Abstract
Objectives
Early transition from intravenous to oral antimicrobial therapy for acute osteomyelitis in children has been suggested as a safe and effective alternative to traditional prolonged intravenous therapy via central venous catheter, but no studies have directly compared these 2 treatment modalities. We sought to compare the effectiveness of early transition from intravenous to oral antimicrobial therapy versus prolonged intravenous antimicrobial therapy for the treatment of children with acute osteomyelitis.Methods
We conducted a retrospective cohort study of children aged 2 months to 17 years diagnosed with acute osteomyelitis between 2000 and 2005 at 29 freestanding children's hospitals in the United States to confirm the extent of variation in the use of early transition to oral therapy. We used propensity scores to adjust for potential differences between children treated with prolonged intravenous therapy and logistic regression to model the association of outcome (treatment failure rates within 6 months of diagnosis) and difference in the mode of therapy within hospitals and across hospitals.Results
Of the 1969 children who met inclusion criteria, 1021 received prolonged intravenous therapy and 948 received oral therapy. The use of prolonged intravenous therapy varied significantly across hospitals (10%-95%). The treatment failure rate was 5% (54 of 1021) in the prolonged intravenous therapy group and 4% (38 of 948) in the oral therapy group. There was no significant association between treatment failure and the mode of antimicrobial therapy. Thirty-five (3.4%) children in the prolonged intravenous therapy group were readmitted for a catheter-associated complication.Conclusions
Treatment of acute osteomyelitis with early transition to oral therapy is not associated with a higher risk of treatment failures and avoids the risks of prolonged intravenous therapy through central venous catheters.Free full text

PROLONGED INTRAVENOUS THERAPY VERSUS EARLY TRANSITION TO ORAL ANTIMICROBIAL THERAPY FOR ACUTE OSTEOMYELITIS IN CHILDREN
Abstract
OBJECTIVES
Early transition from intravenous to oral antimicrobial therapy for acute osteomyelitis in children has been suggested as a safe and effective alternative to traditional prolonged intravenous therapy via central venous catheter, but no studies have directly compared these two treatment modalities. We sought to compare the effectiveness of early transition from intravenous to oral antimicrobial therapy vs. prolonged intravenous antimicrobial therapy for the treatment of children with acute osteomyelitis.
METHODS
We conducted a retrospective cohort study of children ages 2 months to 17 years diagnosed with acute osteomyelitis between 2000 and 2005 at 29 free-standing children’s hospitals in the United States to confirm the extent of variation in use of early transition to oral therapy. We used a propensity scores to adjust for potential differences between children treated with prolonged intravenous therapy, and logistic regression to model the association of outcome (treatment failure rates within 6 months of diagnosis and difference in the mode of therapy within hospitals and across hospitals.
RESULTS
Of the 1969 children who met inclusion criteria, 1021 received prolonged intravenous therapy and 948 received oral therapy. Use of prolonged intravenous therapy varied significantly across hospitals (10% to 95%, P<0.001). The treatment failure rate was 5% (54 of 1021) in the prolonged intravenous therapy group and 4% (38 of 948) in the oral therapy group. There was no significant association between treatment failure and the mode of antimicrobial therapy (adjusted odds ratio=0.77, 95% confidence interval=0.49 to 1.22). Thirty-five children (3.4%) in the prolonged intravenous therapy group were readmitted for a catheter-associated complication.
CONCLUSIONS
Treatment of acute osteomyelitis with early transition to oral therapy is not associated with a higher risk of treatment failures and avoids the risks of prolonged intravenous therapy through central venous catheters.
Each year in the United States, 1 in 5000 children under the age of 13 years is diagnosed with osteomyelitis, which accounts for 1% of all pediatric hospitalizations.1, 2 Osteomyelitis is a bacterial infection of the bone that can occur in children of all ages and usually requires hospitalization for diagnosis and initial management. Although osteomyelitis can result from penetrating trauma or spread from a contiguous site of infection, the most common mechanism of infection in children is hematogenous inoculation of the bone during an episode of bacteremia (acute hematogenous osteomyelitis).
Treatment of acute osteomyelitis requires prolonged administration of antimicrobials. Inadequately treated osteomyelitis can result in progression to chronic infection and loss of function of the affected bone. Until recently, experts recommended that children with acute osteomyelitis receive 4 to 6 weeks of intravenous therapy, usually administered through a central venous catheter. However, recent case series studies have demonstrated successful treatment of acute osteomyelitis with a short-course of intravenous antimicrobial therapy followed by early transition to orally administered antimicrobials for a total duration of therapy of 4 to 6 weeks. Potential advantages of this treatment strategy include lower cost, increased convenience, and reduced risk of complications associated with prolonged insertion of central venous catheters.3–8 Studies have to date not quantified the degree of variation in use of early transition to oral therapy across hospitals and the possible association of use of early transition to oral therapy and treatment failure.
METHODS
Design
We performed a retrospective cohort study to compare the utilization of early transition to oral vs. prolonged intravenous antimicrobial therapy in a large sample of children hospitalized and treated for acute osteomyelitis at 29 free-standing children’s hospitals across the United States and the association of therapy and treatment failure.
Data Source
We used the Pediatric Health Information System (PHIS), an administrative database that contains inpatient data from 40 free-standing children’s hospitals affiliated with the Child Health Corporation of America (CHCA, Overland Park, Kansas). Contributing hospitals are located in 17 of the 20 major metropolitan areas in the United States and account for 70% of all freestanding children’s hospitals in the US (Data from The National Association of Children’s Hospitals and Related Institutions (Alexandria, VA). The database includes detailed information on demographics, diagnoses, procedures, medications, and repeat hospitalizations. Included in the medication files are data on the type and route of administration of all antimicrobials administered during hospitalization. Oversight of PHIS data quality and accuracy is a joint effort between CHCA, Thomson Healthcare (the data manager), and participating hospitals. Data are de-identified at the time of data submission and subjected to 175 reliability and validity checks. Data are accepted into the database when classified errors occur in fewer than 2% of a hospital’s quarterly data.
Assembly of Study Cohort
Our study included children ages 2 months to 17 years with discharge dates between January 1, 2000 and June 30, 2005. Children were included in the study cohort if their index hospitalization was assigned an International Classification of Diseases (ICD-9-CM) diagnosis code for acute osteomyelitis or unspecified osteomyelitis (730.01–730.09 and 730.2–730.29) in any of the 21 diagnosis fields. Children with a hospitalization for chronic osteomyelitis in the 6 months prior to the index admission were excluded. In addition, children were excluded if they were discharged during time periods when CHCA deemed a hospital’s data invalid or missing.
To define a cohort of children with uncomplicated, acute osteomyelitis, we excluded children with specific comorbid conditions documented on the index or prior admissions and those who were hospitalized for 10 or more days. Exclusionary comorbid conditions included those whose presence suggests complicated or difficult to treat osteomylelitis; congenital and acquired immunodeficiencies, sickle cell disease, trauma, osteomyelitis associated with immobilization or pressure ulcers (e.g., spina bifida, quadriplegia, paraplegia, mechanical ventilation, post-operative infections) and osteomyelitis of the head, face and orbits. We also excluded children with conditions that would predispose to inadequate absorption of oral medications (e.g., malabsorption).
In addition, we excluded children with an ICD-9-CM code for any of the following conditions during any admission prior to study entry that might have increased the risk of subsequent complicated osteomyelitis: cellulitis, pyogenic arthritis, sacroilitis, synovitis, myositis, chronic sinusitis, arthropathy, congenital or acquired diseases of bone, fasciitis, postoperative wounds, and placement of orthopedic devices or prosthesis. Finally, we excluded children with less than 6 months of observation time after the initial admission for osteomyelitis, to allow adequate time for observation of study outcomes.
Exposure classification
Children were classified into one of two possible treatment groups at the time of discharge from the hospital-- prolonged intravenous antimicrobial therapy or early transition to oral antimicrobial therapy. The prolonged intravenous therapy group was defined by the presence of a procedure code of 38.93 (venous catheterization, not elsewhere classified), which represents the placement of a central venous catheter. Children without procedure code 38.93 were assumed to have been discharged on oral therapy and comprised the early transition to oral therapy group. We assumed that transition to oral therapy occurred at the time of discharge, which may represent the most conservative estimate of transition time. The assignment of children to the prolonged intravenous or early transition to oral therapy groups was validated by CHCA as part of a data validation project using a 10% random sample of the study cohort from 19 of the 29 hospitals that agreed to participate in the validation study. The charts of children included in the validation sample were reviewed to confirm placement of a central venous catheter during the index hospitalization or early transition to oral antimicrobial therapy as part of the discharge plan.
Outcome measures
The primary outcome was treatment failure, defined as re-hospitalization within 6 months with assigned diagnosis or procedure codes consistent with (1) acute osteomyelitis as the sole diagnosis (730.0–09), (2) chronic osteomyelitis (730.1X), (3) a potential complication of acute osteomyelitis (e.g, myositis, arthritis, etc.), or (4) a surgical procedure related to the musculoskeletal system. Secondary outcomes included re-hospitalization within 6 months for (1) any reason; (2) catheter related complication; and (3) adverse drug reactions associated with antibiotics, C. difficile infection or agranulocytosis, a not uncommon effect of beta-lactam antibiotics. To determine the outcomes, two authors (TZ and RK) who were blinded to treatment group assignment reviewed the ICD-9-CM diagnosis and procedure codes for all children who were re-hospitalized during the study period.
Covariates
We extracted data from the PHIS database on the following potential confounders and effect modifiers: age, sex, race, surgical procedure, anatomic location of the infection, and the presence of an ICD9-CM code for Staphylococcus aureus (S. aureus) infection or methicillin-resistant S. aureus (MRSA). We also collected information on severity of initial illness using the PHIS case mix index (CMI), a widely used risk adjustment measure based upon relative weights derived from Thomson Healthcare’s national pediatric charges per case data and 3M’s All-Patient-Refined Diagnosis-Related Group (APR-DRG) classification system. The relative weights are computed as the ratio of the average charges per patient in an APR-DRG/Severity of Illness group to the average charges for all other children.
Statistical Analysis
Summary statistics were constructed using frequencies and proportions for categorical data elements and means and medians for continuous variables. The chi-square test was used for unadjusted comparisons between children who received prolonged intravenous therapy and children who received oral therapy. Random effects models and likelihood ratio tests were used to determine the significance of inter-hospital variation in use of oral therapy as well as in treatment failure rates. A propensity score model was developed to balance patient-level confounders that may have determined the selected treatment strategy. Propensity score analysis attempts to identify children who are similar except for their treatment or exposure status. The scores represent the probability that a patient will receive a treatment strategy based on his or her observed covariates.9, 10 We calculated propensity scores using multivariable logistic regression with early transition to oral therapy as the outcome of interest. Scores were then grouped into quintiles for later use a covariate in the analysis of mode of administration and treatment failure. In the response model (a logistic regression with early re-hospitalization as the outcome and quintile of propensity score as a covariate), we introduced singly each covariate from the propensity score to identify any residual confounding from that covariate.11 All confidence intervals were adjusted for the clustering of children within hospital using robust variance estimates.12
To determine the effect of exposure misclassification on the observed association between mode of antimicrobial administration and treatment failure, we compared results for the subset of hospitals that participated in the validation study and had no misclassification of exposure with results for the hospitals that did not participate in the validation study or were found to have some degree of misclassification of the exposure identified in the validation study.
We performed additional analyses to determine the within and among hospital effects. Confounding by hospital can occur when both the exposure of interest and outcome are clustered by hospital. To address this potential problem, we decomposed the within- and among-hospital components of the effect of mode of antibiotic administration.13, 14 The within-hospital effect measures the association of early transition to oral therapy and outcome once a child has selected a hospital and been admitted. The among-hospital effect measures the impact on outcome of transferring a given child from a hospital with lower to higher oral therapy use. 15 All analyses were conducted with SAS version 9.1 (Cary, NC) and Stata statistical software version 8.0 (College Station, TX).
Human subjects oversight
The conduct of this study was approved by the Child Health Corporation of America and the Committee for the Protection of Human Subjects at the Children’s Hospital of Philadelphia.
RESULTS
Subject Characteristics
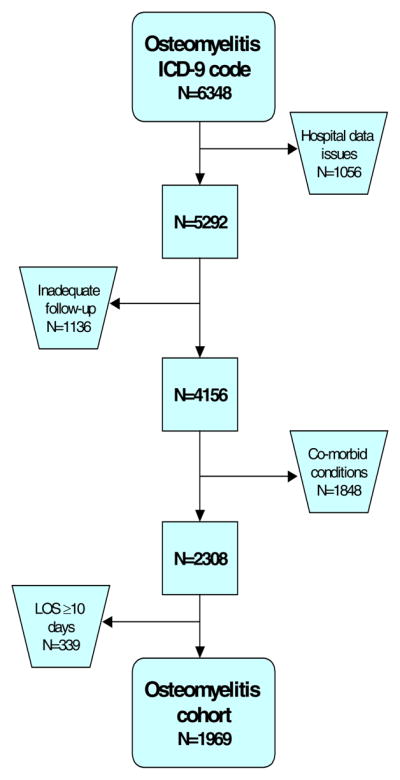
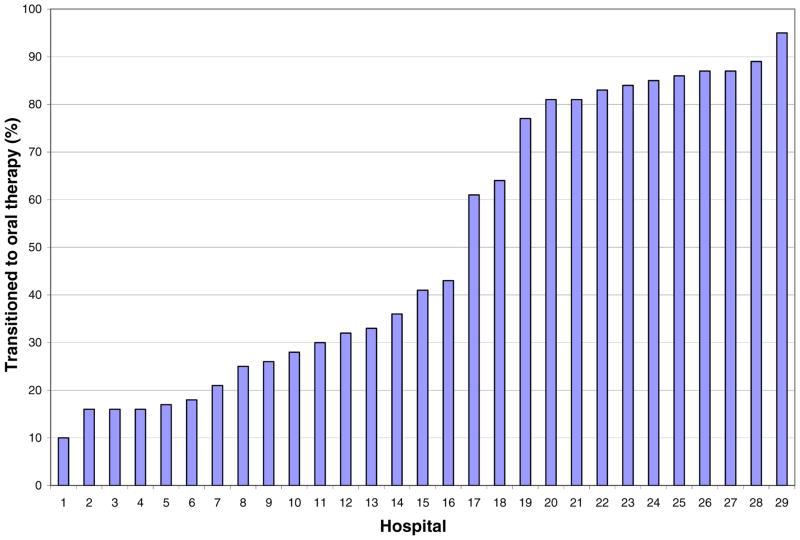
A total of 6348 children had a diagnosis code of acute osteomyelitis or osteomyelitis unspecified during the study period (Figure 1). Children were excluded for significant problems with data completeness or quality as identified by PHIS (1056), insufficient follow-up time (1136), presence of co-morbid conditions (1848), and length of stay greater than or equal to 10 days (339). This left 1969 children in the study cohort, of which 1021 had a central venous catheter placed for prolonged intravenous therapy and 948 did not and were assigned to the oral antimicrobial therapy group. The two study groups were virtually identical in terms of demographic characteristics, length of hospital stay, site of infection, infecting organism, surgical intervention, in-hospital antimicrobial therapy, and disease severity as measured by the case mix index (Table 1). The proportion of children who had a central venous catheter placed for prolonged intravenous therapy varied significantly across hospitals from 10% to 95% (p<0.001) (Figure 2).
Table 1
Demographic and Clinical Characteristics
| Intravenous Therapy | Oral Therapy | |
|---|---|---|
| Characteristics | N=1021 | N=948 |
| Age in Years | ||
 0–1 0–1 | 86 (8%) | 77 (8%) |
 1–5 1–5 | 343 (34%) | 322 (34%) |
 >5 >5 | 592 (58%) | 549 (58%) |
| Length of Hospital Stay (Median, interquartile range) | 5 (3, 6) | 4 (3, 6) |
| Gender | ||
 Male Male | 629 (62%) | 583 (62%) |
| Race | ||
 White White | 725 (71%) | 676 (71%) |
 Black Black | 186 (18%) | 150 (16%) |
 Other Other | 66 (6%) | 80 (8%) |
 Missing Missing | 44 (4%) | 42 (4%) |
| Site | ||
 Shoulder Shoulder | 23 (2%) | 25 (3%) |
 Upper Arm Upper Arm | 48 (5%) | 39 (4%) |
 Forearm Forearm | 31 (3%) | 34 (4%) |
 Hand Hand | 38 (4%) | 38 (4%) |
 Pelvic/Thigh Pelvic/Thigh | 315 (31%) | 287 (30%) |
 Lower Leg Lower Leg | 247 (24%) | 245 (26%) |
 Ankle/Foot Ankle/Foot | 182 (18%) | 178 (19%) |
 Multiple Sites Multiple Sites | 25 (2%) | 25 (3%) |
 Unspecified Unspecified | 112 (11%) | 77 (8%) |
| Organism^ | ||
 Group A Streptococcus Group A Streptococcus | 35 (3%) | 34 (4%) |
 Streptococci-other Streptococci-other | 11 (1%) | 15 (2%) |
 Staphylococcus aureus Staphylococcus aureus | 351 (34%) | 291 (31%) |
 Staphylococcus-other Staphylococcus-other | 51 (5%) | 33 (3%) |
 Methicillin-resistant Staphylococcus aureus Methicillin-resistant Staphylococcus aureus | 83 (8%) | 62 (7%) |
 E. coli E. coli | 2 (<1%) | 2(<1%) |
 Pneumococcus Pneumococcus | 12 (1%) | 9 (1%) |
 Other Gram-Negative Organisms Other Gram-Negative Organisms | 9 (1%) | 5 (1%) |
 >1 Organism >1 Organism | 89 (9%) | 73 (8%) |
| Surgical Procedure | 377 (37%) | 314 (33%) |
| Parental antibiotics received | ||
 Cefazolin Cefazolin | 603 (59%) | 508 (54%) |
 Oxacillin/Nafcillin Oxacillin/Nafcillin | 345 (34%) | 243 (26%) |
 Vancomycin Vancomycin | 188 (18%) | 83 (9%) |
 Clindamycin Clindamycin | 309 (30%) | 321 (34%) |
 TMP-SMX TMP-SMX | 5 (<1%) | 6 (1%) |
 Linezolid Linezolid | 2 (<1%) | 2 (<1%) |
 Other@ Other@ | 355 (35%) | 216 (23%) |
| Case Mix Index* (median, interquartile range) | 1.67 (1.67, 1.67) | 1.67 (1.67, 1.67) |
Treatment failure
The overall treatment failure rate among the 1969 children was 4.7% (95% confidence interval=3.8% to 5.7%) (Table 2). The treatment failure rate varied across hospitals from as low as 1.8% to as high as 12.5% for hospitals with at least 10 children with acute osteomyelitis, but this variation was not statistically significant (likelihood ratio chi square <0.001, p=0.50). The treatment failure rate was 5% (54 of 1021) in the prolonged intravenous therapy group and 4% (38 of 948) in the oral therapy group. There was no significant association between treatment failure and the mode of antimicrobial therapy adjusted for propensity score quintiles and clustering of observations within the 29 hospitals in the sample (Odds Ratio=0.77, 95% confidence interval=0.49 to 1.22) (Table 2). The median time to treatment failure was similar in both groups, 16.5 days (interquartile range, 6–35 days) for the prolonged intravenous therapy group compared to 14 days (interquartile range, 3–45 days) for the oral therapy group (p=0.65). Further, we did not detect any residual confounding after re-inclusion of all covariates from the propensity score in the final multivariate analysis.
Table 2
Treatment Outcomes of Acute Osteomyelitis.*
| Primary Outcome | Intravenous Therapy (1021) N (%) | Oral Therapy (948) N (%) | Propensity-score adjusted odds ratio (95% confidence interval) for those children treated with early transition to oral therapy |
|---|---|---|---|
|
| |||
| Treatment Failure within 6 months of diagnosis | 54 (5) | 38 (4) | 0.77 (0.49, 1.22) |
 Chronic osteomyelitis Chronic osteomyelitis | 13 (1.3) | 8 (0.8) | 0.84 (0.33, 2.13) |
 Musculoskeletal surgery Musculoskeletal surgery | 18 (1.8) | 15 (1.6) | 0.80 (0.38, 1.70) |
 Complication of osteomyelitis** Complication of osteomyelitis** | 11 (1.1) | 6 (0.6) | 0.75 (0.27, 2.07) |
 Acute osteomyelitis as sole readmission diagnosis Acute osteomyelitis as sole readmission diagnosis | 12 (1.2) | 9 (0.9) | 0.72 (0.25, 2.08) |
|
| |||
| Secondary Outcomes | |||
|
| |||
| Any re-hospitalization within 6 months of diagnosis | 102 (10) | 56 (5.9) | 0.6 (0.38, 0.96) |
|
| |||
| Catheter-associated complication | 35 (3) | 0 (0) | --- |
|
| |||
| Adverse effect of antimicrobials^ | 15 (1.5) | 4 (0.4) | 0.39 (0.14, 1.1) |
Validation study
Nineteen of the 29 hospitals agreed to participate in the validation study. Thirteen hospitals found no misclassification of the exposure and 6 hospitals found that some children who were treated with prolonged intravenous therapy were misclassified and assigned to the oral therapy group. The rate of misclassification for these hospitals ranged from 11 to 50%. None of the hospitals reported misclassification of children assigned to the intravenous therapy group. Among the hospitals reporting no misclassification, the odds ratio for treatment failure of oral therapy compared with intravenous therapy was 0.74 (95% confidence interval, 0.27 to 2.02). Similar results were observed in the cohort of 16 hospitals that did not participate in the validation study or reported misclassification of the exposure (odds ratio, 0.73; 95% confidence interval: 0.47 to 1.13).
Within and among hospital effects
The effect of mode of antimicrobial administration was decomposed into within and among hospital effects. The within hospital effect favoring oral therapy was 0.73 (95% confidence interval: 0.39, 1.35). There was also no among-hospital association of oral therapy and outcome (odds ratio, 0.98; 95% confidence interval: 0.93, 1.04).
Secondary Outcomes
Children treated with prolonged intravenous therapy were more likely to experience a treatment related complication (Table 2). Thirty-five children (3%) in the prolonged intravenous treatment group were readmitted for a catheter complication. The rate of readmission for antimicrobial complications was significantly higher in the prolonged intravenous therapy group (1.6% vs. 0.4%, p=0.005). The overall 6-month re-hospitalization rate, which included hospitalizations for any reason (treatment failures, catheter complications, etc.) was significantly higher in the prolonged intravenous therapy group compared to the oral therapy group (10%, vs. 6%; p=0.017).
DISCUSSION
In this cohort of children with acute osteomyelitis, we found that utilization of prolonged intravenous therapy and early transition to oral therapy were equally effective. There was wide variation in the choice of treatment strategy across the 29 hospitals included in the cohort despite the fact that the children in the two treatment groups had identical clinical and demographic characteristics. The distribution of patients’ age, sex, affected bone, and identified pathogen closely mirrors previous descriptions of the epidemiology of acute osteomyelitis in children.16–21 The overall treatment failure rate of 4.2% in our study and in other reports 6, 7 suggests that the study cohort is representative of children with acute, uncomplicated osteomyelitis and that our results, therefore, are likely generalizable to other cohorts of children with these clinical characteristics.
We anticipated that treatment selection decisions across hospitals may be influenced by observed severity of illness or other patient characteristics but this was not the case. The children in the two treatment groups were virtually identical in terms of their measured clinical and demographic characteristics. Thus, adjusted rates of treatment failure did not differ from crude rates. With identical patient characteristics and outcomes in the 2 treatment groups and such wide variability in treatment modality choice across hospitals, it appears that institutional culture and tradition, rather than patient characteristics, is driving therapeutic choices.
The lack of high quality evidence supporting early transition to oral therapy has likely contributed significantly to the wide variability in its adoption. Several small observational studies have supported the use of short-course intravenous with early transition to oral therapy as an adequate and alternative to traditional management.3–8 A systematic review that included 12 small studies (sample size ranging between 5–50 children), the majority of which were case series, demonstrated no statistically significant difference in clinical cure rate at 6 months between children receiving 7 days or greater (98.8%) versus less than one week (95.2%) of intravenous antibiotics (p=0.838) prior to transition to oral antimicrobials.7 The treatment failure rates found in our large study were similar to those described in the systematic review.
We found a significant difference in the rate of re-hospitalization for central venous catheter-associated complications in children treated with prolonged intravenous therapy after discharge. Approximately 4% of children treated with prolonged intravenous therapy experienced a catheter-associated complication requiring hospitalization. Previous studies that evaluated the complications of outpatient intravenous antimicrobial therapy showed high rates of central venous catheter-associated complications, ranging between 29% and 41%.8, 22 In this study, the rate of central venous catheter-associated complications is lower than these prior estimates but is likely an underestimate because it only captures children who required re-hospitalization; emergency department visits were not captured.
A potential limitation of our study lies in misclassification in the administrative data such as PHIS due to miscoded or inaccurate information. For that reason, we validated the classification of exposure (intravenous or oral therapy) using chart review and showed that limiting the analysis to children from centers with no misclassification of the exposure variable did not change the relative risk of treatment failure. We recognize that misclassification of the outcome is also possible. Children could have been admitted to hospitals other than the PHIS hospitals for treatment failure or complication. However, we believe that most children who receive treatment for diseases such as osteomyelitis would likely return to the institution at which they received initial therapy. In addition, we cannot completely rule out a late occurrence of recurrence osteomyelitis or chronic osteomyelitis, however, we believe the majority of complications should be captured in the 6 month follow-up period used in our study. Finally, some investigators have suggested that certain conditions must be met for oral therapy to be considered for treatment of acute osteomyelitis, including an identified organism, patient compliance, surgical debridement, and the ability to follow serum levels of the oral antibiotic.5, 6, 23, 24 We are unable to comment on patient compliance, however, we did not identify a difference between the two study groups in the number of patients with an identified organism or those who underwent surgery. In addition, serum levels of oral antibiotics are no longer routinely measured.
CONCLUSIONS
We found that treatment of acute, uncomplicated osteomyelitis with early transition to oral therapy did not increase the risk of treatment failure. Our study may provide the best evidence to support wider use of this treatment strategy because, given the low treatment failure rates, a randomized clinical trial might not be feasible. Clinical practice guidelines outlining parameters and a protocol for early transition to oral therapy need to be implemented across these hospitals to reduce variation and to determine if good clinical outcomes are sustained.
Acknowledgments
Financial support. Dr. Theoklis E. Zaoutis was supported by grant K23 AI0629753-01 from the National Institutes of Health. Dr. A. Russell Localio was supported in part by an Agency for Healthcare Research and Quality (AHRQ) Centers for Education and Research on Therapeutics cooperative agreement (grant #HS10399). Dr. Ron Keren was supported by grant K23 HD043179 from the National Institute of Child Health and Human Development, Bethesda, MD.
Abbreviations
| PHIS | Pediatric Health Information System |
| CHCA | Child Health Corporation of America |
| ICD-9-CM | International Classification of Diseases |
| S. aureus | Staphylococcus aureus |
| MRSA | methicillin-resistant S. aureus |
| CMI | case mix index |
| APR-DRG | All-Patient-Refined Diagnosis-Related Group |
Footnotes
Potential conflicts of interest: All authors report no conflicts of interest relevant to this article.
References
Full text links
Read article at publisher's site: https://doi.org/10.1542/peds.2008-0596
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3774269?pdf=render
Free after 12 months at intl-pediatrics.aappublications.org
http://intl-pediatrics.aappublications.org/cgi/content/full/123/2/636
Free to read at intl-pediatrics.aappublications.org
http://intl-pediatrics.aappublications.org/cgi/content/abstract/123/2/636
Free after 12 months at intl-pediatrics.aappublications.org
http://intl-pediatrics.aappublications.org/cgi/reprint/123/2/636.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/102357636
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1542/peds.2008-0596
Article citations
Developing a nomogram for predicting acute complicated course in pediatric acute hematogenous osteomyelitis.
Ital J Pediatr, 50(1):130, 29 Jul 2024
Cited by: 1 article | PMID: 39075514 | PMCID: PMC11287884
[Arthritis and osteomyelitis in childhood and adolescence-Bacterial and nonbacterial].
Z Rheumatol, 23 Apr 2024
Cited by: 0 articles | PMID: 38653784
First-Generation Cephalosporins for Treatment of Acute Hematogenous Osteomyelitis in Children: A Study of Efficacy and Adverse Effects.
Open Forum Infect Dis, 10(12):ofad610, 14 Dec 2023
Cited by: 0 articles | PMID: 38156049 | PMCID: PMC10753912
Defining Variability in Evaluation and Management of Children with Chronic Osteomyelitis.
J Pediatric Infect Dis Soc, 12(4):226-229, 01 Apr 2023
Cited by: 0 articles | PMID: 36688512 | PMCID: PMC10146934
Impact of guidelines implementation on empiric antibiotic treatment for pediatric uncomplicated osteomyelitis and septic arthritis over a ten-year period: Results of the ELECTRIC study (ostEomyeLitis and sEptiC arThritis tReatment in children).
Front Pediatr, 11:1135319, 23 Feb 2023
Cited by: 0 articles | PMID: 36911022 | PMCID: PMC9997840
Go to all (100) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Comparative effectiveness of intravenous vs oral antibiotics for postdischarge treatment of acute osteomyelitis in children.
JAMA Pediatr, 169(2):120-128, 01 Feb 2015
Cited by: 80 articles | PMID: 25506733
Complications of central venous catheters used for the treatment of acute hematogenous osteomyelitis.
Pediatrics, 117(4):1210-1215, 01 Apr 2006
Cited by: 50 articles | PMID: 16585317
Intravenous versus oral outpatient antibiotic therapy for pediatric acute osteomyelitis.
Iowa Orthop J, 33:208-212, 01 Jan 2013
Cited by: 7 articles | PMID: 24027485 | PMCID: PMC3748882
Funding
Funders who supported this work.
AHRQ HHS (2)
Grant ID: HS10399
Grant ID: U18 HS010399
NIAID NIH HHS (2)
Grant ID: K23 AI0629753-01
Grant ID: K23 AI062975
NICHD NIH HHS (1)
Grant ID: K23 HD043179