akashi K, Matsumoto K. Frequent infection with human T-cell lymphotropic virus type I in patients with AIDS but not in carriers of human immunodeficiency virus type 1. J Acquir Immune Defic Syndr. 1989;2(3):272–276. [Abstract] [Google Scholar]
- Horvat RT, Wood C, Balachandran N. Transactivation of human immunodeficiency virus promoter by human herpesvirus 6. J Virol. 1989 Feb;63(2):970–973. [Europe PMC free article] [Abstract] [Google Scholar]
- Kunsch C, Hartle HT, Wigdahl B. Infection of human fetal dorsal root ganglion glial cells with human immunodeficiency virus type 1 involves an entry mechanism independent of the CD4 T4A epitope. J Virol. 1989 Dec;63(12):5054–5061. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee H, Swanson P, Shorty VS, Zack JA, Rosenblatt JD, Chen IS. High rate of HTLV-II infection in seropositive i.v. drug abusers in New Orleans. Science. 1989 Apr 28;244(4903):471–475. [Abstract] [Google Scholar]
- Lodish HF, Porter M. Specific incorporation of host cell surface proteins into budding vesicular stomatitis virus particles. Cell. 1980 Jan;19(1):161–169. [Abstract] [Google Scholar]
- Lusso P, di Marzo Veronese F, Ensoli B, Franchini G, Jemma C, DeRocco SE, Kalyanaraman VS, Gallo RC. Expanded HIV-1 cellular tropism by phenotypic mixing with murine endogenous retroviruses. Science. 1990 Feb 16;247(4944):848–852. [Abstract] [Google Scholar]
- Malim MH, Hauber J, Fenrick R, Cullen BR. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988 Sep 8;335(6186):181–183. [Abstract] [Google Scholar]
- Mann R, Mulligan RC, Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. [Abstract] [Google Scholar]
- Metsikkö K, Garoff H. Role of heterologous and homologous glycoproteins in phenotypic mixing between Sendai virus and vesicular stomatitis virus. J Virol. 1989 Dec;63(12):5111–5118. [Europe PMC free article] [Abstract] [Google Scholar]
- Mosca JD, Bednarik DP, Raj NB, Rosen CA, Sodroski JG, Haseltine WA, Pitha PM. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987 Jan 1;325(6099):67–70. [Abstract] [Google Scholar]
- Ostrove JM, Leonard J, Weck KE, Rabson AB, Gendelman HE. Activation of the human immunodeficiency virus by herpes simplex virus type 1. J Virol. 1987 Dec;61(12):3726–3732. [Europe PMC free article] [Abstract] [Google Scholar]
- Page KA, Landau NR, Littman DR. Construction and use of a human immunodeficiency virus vector for analysis of virus infectivity. J Virol. 1990 Nov;64(11):5270–5276. [Europe PMC free article] [Abstract] [Google Scholar]
- Palker TJ, Tanner ME, Scearce RM, Streilein RD, Clark ME, Haynes BF. Mapping of immunogenic regions of human T cell leukemia virus type I (HTLV-I) gp46 and gp21 envelope glycoproteins with env-encoded synthetic peptides and a monoclonal antibody to gp46. J Immunol. 1989 Feb 1;142(3):971–978. [Abstract] [Google Scholar]
- Pedersen NC, Torten M, Rideout B, Sparger E, Tonachini T, Luciw PA, Ackley C, Levy N, Yamamoto J. Feline leukemia virus infection as a potentiating cofactor for the primary and secondary stages of experimentally induced feline immunodeficiency virus infection. J Virol. 1990 Feb;64(2):598–606. [Europe PMC free article] [Abstract] [Google Scholar]
- Perez LG, Davis GL, Hunter E. Mutants of the Rous sarcoma virus envelope glycoprotein that lack the transmembrane anchor and cytoplasmic domains: analysis of intracellular transport and assembly into virions. J Virol. 1987 Oct;61(10):2981–2988. [Europe PMC free article] [Abstract] [Google Scholar]
- Pumarola-Sune T, Navia BA, Cordon-Cardo C, Cho ES, Price RW. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987 May;21(5):490–496. [Abstract] [Google Scholar]
- Raffi F, Boudart D, Billaudel S. Acute co-infection with human immunodeficiency virus (HIV) and cytomegalovirus. Ann Intern Med. 1990 Feb 1;112(3):234–235. [Abstract] [Google Scholar]
- RusS G, Poláková K, Závada J. Assembly of xenotropic murine leukaemia virus-related antigens from the surface of mouse L cells by vesicular stomatitis virus. Acta Virol. 1983 Mar;27(2):105–109. [Abstract] [Google Scholar]
- Salahuddin SZ, Ablashi DV, Markham PD, Josephs SF, Sturzenegger S, Kaplan M, Halligan G, Biberfeld P, Wong-Staal F, Kramarsky B, et al. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. [Abstract] [Google Scholar]
- Seto E, Yen TS, Peterlin BM, Ou JH. Trans-activation of the human immunodeficiency virus long terminal repeat by the hepatitis B virus X protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8286–8290. [Europe PMC free article] [Abstract] [Google Scholar]
- Shimotohno K, Wachsman W, Takahashi Y, Golde DW, Miwa M, Sugimura T, Chen IS. Nucleotide sequence of the 3' region of an infectious human T-cell leukemia virus type II genome. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6657–6661. [Europe PMC free article] [Abstract] [Google Scholar]
- Siekevitz M, Josephs SF, Dukovich M, Peffer N, Wong-Staal F, Greene WC. Activation of the HIV-1 LTR by T cell mitogens and the trans-activator protein of HTLV-I. Science. 1987 Dec 11;238(4833):1575–1578. [Abstract] [Google Scholar]
- Sommerfelt MA, Williams BP, Clapham PR, Solomon E, Goodfellow PN, Weiss RA. Human T cell leukemia viruses use a receptor determined by human chromosome 17. Science. 1988 Dec 16;242(4885):1557–1559. [Abstract] [Google Scholar]
- Southern PJ, Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [Abstract] [Google Scholar]
- Spector DH, Wade E, Wright DA, Koval V, Clark C, Jaquish D, Spector SA. Human immunodeficiency virus pseudotypes with expanded cellular and species tropism. J Virol. 1990 May;64(5):2298–2308. [Europe PMC free article] [Abstract] [Google Scholar]
- Starcich B, Ratner L, Josephs SF, Okamoto T, Gallo RC, Wong-Staal F. Characterization of long terminal repeat sequences of HTLV-III. Science. 1985 Feb 1;227(4686):538–540. [Abstract] [Google Scholar]
- Stuve LL, Brown-Shimer S, Pachl C, Najarian R, Dina D, Burke RL. Structure and expression of the herpes simplex virus type 2 glycoprotein gB gene. J Virol. 1987 Feb;61(2):326–335. [Europe PMC free article] [Abstract] [Google Scholar]
- Thomas EK, Weber JN, McClure J, Clapham PR, Singhal MC, Shriver MK, Weiss RA. Neutralizing monoclonal antibodies to the AIDS virus. AIDS. 1988 Feb;2(1):25–29. [Abstract] [Google Scholar]
- Turner JM, Brodsky MH, Irving BA, Levin SD, Perlmutter RM, Littman DR. Interaction of the unique N-terminal region of tyrosine kinase p56lck with cytoplasmic domains of CD4 and CD8 is mediated by cysteine motifs. Cell. 1990 Mar 9;60(5):755–765. [Abstract] [Google Scholar]
- Webster A, Lee CA, Cook DG, Grundy JE, Emery VC, Kernoff PB, Griffiths PD. Cytomegalovirus infection and progression towards AIDS in haemophiliacs with human immunodeficiency virus infection. Lancet. 1989 Jul 8;2(8654):63–66. [Abstract] [Google Scholar]
- Weiss RA, Bennett PL. Assembly of membrane glycoproteins studied by phenotypic mixing between mutants of vesicular stomatitis virus and retroviruses. Virology. 1980 Jan 30;100(2):252–274. [Abstract] [Google Scholar]
- Weiss RA, Payne LN. The heritable nature of the factor in chicken cells which acts as a helper virus for Rous sarcoma virus. Virology. 1971 Aug;45(2):508–515. [Abstract] [Google Scholar]
- Wiley CA, Nelson JA. Role of human immunodeficiency virus and cytomegalovirus in AIDS encephalitis. Am J Pathol. 1988 Oct;133(1):73–81. [Europe PMC free article] [Abstract] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. [Europe PMC free article] [Abstract] [Google Scholar]
- Willey RL, Bonifacino JS, Potts BJ, Martin MA, Klausner RD. Biosynthesis, cleavage, and degradation of the human immunodeficiency virus 1 envelope glycoprotein gp160. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9580–9584. [Europe PMC free article] [Abstract] [Google Scholar]
- Witte ON, Baltimore D. Mechanism of formation of pseudotypes between vesicular stomatitis virus and murine leukemia virus. Cell. 1977 Jul;11(3):505–511. [Abstract] [Google Scholar]
- Yamamoto N, Okada M, Koyanagi Y, Kannagi M, Hinuma Y. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science. 1982 Aug 20;217(4561):737–739. [Abstract] [Google Scholar]
- Zhu ZH, Chen SS, Huang AS. Phenotypic mixing between human immunodeficiency virus and vesicular stomatitis virus or herpes simplex virus. J Acquir Immune Defic Syndr. 1990;3(3):215–219. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Virology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jvi.65.1.162-169.1991
Read article for free, from open access legal sources, via Unpaywall:
https://jvi.asm.org/content/jvi/65/1/162.full.pdf
Free to read at jvi.asm.org
http://jvi.asm.org/cgi/content/abstract/65/1/162
Free after 4 months at jvi.asm.org
http://jvi.asm.org/cgi/reprint/65/1/162
Citations & impact
Impact metrics
Article citations
The HIV-1 capsid serves as a nanoscale reaction vessel for reverse transcription.
PLoS Pathog, 20(9):e1011810, 03 Sep 2024
Cited by: 1 article | PMID: 39226318 | PMCID: PMC11398657
SARS-CoV-2 infection of human lung epithelial cells induces TMPRSS-mediated acute fibrin deposition.
Nat Commun, 14(1):6380, 11 Oct 2023
Cited by: 0 articles | PMID: 37821447 | PMCID: PMC10567911
Neutral sphingomyelinase 2 is required for HIV-1 maturation.
Proc Natl Acad Sci U S A, 120(28):e2219475120, 05 Jul 2023
Cited by: 7 articles | PMID: 37406093 | PMCID: PMC10334776
Discovery of Highly Potent Small Molecule Pan-Coronavirus Fusion Inhibitors.
Viruses, 15(4):1001, 19 Apr 2023
Cited by: 2 articles | PMID: 37112982 | PMCID: PMC10141620
Infection of the Ex Vivo Tonsil Model by HTLV-1 Envelope-Pseudotyped Viruses.
Pathogens, 12(2):182, 24 Jan 2023
Cited by: 3 articles | PMID: 36839454 | PMCID: PMC9958901
Go to all (194) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pseudotyping of HIV-1 with Human T-Lymphotropic Virus 1 (HTLV-1) Envelope Glycoprotein during HIV-1-HTLV-1 Coinfection Facilitates Direct HIV-1 Infection of Female Genital Epithelial Cells: Implications for Sexual Transmission of HIV-1.
mSphere, 3(2):e00038-18, 04 Apr 2018
Cited by: 4 articles | PMID: 29624497 | PMCID: PMC5885023
Reciprocal functional pseudotyping of HIV-1 and HTLV-1 viral genomes by the heterologous counterpart envelope proteins.
Virology, 443(1):106-112, 05 Jun 2013
Cited by: 4 articles | PMID: 23747197 | PMCID: PMC3728900
Identification of human immunodeficiency virus envelope gene sequences influencing viral entry into CD4-positive HeLa cells, T-leukemia cells, and macrophages.
J Virol, 65(11):5782-5789, 01 Nov 1991
Cited by: 123 articles | PMID: 1920616 | PMCID: PMC250239
Molecular biology of human T-lymphotropic retroviruses.
Cancer Res, 45(9 suppl):4539s-4544s, 01 Sep 1985
Cited by: 4 articles | PMID: 2990683
Review