Abstract
Free full text

Common variants on 9q22.33 and 14q13.3 predispose to thyroid cancer in European populations
Abstract
In order to search for sequence variants conferring risk of thyroid cancer we conducted a genome-wide association study in 192 and 37,196 Icelandic cases and controls, respectively, followed by a replication study in individuals of European descent. Here we show that two common variants, located on 9q22.33 and 14q13.3, are associated with the disease. Overall, the strongest association signals were observed for rs965513 on 9q22.33 (OR = 1.75; P = 1.7 × 10−27) and rs944289 on 14q13.3 (OR = 1.37; P = 2.0 × 10−9). The gene nearest to the 9q22.33 locus is FOXE1 (TTF2) and NKX2-1 (TTF1) is among the genes located at the 14q13.3 locus. Both variants contribute to an increased risk of both papillary and follicular thyroid cancer. Approximately 3.7% of individuals are homozygous for both variants, and their estimated risk of thyroid cancer is 5.7-fold greater than that of noncarriers. In a study on a large sample set from the general population, both risk alleles are associated with low concentrations of thyroid stimulating hormone (TSH), and the 9q22.33 allele is associated with low concentration of thyroxin (T4) and high concentration of triiodothyronine (T3).
Thyroid carcinoma is the most common endocrine malignancy, and its incidence in industrialized countries has been rising over the past few decades: at present, its incidence is 4.9 and 14.1 per 100,000 in the United States for males and females of European ancestry, respectively (see URLs section in Methods). It has been determined that the risk of thyroid cancer has a greater genetic component than the risk of any other cancer, and the effect has been shown to extend beyond the nuclear family1–3. The risk has been reported to be highest for first-degree male relatives of male probands but lowest for first-degree female relatives of female probands4. Not much is known about variants in the sequence of the genome that affect the risk of thyroid cancer. However, recently variants at 1p12, 8q24 and in the pre-miR146a at 5q33 have been implicated in the disease5–8.
Thyroid cancer is classified histologically into four main groups: papillary (PTC), follicular (FTC), medullary (MTC) and undifferentiated or anaplastic thyroid carcinomas. Most of all thyroid tumors are PTC (80–85%) or FTC (10–15%)9,10. Among established risk factors for FTC is deficiency in iodine intake, and for PTC the risk factors include ionizing radiation, nodular disease of the thyroid as well as family history9. Medullary thyroid cancer is a part of the multiple endocrine neoplasia type 2 syndrome (MEN2) and accounts for about 1–3% of all thyroid cancer cases. Its pathogenesis can mainly be explained by mutations in the RET oncogene11. Anaplastic thyroid cancer accounts for between 1% and 5% of all carcinomas of the thyroid. This subphenotype is one of the most aggressive of all human malignancies, but little is known about its pathogenesis12.
In Iceland, the annual incidence of thyroid cancer is similar to that in the United States, at 4.6 and 12.1 per 100,000 for males and females, respectively, according to the Icelandic Cancer Registry (see URLs section below). In order to search for sequence variants conferring risk of thyroid cancer, we conducted a genome-wide association study (GWAS) with 192 histopathologically confirmed Icelandic thyroid cancer cases and 37,196 controls genotyped using the Illumina HumanHap300 and HumanCNV370-duo Bead Chip genotyping platform (Supplementary Methods online). Furthermore, we used a method in which known genotypes of relatives are used to provide information on individuals with thyroid cancer who were not genotyped (in silico genotyping), in order to add genotypes that represent, on average per SNP, an additional 186 individuals with thyroid cancer13. After removal of SNPs that failed quality checks, a total of 304,083 SNPs were tested for association. We calculated the allelic odds ratio (OR) for each SNP assuming the multiplicative model, and computed a standard likelihood ratio χ2 statistic for the purpose of testing. The results were adjusted for familial relatedness between individuals and for potential population stratification using the method of genomic control14; the χ2 statistics were divided by an estimated inflation factor of 1.09. Quantile-quantile plots of the χ2 statistics before and after adjustment are shown in Supplementary Figure 1 online.
The nine strongest signals (P < 1 × 10−6) are shown in Supplementary Table 1 online; of these, seven were located in the same linkage disequilibrium (LD) region as the FOXE1 (forkhead factor E1) gene on 9q22.33 (Fig. 1). In an attempt to confirm these results we proceeded to genotype the nine SNPs in additional 241 Icelandic thyroid cancer cases using Centaurus15 single-track assay genotyping. Combining these results and the results from the GWAS, we observed that the strongest association signals were for allele A of rs965513 (rs965513[A]) and allele A of rs10759944 (rs10759944[A]), with an OR of 1.77 for both variants (P = 6.8 × 10−20 and P = 1.7 × 10−19 for rs965513 and rs10759944, respectively; Table 1 and Supplementary Table 1). These two SNPs are nearly perfect surrogates of each other (r2 = 1 in the Utah CEPH (CEU) HapMap samples and r2 = 0.998 in the Icelandic samples), and because the effects of the variants cannot be distinguished from each other, we elected to focus on rs965513[A] in subsequent investigations. When we controlled for rs965513[A] in a multivariate analysis, none of the remaining SNPs on 9q22.33 (ranking 3 to 7 in Supplementary Table 1) is significant. In the combined analysis of the Icelandic data, the eighth-ranking association signal was observed for allele T of rs944289 (rs944289[T]) located on 14q13.3. This signal reached a genome-wide significance, with an OR of 1.37 and P = 2.5 × 10−8 (Table 1 and Supplementary Table 1). The ninth SNP, rs622450 on 1p36.13, failed to replicate in the Icelandic samples and was not studied further (Supplementary Table 1).
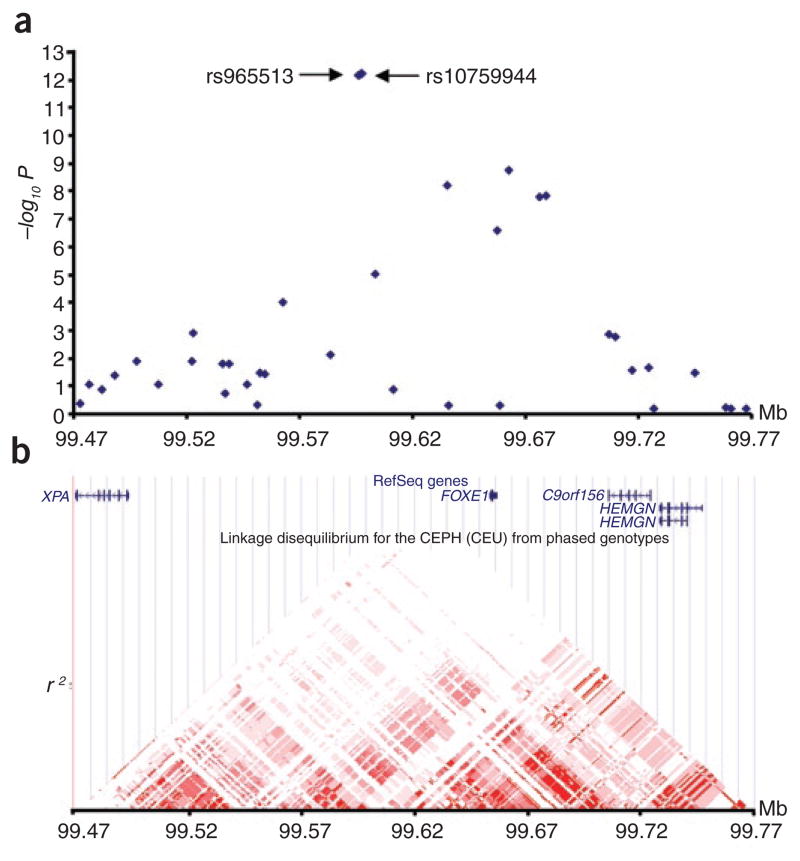
A schematic view of the association results and LD structure in a region on chromosome 9q22.33. (a) Single-marker (blue diamonds) association results for SNPs from the Illumina Hap300/370 chip. The results are based on analysis of 192 and 37,217 Icelandic thyroid cancer cases and controls, respectively, and shown are P values corrected for relatedness. (b) Pairwise correlation coefficient (r2) from the CEU HapMap population and the relative location of genes in the region, based on the UCSC Genome Browser, build 36. The locations of rs965513 and rs10759944 are not resolved in this image.
Table 1
Association results for rs965513, rs944289 and thyroid cancer in Iceland, Spain and the United States
| Study population (n cases/n controls) Variant (allele) | Frequency
| OR (95% CI) | P value | |
|---|---|---|---|---|
| Cases | Controls | |||
| Results for rs965513[A] on 9q22.33 | ||||
| Iceland genome-wide scan (378a/37,196) | 0.484 | 0.352 | 1.73 (1.49, 2.01) | 7.5 × 10−13 |
| Iceland all (579b/37,196) | 0.490 | 0.352 | 1.77 (1.57, 2.00) | 6.8 × 10−20 |
| Columbus, Ohio, US (294/384) | 0.471 | 0.329 | 1.81 (1.45, 2.26) | 1.2 × 10−7 |
| Spain (89/1,343) | 0.444 | 0.342 | 1.54 (1.13, 2.09) | 6.5 × 10−3 |
| Combined Columbus and Spain (383/1,727) | – | 0.336 | 1.72 (1.43, 2.05) | 3.7 × 10−9 |
| All combined (962/38,923)c | – | 0.341 | 1.75 (1.59, 1.94) | 1.7 × 10−27 |
| Results for rs944289[T] on 14q13.3 | ||||
| Iceland genome-wide scan (378a/37,083) | 0.650 | 0.558 | 1.48 (1.26, 1.72) | 8.6 × 10−7 |
| Iceland all (574b/37,083) | 0.644 | 0.558 | 1.44 (1.26, 1.63) | 2.5 × 10−8 |
| Columbus, Ohio, US (342/381) | 0.654 | 0.591 | 1.32 (1.06, 1.63) | 1.2 × 10−2 |
| Spain (90/881) | 0.600 | 0.569 | 1.14 (0.83, 1.55) | 4.3 × 10−1 |
| Combined Columbus and Spain (432/1,262) | – | 0.580 | 1.26 (1.05, 1.50) | 1.1 × 10−2 |
| All combined (1,006/38,345)c | – | 0.573 | 1.37 (1.24, 1.52) | 2.0 × 10−9 |
Shown are the corresponding numbers of cases and controls (n), allelic frequencies of variants in affected and control individuals, the allelic odds ratio (OR) with 95% confidence interval (95% CI) and P values based on the multiplicative model. All P values shown are two-sided.
We next tested the association of the two genome-wide significant variants, rs965513 on 9q22.33 and rs944289 on 14q13.3, with thyroid cancer in two case-control groups of European descent, with populations from Columbus, Ohio, United States (342 cases and 384 controls) and Spain (90 cases and 1,343 controls). The results for both SNPs were replicated in both study groups (Table 1). A test of heterogeneity in the ORs between the three study populations showed no significant difference (P = 0.58 for rs965513 and P = 0.35 for rs944289). Combination of the results from Iceland, Columbus and Spain gave an estimated OR of 1.75 for rs965513[A] (P = 1.7 × 10−27), whereas rs944289[T] gave an OR of 1.37 (P = 2.0 × 10−9) (Table 1).
In order to investigate the mode of inheritance, we computed the genotype-specific ORs and found that the multiplicative model provided an adequate fit for both variants (Supplementary Table 2 online). Approximately 11% and 32% of individuals in the general population are homozygous carriers of rs965513[A] or rs944289[T], respectively, and 3.7% are homozygous for both variants. Homozygous carriers of rs965513[A] or rs944289[T] are estimated to have 3.1- and 1.9-fold greater risk, respectively, of developing the disease than noncarriers. Doubly homozygous individuals have an estimated 5.7-fold greater risk. Furthermore, we observed that the frequency of rs965513[A] was higher among subjects diagnosed at a younger age in all three populations. With the data combined, it is estimated that, for each allele carried, age at diagnosis is reduced by 2.42 y (P = 0.0014; Supplementary Table 3 online). No significant age effect was observed for rs944289 and no significant difference was seen, for either variant, between the observed effects on males and females (P = 0.19 and P = 0.56 for rs965513[A] and rs944289[T], respectively; Supplementary Table 3).
We analyzed the effect of rs965513 and rs944289 in the four main histological classes of thyroid cancer. Most of the Spanish and Icelandic sample collections consist of PTC (~85%) and FTC (~12%) and all of the cases from Columbus were PTC. For rs965513[A] on 9q22.33, the observed OR for PTC in the combined analysis of the three populations was 1.80 (P = 4.7 × 10−23) and for FTC the OR was 1.55, calculated using the Icelandic and Spanish samples only (P = 0.016) (Supplementary Table 4 online). Similarly, for rs944289[T] on 14q13.3, the OR for the PTC in the combined analysis of all three study populations was 1.32 (P = 2.0 × 10−6) and for FTC the OR was 1.63, calculated from the Icelandic and Spanish samples only (P = 0.0071) (Supplementary Table 4). This demonstrates that both variants affect the risk of the two main histological types of thyroid cancer. The numbers of other histological thyroid cancer types were too limited to draw meaningful conclusions.
The SNP rs965513 resides on 9q22.33 within a LD region where the following genes have been localized: XPA, FOXE1, C9orf156 and HEMGN (Fig. 1). The closest gene is FOXE1, located about 57 kb telomeric to rs965513. FOXE1 is important for both pituitary- and thyroid-gland formation16,17 and is at the center of a regulatory network of transcription factors and cofactors that initiate thyroid differentiation at the embryonic stage18. Furthermore, mutations of the FOXE1 gene cause human syndromes that are associated with thyroid agenesis, among other phenotypes17,19. FOXE1 is also necessary for the maintenance of the differentiated state of the thyroid, as it is involved in regulating the transcription of thyroid-specific genes, such as the TG (thyroglobulin) and TPO (thyroperoxidase) genes. Regulated expression of both of these genes is pivotal for the synthesis of the thyroid hormones triiodothyronine (T3) and thyroxine (T4), as Tg is the precursor of the T3 and T4, and their synthesis is catalyzed by TPO. Central to the thyroid hormone synthesis and secretion control is the thyroid stimulating hormone (TSH), which acts as principal regulator.
The 14q13.3 SNP rs944289 is in a 249-kb LD-region with no RefSeq genes. The closest genes are: BRMS1L, MBIP, SFTA3 and NKX2-1(TTF1) (Supplementary Fig. 2 online). Although several of these genes have been implicated in cancers at various sites, NKX2-1 is probably the best candidate as a source of the association signal, because it has a prominent role in the development of the thyroid18 and its expression is altered in thyroid tumors20.
Given the involvement of FOXE1 and NKX2-1 in the biology of the thyroid gland, we assessed the effect of rs965513[A] and rs944289[T] on circulating levels in serum of TSH (N = 12,035), free T4 (N = 7,108) and free T3 (N = 3,593). The data used came from a series of measurements collected over a period of 11 y (from 1997 to 2008) from Icelanders not known to have thyroid cancer (Supplementary Table 5 online). Both variants were associated with (P = 2.90 × 10−14 and P = 0.03 for rs965513[A] and rs944289[T], respectively) a decrease in serum concentrations of TSH, by 5.9% and 1.7% per copy of rs965513[A] and rs944289[T], respectively (Table 2). rs965513[A] on 9q22.33 was also associated with serum concentrations of T3 and T4, but in opposite directions; each copy of rs965513[A] was associated with an increase in T3 levels by 1.2% and a decrease in T4 levels by 1.2% (P = 3.00 × 10−3 and 6.10 × 10−5 for T3 and T4, respectively) (Table 2). For rs944289[T] on 14q13.3, no significant effect on either T3 or T4 was observed (Table 2). These data demonstrate that at least the 9q22.33 variant affects some aspects of the endocrine function of the thyroid.
Table 2
Association results for rs965513, rs944289 and levels of thyroid related hormones in Icelandic individuals
| Type of measurement | Individuals (n) | Effect per risk allele (95% CI) | P value |
|---|---|---|---|
| Results for rs965513[A] on 9q22.33 | |||
| Thyroid stimulating hormone (TSH) | 12,035 | −5.9% (−7.4%, −4.4%) | 2.9 × 10−14 |
| Free thyroxine (T4) | 7,108 | −1.2% (−1.8%, −0.6%) | 6.1 × 10−5 |
| Free triiodothyronine (T3) | 3,593 | +1.2% (+0.4%, +2.0%) | 3.0 × 10−3 |
| Results for rs944289[T] on 14q13.3 | |||
| Thyroid stimulating hormone (TSH) | 11,925 | −1.7% (−3.2%, −0.2%) | 0.030 |
| Free thyroxine (T4) | 6,931 | +0.5% (−0.1%, +1.0%) | 0.098 |
| Free triiodothyronine (T3) | 3,564 | −0.3% (−1.1%, +0.5%) | 0.44 |
Shown are association results (per risk allele) for individuals (n) with a given type of measurement and a known carrier status for rs965513 and rs944289. The minus sign (−) denotes a decreased and the plus sign (+) an increased concentration of thyroid related hormones (see Supplementary Methods and Supplementary Table 5).
Taken together, the effect of the 9q22.33 SNP (rs965513) on thyroid and thyroid-related hormones, the proximity of rs965513 to FOXE1, and the controlling effect of FOXE1 on thyroid-specific genes strongly suggest that the association between thyroid cancer and rs965513 is mediated through processes involving FOXE1. Furthermore, the expression of FOXE1 has been shown to be abnormal in thyroid tumors21. The explanation of the 14q13.3 association signal seems to be similar and mediated through NKX2-1, although the data are less compelling. Compared to the common susceptibility variants recently discovered for complex diseases, the relative risks associated with the variants reported here are quite high. Together, they have a population attributable risk (PAR) of 57% for populations of European descent. Furthermore, we have demonstrated that these variants influence crucial players in the thyroid hormonal pathway. These variants are therefore likely to be among the most important determinants of genetic susceptibility to thyroid cancer.
METHODS
Subjects
Icelandic study population
Individuals diagnosed with thyroid cancer were identified on the basis of a nationwide list from the Icelandic Cancer Registry (ICR) (see URLs section below) that contained all 1,110 Icelandic subjects with thyroid cancer diagnosed from January 1, 1955 to December 31, 2007. Of these, 1,097 were nonmedullary thyroid cancers. The Icelandic thyroid cancer study population consists of 460 subjects (diagnosed from December 1974 to June 2007) recruited from November 2000 until April 2008, of whom 454 (98%) were successfully genotyped in this study. The histology of all thyroid carcinomas used in the present study has been reviewed and confirmed by one of the authors of this article (J.G.J.). A total of 192 subjects were included in a genome-wide SNP genotyping effort, using Illumina Sentrix HumanHap300 (n = 96) and HumanCNV370-duo Bead Chip (n = 96) microarrays (Illumina) and were successfully genotyped according to our quality control criteria (see below) and used in the present case-control association analysis. The remaining 241 cases were genotyped using the Centaurs single-track genotyping platform (see below). The mean age at diagnosis for the consenting subjects was 44 y (median 43 y) and the range was from 13 to 87 y. The mean age at diagnosis was 56 y for all subjects with thyroid cancer in the ICR. The median time from diagnosis to blood sampling was 10 y (range 0 to 46 y). When we compared the frequency of rs965513[A] between individuals diagnosed before 1998 and those diagnosed 1998 or later no significant difference was observed (P = 0.97). The 37,202 controls (16,109 males (43.3%) and 21,093 females (56.7%)) used in this study consisted of individuals belonging to different genetic research projects at deCODE. The individuals have been diagnosed with common diseases of the cardiovascular system (for example, stroke or myocardial infarction), psychiatric and neurological diseases (for example, schizophrenia, bipolar disorder), endocrine and autoimmune system diseases (for example, type 2 diabetes, asthma) and malignant diseases (for example, cancer of the breast or prostate), and also included are individuals randomly selected from the Icelandic genealogical database. No single disease project represented more than 6% of the total number of controls. The controls had a mean age of 84 y and the range was from 8 to 105 y. A linear regression analysis showed no correlation between allele frequency of rs965513[A] and year of birth among the Icelandic controls (P > 0.2). The controls were absent from the nationwide list of individuals with thyroid cancer according to the ICR. The DNA for both the Icelandic cases and controls was isolated from whole blood using standard methods.
The study was approved by the Data Protection Commission of Iceland and the National Bioethics Committee of Iceland. Written informed consent was obtained from all subjects. Personal identifiers associated with medical information and blood samples were encrypted with a third-party encryption system as previously described22.
Columbus, Ohio, United States
The study was approved by the institutional review board of Ohio State University. All subjects provided written informed consent. Cases (n = 342) were individuals with histologically confirmed papillary thyroid carcinoma (including traditional PTC and follicular variant PTC). These subjects were admitted to the Ohio State University Comprehensive Cancer Center, with the exception of one case obtained through Cooperative Human Tissue Network (CHTN); this case was admitted to the University of Pennsylvania Medical Center. All cases were of European descent (92 men, 250 women, median age 40 y, range 13 to 88 y). The genomic DNA was extracted either from blood samples, or fresh frozen normal thyroid tissues from individuals with PTC. Controls (n = 384) were individuals without clinically diagnosed thyroid cancers from central Ohio area. All controls were of European descent (143 men, 241 women, median age 51 y, range 18 to 94 y).
Spain
The Spanish study population consisted of 90 individuals with thyroid cancer. The subjects were recruited from the Oncology Department of Zaragoza Hospital in Zaragoza, Spain, from October 2006 to June 2007. All subjects were of self-reported European descent. Clinical information including age at onset, grade and stage was obtained from medical records. The average age at diagnosis for the subjects was 48 y (median 49 y) and the range was from 22 to 79 y. The 1,343 Spanish control individuals (579 (43%) males and 764 (57%) females), who had a mean age of 51 (median age 50 and range 12–87 y) were approached at the University Hospital in Zaragoza, Spain, and were not known to have thyroid cancer. The DNA for both the Spanish cases and controls was isolated from whole blood using standard methods. Study protocols were approved by the institutional review board of Zaragoza University Hospital. All subjects gave written informed consent.
Statistical analysis
Association analysis
We used a likelihood procedure described in a previous publication23 and implemented in the NEMO software for the association analyses. An attempt was made to genotype all individuals for the SNPs reported in Table 1. The yield was higher than 95% for the SNPs in every group. We tested the association of an allele with thyroid cancer using a standard likelihood ratio statistic that, if the subjects were unrelated, would have asymptotically a χ;2 distribution with one degree of freedom under the null hypothesis. Allelic frequencies rather than carrier frequencies are presented for the markers in the main text. Allele-specific ORs and associated P values were calculated assuming a multiplicative model for the two chromosomes of an individual24. For each of the three case-control groups there was no significant deviation from Hardy-Weinberg equilibrium in the controls (P > 0.3). Results from multiple case-control groups were combined using a Mantel-Haenszel model25 in which the groups were allowed to have different population frequencies for alleles and genotypes but were assumed to have common relative risks (see ref. 26 for a more detailed description of the association analysis).
Correction for relatedness and genomic control
Some individuals in the Icelandic GWAS group were related to each other, causing the aforementioned χ2 test statistic to have a mean >1. We estimated the inflation factor by using a method of genomic control14, calculating the average of the 304,083 χ2 statistics. According to this method the inflation factor was estimated to be 1.09. On the basis of the change in sample size of genotyped and in silico genotyped cases due to single assay genotyping, we estimated the inflation factor in the combined Icelandic sample set to be 1.12 (ref. 27). The χ2 statistics for the test for association with thyroid cancer in the combined Icelandic samples presented in the main text and Table 1 were adjusted accordingly.
URLs
National Cancer Institute Surveillance Epidemiology and End Results (SEER), http://seer.cancer.gov/; Icelandic Cancer Registry, http://www.krabbameinsskra.is/indexen.jsp?icd=C73; University of California Santa Cruz Genome Browser, http://genome.ucsc.edu/.
Acknowledgments
We thank the study participants whose contribution made this work possible. This project was funded in part by the following contract numbers: US National Institutes of Health CA16058 and CA124570.
Footnotes
Note: Supplementary information is available on the Nature Genetics website.
AUTHOR CONTRIBUTIONS
The study was designed and results were interpreted by J.G., P.S., D.F.G., J.T.B., A.K. and K.S. Statistical analysis was carried out by P.S., D.F.G., F.G., J.G., J.T.B., M.L.F. and A.K. Subject recruitment, biological material collection and handling was organized and carried out by J.G., J.G.J., J.T.B., S.N.S., H. He, R.N., E.A., E.F., E.P., B.S., M.M., G.I.E., U.S.B., H. Holm, K.K., H.K., J.R.G., T.J., T.R., H. Hjartarsson, J.I.M., A.d.l.C., J.H. and U.T. Genotyping was supervised and carried out by J.G, J.T.B., A.S., H. He, M.J., D.N.M., S.M., O.B.S., H. Helgadottir, W.L., T.B., A.d.l.C., T.R. and U.T. Authors J.G., P.S., D.F.G. and K.S. drafted the manuscript. All authors contributed to the final version of the paper. Principal collaborators for the replication case-control samples were J.I.M. (Spain) and A.d.l.C. (US).
COMPETING INTERESTS STATEMENT
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturegenetics/.
Reprints and permissions information is available online at http://npg.nature.com/reprintsandpermissions/
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/ng.339
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3664837?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/ng.339
Article citations
Association of telomerase reverse transcriptase gene rs10069690 variant with cancer risk: an updated meta-analysis.
BMC Cancer, 24(1):1059, 27 Aug 2024
Cited by: 0 articles | PMID: 39192222 | PMCID: PMC11350973
Chromosomal localization of mutated genes in non-syndromic familial thyroid cancer.
Front Oncol, 14:1286426, 20 Mar 2024
Cited by: 0 articles | PMID: 38571492 | PMCID: PMC10987779
Review Free full text in Europe PMC
Identification of Germline FOXE1 and Somatic MAPK Pathway Gene Alterations in Patients with Malignant Struma Ovarii, Cleft Palate and Thyroid Cancer.
Int J Mol Sci, 25(4):1966, 06 Feb 2024
Cited by: 0 articles | PMID: 38396644 | PMCID: PMC10888156
LncRNA HAGLROS contribute to papillary thyroid cancer progression by modulating miR-206/HMGA2 expression.
Aging (Albany NY), 15(24):14930-14944, 18 Dec 2023
Cited by: 2 articles | PMID: 38112616 | PMCID: PMC10781464
Radiation-Related Thyroid Cancer.
Endocr Rev, 45(1):1-29, 01 Jan 2024
Cited by: 6 articles | PMID: 37450579 | PMCID: PMC10765163
Review Free full text in Europe PMC
Go to all (246) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
SNPs (4)
- (11 citations) dbSNP - rs965513
- (9 citations) dbSNP - rs944289
- (3 citations) dbSNP - rs10759944
- (1 citation) dbSNP - rs622450
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Common variants at 9q22.33, 14q13.3, and ATM loci, and risk of differentiated thyroid cancer in the French Polynesian population.
PLoS One, 10(4):e0123700, 07 Apr 2015
Cited by: 24 articles | PMID: 25849217 | PMCID: PMC4388539
Fine-mapping of two differentiated thyroid carcinoma susceptibility loci at 9q22.33 and 14q13.3 detects novel candidate functional SNPs in Europeans from metropolitan France and Melanesians from New Caledonia.
Int J Cancer, 139(3):617-627, 30 Mar 2016
Cited by: 7 articles | PMID: 26991144
Confirmation of papillary thyroid cancer susceptibility loci identified by genome-wide association studies of chromosomes 14q13, 9q22, 2q35 and 8p12 in a Chinese population.
J Med Genet, 50(10):689-695, 11 Jul 2013
Cited by: 53 articles | PMID: 23847140
Common variants at the 9q22.33, 14q13.3 and ATM loci, and risk of differentiated thyroid cancer in the Cuban population.
BMC Genet, 16:22, 01 Mar 2015
Cited by: 24 articles | PMID: 25879635 | PMCID: PMC4354996
Funding
Funders who supported this work.
NCI NIH HHS (4)
Grant ID: CA124570
Grant ID: P01 CA124570
Grant ID: P30 CA016058
Grant ID: CA16058





