Abstract
Objective
Existing evidence suggests telomerase activation is a crucial step in tumorigenesis. The telomerase reverse transcriptase (TERT), encoded by the human TERT gene, is critical for telomerase expression. The TERT rs10069690 (C > T) variant was identified to be associated with the risk of cancer, however, there have been inconsistent results. Therefore, we performed a comprehensive meta-analysis aiming to clarify the association between this variant and cancer susceptibility.Methods
We conducted literature search in PubMed, EMbase, MEDLINE and Cochrane Library up to April 30, 2024. Overall, there are 55 studies involving 334,196 patients with cancer and 741,187 controls included in the present study. All statistical analyses were performed by STATA software (version 11.0).Results
The pooled results showed a significant association between rs10069690 and an increased risk of cancer under allele model (OR = 1.10, 95% CI: 1.07-1.13, P < 0.001), especially in European and Asian populations. When stratified by cancer types, this variant was associated with elevated risk of breast cancer (OR = 1.11, 95% CI: 1.07-1.15, P < 0.001), ovarian cancer (OR = 1.14, 95% CI: 1.10-1.19, P < 0.001), lung cancer (OR = 1.20, 95% CI: 1.07-1.35, P = 0.003), thyroid cancer (OR = 1.23, 95% CI: 1.15-1.32, P < 0.001), gastric cancer (OR = 1.31, 95% CI: 1.19-1.45, P < 0.001), and renal cell carcinoma (OR = 1.29, 95% CI: 1.07-1.55, P = 0.007), while decreased risk was found for hepatocellular carcinoma, prostate cancer and pancreatic cancer. Our results also indicated that this variant was significantly associated with solid cancer (OR = 1.11, 95% CI: 1.07-1.14, P < 0.001), but not with hematological tumor.Conclusion
This systematic meta-analysis demonstrated that the TERT rs10069690 variant was a risk factor for cancer. However, the effects of this variant may vary in different types of cancer and differ across ethnic populations.Free full text

Association of telomerase reverse transcriptase gene rs10069690 variant with cancer risk: an updated meta-analysis
Abstract
Objective
Existing evidence suggests telomerase activation is a crucial step in tumorigenesis. The telomerase reverse transcriptase (TERT), encoded by the human TERT gene, is critical for telomerase expression. The TERT rs10069690 (C >
> T) variant was identified to be associated with the risk of cancer, however, there have been inconsistent results. Therefore, we performed a comprehensive meta-analysis aiming to clarify the association between this variant and cancer susceptibility.
T) variant was identified to be associated with the risk of cancer, however, there have been inconsistent results. Therefore, we performed a comprehensive meta-analysis aiming to clarify the association between this variant and cancer susceptibility.
Methods
We conducted literature search in PubMed, EMbase, MEDLINE and Cochrane Library up to April 30, 2024. Overall, there are 55 studies involving 334,196 patients with cancer and 741,187 controls included in the present study. All statistical analyses were performed by STATA software (version 11.0).
Results
The pooled results showed a significant association between rs10069690 and an increased risk of cancer under allele model (OR =
= 1.10, 95% CI: 1.07–1.13, P
1.10, 95% CI: 1.07–1.13, P <
< 0.001), especially in European and Asian populations. When stratified by cancer types, this variant was associated with elevated risk of breast cancer (OR
0.001), especially in European and Asian populations. When stratified by cancer types, this variant was associated with elevated risk of breast cancer (OR =
= 1.11, 95% CI: 1.07–1.15, P
1.11, 95% CI: 1.07–1.15, P <
< 0.001), ovarian cancer (OR
0.001), ovarian cancer (OR =
= 1.14, 95% CI: 1.10–1.19, P
1.14, 95% CI: 1.10–1.19, P <
< 0.001), lung cancer (OR
0.001), lung cancer (OR =
= 1.20, 95% CI: 1.07–1.35, P
1.20, 95% CI: 1.07–1.35, P =
= 0.003), thyroid cancer (OR
0.003), thyroid cancer (OR =
= 1.23, 95% CI: 1.15–1.32, P
1.23, 95% CI: 1.15–1.32, P <
< 0.001), gastric cancer (OR
0.001), gastric cancer (OR =
= 1.31, 95% CI: 1.19–1.45, P
1.31, 95% CI: 1.19–1.45, P <
< 0.001), and renal cell carcinoma (OR
0.001), and renal cell carcinoma (OR =
= 1.29, 95% CI: 1.07–1.55, P
1.29, 95% CI: 1.07–1.55, P =
= 0.007), while decreased risk was found for hepatocellular carcinoma, prostate cancer and pancreatic cancer. Our results also indicated that this variant was significantly associated with solid cancer (OR
0.007), while decreased risk was found for hepatocellular carcinoma, prostate cancer and pancreatic cancer. Our results also indicated that this variant was significantly associated with solid cancer (OR =
= 1.11, 95% CI: 1.07–1.14, P
1.11, 95% CI: 1.07–1.14, P <
< 0.001), but not with hematological tumor.
0.001), but not with hematological tumor.
Conclusion
This systematic meta-analysis demonstrated that the TERT rs10069690 variant was a risk factor for cancer. However, the effects of this variant may vary in different types of cancer and differ across ethnic populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12833-2.
Introduction
Telomeres are located on the ends of chromosomes which play a crucial role in maintaining chromosome integrity and genomic stability [1]. Telomeres comprise tandem (TTAGGG)n nucleotide repeats and shorten gradually with each cycle of cell division [2]. The shortening of telomeres restricts the proliferation of normal somatic cells. On the contrary, cancer cells can maintain long telomeres and then proliferate indefinitely [3]. It is demonstrated that telomere attrition in leukocytes is inversely correlated with aging, and the length of leucocyte telomere is related with age-related diseases including cancer [4, 5]. As is known now, telomere length is maintained by telomerase, a cellular ribonucleoprotein with reverse transcriptase activity, which is required for synthesis and elongation of telomeric ends. The expression of telomerase is extremely low in most normal somatic cells, but the activation of telomerase is widely observed in malignancies [6]. The human TERT gene at 5p13.33 encodes the telomerase reverse transcriptase (TERT), which is the catalytic subunit of telomerase [7]. As the determining factor for telomerase activity, TERT can exert a significant influence on maintaining cell immortality and cancer development. Genetic variants in the TERT gene were shown to increase the susceptibility to cancer [8]. Common TERT variations were also identified to associate with telomere length in leukocytes [9]. Genome-wide association studies (GWAS) indicated that the 5p13.33 region (harboring the TERT and CLPTM1L genes) was a risk locus for tumor [10, 11]. The TERT rs10069690 (C >
> T) variant has been found associated with the risk of various types of cancer, such as breast cancer, lung cancer, ovarian cancer, thyroid cancer and gastric cancer [9, 12–14]. Nevertheless, there was evidence to the contrary. The study by Lawi et al. demonstrated that there was no significant difference in the allele and genotype distribution of rs10069690 between Iraqi patients with non-small cell lung carcinoma (NSCLC) and healthy controls [15]. Additional reports demonstrated that this variant was not associated with risk of melanoma, endometrial cancer or renal cell carcinoma (RCC) in European population [16–18]. Although a meta-analysis has been performed on the overall effect of rs10069690 on cancer risk, multiple lines of new evidence from different tumors and diverse ethnic populations have emerged in recent years [13, 15, 19–26]. To provide a more comprehensive understanding and clarify the inconsistency, we therefore conducted an updated meta-analysis to systematically evaluate the association of the TERT rs10069690 variant with the susceptibility to cancer.
T) variant has been found associated with the risk of various types of cancer, such as breast cancer, lung cancer, ovarian cancer, thyroid cancer and gastric cancer [9, 12–14]. Nevertheless, there was evidence to the contrary. The study by Lawi et al. demonstrated that there was no significant difference in the allele and genotype distribution of rs10069690 between Iraqi patients with non-small cell lung carcinoma (NSCLC) and healthy controls [15]. Additional reports demonstrated that this variant was not associated with risk of melanoma, endometrial cancer or renal cell carcinoma (RCC) in European population [16–18]. Although a meta-analysis has been performed on the overall effect of rs10069690 on cancer risk, multiple lines of new evidence from different tumors and diverse ethnic populations have emerged in recent years [13, 15, 19–26]. To provide a more comprehensive understanding and clarify the inconsistency, we therefore conducted an updated meta-analysis to systematically evaluate the association of the TERT rs10069690 variant with the susceptibility to cancer.
Methods
Identification and eligibility of relevant studies
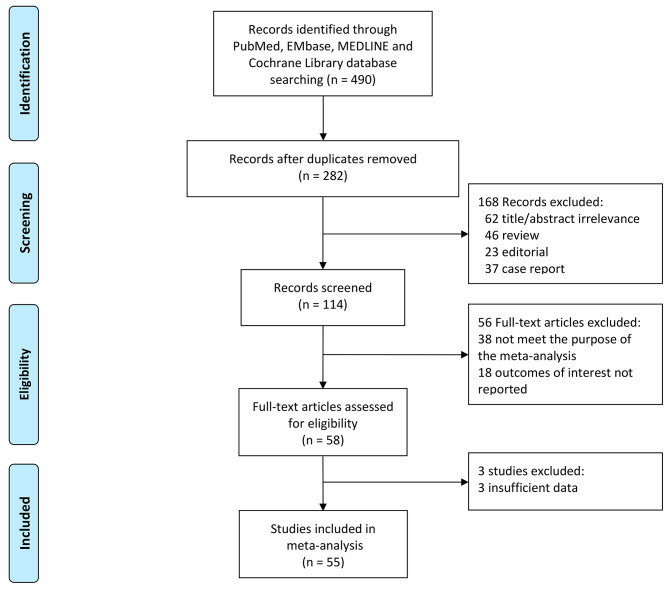
We performed a comprehensive literature search in PubMed, EMbase, MEDLINE and Cochrane Library without language restriction up to April 30, 2024. Eligible studies were selected based on the PICO (Population, Intervention/exposure, Comparison, Outcome/event) criteria (Supplementary Table S1). The search term included combinations of keywords as follows: “TERT”, “telomerase reverse transcriptase”, “polymorphism”, “single nucleotide polymorphism (SNP)”, “variant”, “rs10069690”, “cancer” and “tumor”. Included studies were required to meet the following criteria: being case-control or cohort studies; investigating the relationship between TERT rs10069690 and the risk of cancer; reporting odds ratios (ORs) with 95% confidence intervals (CIs) or sufficient information for effect size calculation. The titles and abstracts of potential articles were screened by two authors independently. The full-texts of relevant articles were then assessed, while the references of included studies were scrutinized and hand-searched for additional eligible studies. The exclusion criteria were commentaries, reviews, non-research letters, case reports, and studies with overlapping or insufficient data. The detailed strategy of study selection is shown in Fig. 1.
Quality assessment and data extraction
To assess the risk of introducing bias, the 9-point Newcastle–Ottawa Scale (NOS) was used to assess the methodological quality of included studies (0–3 as low; 4–6 as moderate and 7–9 as high quality) [27]. Two authors extracted the data in Excel from all the included studies independently, according to the criteria of inclusion and exclusion. Extracted information includes authorship, country, publication year, study design, cancer type, patient ethnicity, number of cases and controls, genotyping method, and risk estimates with corresponding 95% CIs. Any discrepancies were resolved by discussion and consensus.
Statistical analysis
The association between the TERT rs10069690 variant and cancer risk was estimated in OR with corresponding 95% CIs in the allele model. Subgroup analyses were performed by patient ethnicity (stratified as European, Asian and the others), type of cancer and cancer classification (stratified as solid cancer and hematological tumor). For a certain type of cancer, we also conducted stratified analyses based on patient ethnicity when there was at least one ethnic subgroup including more than 2 studies. Since the rs10069690 variant was reported to be specific to estrogen receptor (ER)-negative breast cancer and triple-negative breast cancer (TNBC), we performed a further analysis based on these two subtypes of breast cancer. Cochran’s Q test and I2 index were calculated to explore heterogeneity across included studies [28]. The DerSimonian-Laird random effects model was used to calculate pooled effect estimates if heterogeneity was present (p <
< 0.05) [29]. Otherwise, the fixed-effects model was adopted. To assess the stability of the results, a sensitivity analysis was conducted by removing each individual study in turn from the total and reanalyzing the remainder. Egger’s tests and funnel plots were used to identify potential publication bias [30]. Certainty of the evidence was assessed by GRADE approach [31]. Type I error rate was set at 0.05 for two-sided analysis. All statistical analyses were performed using the STATA software (version 11.0).
0.05) [29]. Otherwise, the fixed-effects model was adopted. To assess the stability of the results, a sensitivity analysis was conducted by removing each individual study in turn from the total and reanalyzing the remainder. Egger’s tests and funnel plots were used to identify potential publication bias [30]. Certainty of the evidence was assessed by GRADE approach [31]. Type I error rate was set at 0.05 for two-sided analysis. All statistical analyses were performed using the STATA software (version 11.0).
Results
Characteristics of included studies
A total of 55 studies involving 334,196 patients with cancer and 741,187 controls were finally included in our study [9, 13–18, 20–26, 32–72]. The main characteristics of included studies are shown in Supplementary Table S2. As for methodological quality assessment, 34 studies were awarded ≥
≥ 8 points, and 21 studies were awarded 6 to 7 points, indicating that included studies were of median-to-high quality. When stratified by the types of cancer, there are 12 studies on breast cancer, 7 on lung cancer, 6 on ovarian cancer, 4 on leukemia, 3 on thyroid cancer, 3 on gastric cancer, 2 on hepatocellular carcinoma (HCC), 2 on RCC, 2 on prostate cancer, 2 on pancreatic cancer, 2 on colorectal cancer, 2 on glioma and one each on other eleven kinds of cancers.
8 points, and 21 studies were awarded 6 to 7 points, indicating that included studies were of median-to-high quality. When stratified by the types of cancer, there are 12 studies on breast cancer, 7 on lung cancer, 6 on ovarian cancer, 4 on leukemia, 3 on thyroid cancer, 3 on gastric cancer, 2 on hepatocellular carcinoma (HCC), 2 on RCC, 2 on prostate cancer, 2 on pancreatic cancer, 2 on colorectal cancer, 2 on glioma and one each on other eleven kinds of cancers.
Association between rs10069690 and cancer risk
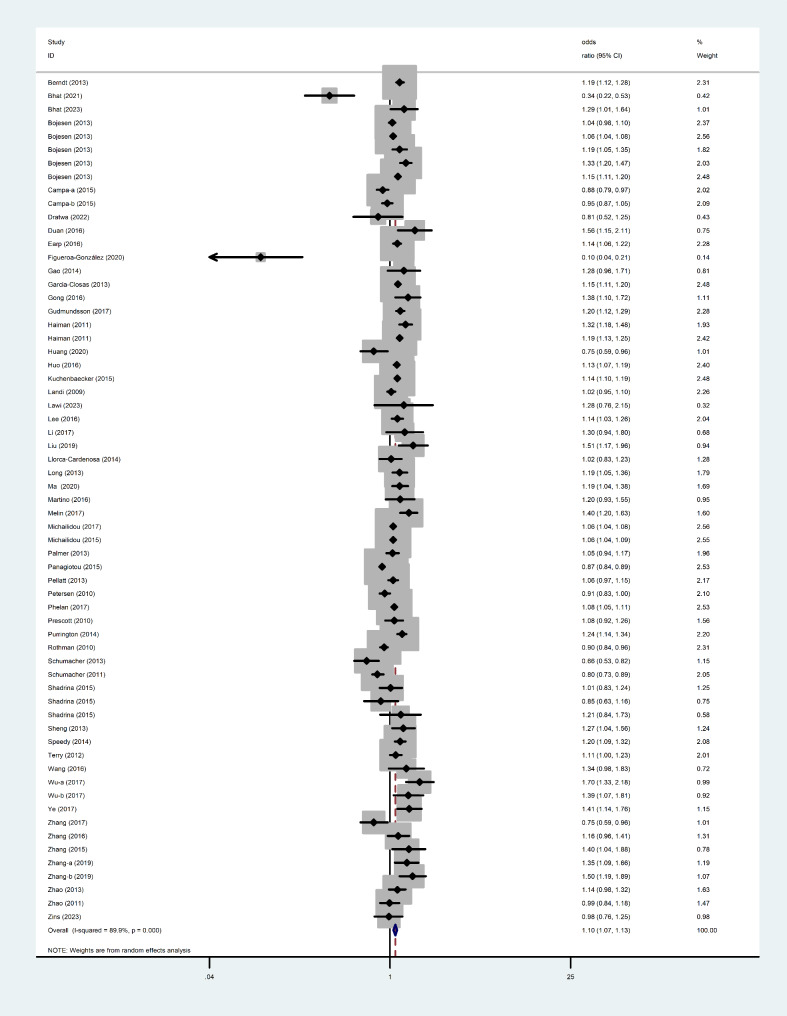
The main results of this meta-analysis were listed in Table 1. Overall, we found the TERT rs10069690 variant was significantly associated with the risk of cancer under allele model (OR =
= 1.10, 95% CI: 1.07–1.13, P
1.10, 95% CI: 1.07–1.13, P <
< 0.001; I2
0.001; I2 =
= 89.9%) (Fig. 2). When stratified by ethnicity, we found significant associations between this variant and increased risk of cancer in European population (OR
89.9%) (Fig. 2). When stratified by ethnicity, we found significant associations between this variant and increased risk of cancer in European population (OR =
= 1.06, 95% CI: 1.02–1.10, P
1.06, 95% CI: 1.02–1.10, P <
< 0.001; I2
0.001; I2 =
= 93.0%) and Asians (OR
93.0%) and Asians (OR =
= 1.23, 95% CI: 1.13–1.34, P
1.23, 95% CI: 1.13–1.34, P =
= 0.004; I2
0.004; I2 =
= 75.0%), but not in other populations (OR
75.0%), but not in other populations (OR =
= 1.07, 95% CI: 0.97–1.17, P
1.07, 95% CI: 0.97–1.17, P =
= 0.173; I2
0.173; I2 =
= 89.0%) (Supplementary Figure S1-S3). When stratified by cancer classification, the analysis indicated that the variant was associated with enhanced risk of solid cancer (OR
89.0%) (Supplementary Figure S1-S3). When stratified by cancer classification, the analysis indicated that the variant was associated with enhanced risk of solid cancer (OR =
= 1.11, 95% CI: 1.07–1.14, P
1.11, 95% CI: 1.07–1.14, P <
< 0.001; I2
0.001; I2 =
= 90.2%), rather than hematological tumor (OR
90.2%), rather than hematological tumor (OR =
= 0.99, 95% CI: 0.85–1.16, P
0.99, 95% CI: 0.85–1.16, P =
= 0.917; I2
0.917; I2 =
= 88.3%).
88.3%).
Table 1
Main results of the meta-analysis of the association between rs10069690 and cancer risk
| Category | No. of studies | OR (95% CI) | P(Z) | P(Q) | I2(%) | Model |
|---|---|---|---|---|---|---|
| Total | 55 | 1.10 (1.07, 1.13) | < 0.001 0.001 | < 0.001 0.001 | 89.9 | RE |
| Ethnicity | ||||||
 European European | 24 | 1.06 (1.02, 1.10) | < 0.001 0.001 | < 0.001 0.001 | 93.0 | RE |
 Asian Asian | 20 | 1.23 (1.13, 1.34) | 0.004 | < 0.001 0.001 | 75.0 | RE |
 Others Others | 15 | 1.07 (0.97, 1.17) | 0.173 | < 0.001 0.001 | 89.0 | RE |
| Cancer type | ||||||
| Breast cancer | 12 | 1.11 (1.07, 1.15) | < 0.001 0.001 | < 0.001 0.001 | 86.0 | RE |
 European European | 7 | 1.09 (1.06, 1.13) | < 0.001 0.001 | < 0.001 0.001 | 82.7 | RE |
 Asian Asian | 1 | 1.04 (0.98, 1.11) | 0.194 | / | / | / |
 Others Others | 7 | 1.13 (1.01, 1.27) | 0.031 | < 0.001 0.001 | 86.8 | RE |
| Ovarian cancer | 6 | 1.14 (1.10, 1.19) | < 0.001 0.001 | 0.002 | 70.8 | RE |
 European European | 4 | 1.15 (1.10, 1.20) | < 0.001 0.001 | < 0.001 0.001 | 80.4 | RE |
 Others Others | 2 | 1.13 (1.05, 1.21) | 0.001 | 0.717 | 0 | FE |
| Lung cancer | 7 | 1.20 (1.07, 1.35) | 0.003 | 0.031 | 56.8 | RE |
 European European | 1 | 1.02 (0.95, 1.10) | 0.596 | / | / | / |
 Asian Asian | 4 | 1.24 (1.12, 1.38) | < 0.001 0.001 | 0.415 | 0 | FE |
 Others Others | 2 | 1.29 (1.03, 1.61) | 0.024 | 0.979 | 0 | FE |
| Gastric cancer | 3 | 1.31 (1.19, 1.45) | < 0.001 0.001 | 0.218 | 32.4 | FE |
 Asian Asian | 3 | 1.31 (1.19, 1.45) | < 0.001 0.001 | 0.218 | 32.4 | FE |
| Thyroid cancer | 3 | 1.23 (1.15, 1.32) | < 0.001 0.001 | 0.141 | 48.9 | FE |
| HCC | 2 | 0.75 (0.63, 0.89) | 0.001 | 1 | 0.0 | FE |
| RCC | 2 | 1.29 (1.07, 1.55) | 0.007 | 0.432 | 0.0 | FE |
| Prostate cancer | 2 | 0.86 (0.84, 0.89) | < 0.001 0.001 | 0.111 | 60.6 | FE |
| Pancreatic cancer | 2 | 0.93 (0.87, 0.99) | 0.031 | 0.524 | 0.0 | FE |
| Leukemia | 4 | 1.04 (0.86, 1.26) | 0.702 | < 0.001 0.001 | 90.5 | RE |
| Colorectal cancer | 2 | 1.07 (0.99, 1.17) | 0.089 | 0.234 | 29.5 | FE |
| Glioma | 2 | 1.18 (0.84, 1.66) | 0.34 | 0.003 | 88.7 | RE |
| Cancer classification | ||||||
| Solid cancer | 49 | 1.11 (1.07, 1.14) | < 0.001 0.001 | < 0.001 0.001 | 90.2 | RE |
| Hematological tumor | 6 | 0.99 (0.85, 1.16) | 0.917 | < 0.001 0.001 | 88.3 | RE |
OR: odds ratios; CI: confidence interval; HCC: hepatocellular carcinoma; RCC: renal cell carcinoma; RE: random effects model; FE: fixed effects model
In the subgroup analyses based on cancer type, the rs10069690 variant had significantly associated with an elevated risk of breast cancer (OR =
= 1.11, 95% CI: 1.07–1.15, P
1.11, 95% CI: 1.07–1.15, P <
< 0.001; I2
0.001; I2 =
= 86.0%) (Supplementary Figure S4). And then, a further stratified analysis based on histopathological subtypes of breast cancer showed similar results (ER-negative breast cancer: OR
86.0%) (Supplementary Figure S4). And then, a further stratified analysis based on histopathological subtypes of breast cancer showed similar results (ER-negative breast cancer: OR =
= 1.18, 95% CI: 1.15–1.22, P
1.18, 95% CI: 1.15–1.22, P <
< 0.001, I2
0.001, I2 =
= 48.6%; triple negative breast cancer: OR
48.6%; triple negative breast cancer: OR =
= 1.24, 95% CI: 1.18–1.31, P
1.24, 95% CI: 1.18–1.31, P <
< 0.001, I2
0.001, I2 =
= 28.8%) (Supplementary Table S3 and Figure S5-S6). Significant association was also observed for ovarian cancer (OR
28.8%) (Supplementary Table S3 and Figure S5-S6). Significant association was also observed for ovarian cancer (OR =
= 1.14, 95% CI: 1.10–1.19, P
1.14, 95% CI: 1.10–1.19, P <
< 0.001; I2
0.001; I2 =
= 70.8%), lung cancer (OR
70.8%), lung cancer (OR =
= 1.20, 95% CI: 1.07–1.35, P
1.20, 95% CI: 1.07–1.35, P =
= 0.003; I2
0.003; I2 =
= 56.8%), gastric cancer (OR
56.8%), gastric cancer (OR =
= 1.31, 95% CI: 1.19–1.45, P
1.31, 95% CI: 1.19–1.45, P <
< 0.001; I2
0.001; I2 =
= 32.4%), thyroid cancer (OR
32.4%), thyroid cancer (OR =
= 1.23, 95% CI: 1.15–1.32, P
1.23, 95% CI: 1.15–1.32, P <
< 0.001; I2
0.001; I2 =
= 48.9%) and RCC (OR
48.9%) and RCC (OR =
= 1.29, 95% CI: 1.07–1.55, P
1.29, 95% CI: 1.07–1.55, P =
= 0.007; I2
0.007; I2 =
= 0%) (Supplementary Figure S7-S11). On the contrary, significant decreased risk was observed for HCC (OR
0%) (Supplementary Figure S7-S11). On the contrary, significant decreased risk was observed for HCC (OR =
= 0.75, 95% CI: 0.63–0.89, P
0.75, 95% CI: 0.63–0.89, P =
= 0.001; I2
0.001; I2 =
= 0%), prostate cancer (OR
0%), prostate cancer (OR =
= 0.86, 95% CI: 0.84–0.89, P
0.86, 95% CI: 0.84–0.89, P <
< 0.001; I2
0.001; I2 =
= 60.6%) and pancreatic cancer (OR
60.6%) and pancreatic cancer (OR =
= 0.93, 95% CI: 0.87–0.99, P
0.93, 95% CI: 0.87–0.99, P =
= 0.031; I2
0.031; I2 =
= 0%) (Supplementary Figure S12-S14). There was no significant association found in the pooled analyses for leukemia, colorectal cancer and glioma (Table 1). However, a correlation between this variant and leukemia was identified after removing the study by Bhat et al. [20] (OR
0%) (Supplementary Figure S12-S14). There was no significant association found in the pooled analyses for leukemia, colorectal cancer and glioma (Table 1). However, a correlation between this variant and leukemia was identified after removing the study by Bhat et al. [20] (OR =
= 1.20, 95% CI: 1.14–1.26, P
1.20, 95% CI: 1.14–1.26, P <
< 0.001; I2
0.001; I2 =
= 0%).
0%).
Sensitivity analysis, publication bias and certainty of the evidence
Sensitivity analysis indicated that no single study yield to obvious influence on the pooled ORs, suggesting that these results are robust (Supplementary Figure S15). Egger’s tests and the funnel-plot analysis showed no publication bias detected for the overall meta-analysis (P >
> 0.05, Supplementary Figure S16). The quality of the evidence was rated as moderate for the overall meta-analysis due to the inconsistency according to the GRADE approach (Supplementary Table S4).
0.05, Supplementary Figure S16). The quality of the evidence was rated as moderate for the overall meta-analysis due to the inconsistency according to the GRADE approach (Supplementary Table S4).
Discussion
In the present study, we performed a meta-analysis to evaluate the impact of TERT rs10069690 variant on cancer susceptibility. The results indicated that this variant was significantly associated with the risk of cancer, which is consistent with previous findings. Due to the ethnic differences, this association retained in Europeans and Asians, but not in other populations. Compared with the previous meta-analysis [19], our study comprised a larger number of studies which allows a more comprehensive assessment. In addition, we performed more refined subgroup analyses based on ethnicity and type/subtype of cancer which provides new insights to the relationship between rs10069690 and cancer risk.
There was substantial heterogeneity in the overall meta-analysis, which was significantly reduced in the subgroup analyses by cancer type. This is plausible that the effects of rs10069690 may vary among different tumors. Previous evidence demonstrated that the risk T allele at rs10069690 resulted in co-production of full-length TERT and an additional splice site in intron 4 of TERT, which created an alternatively splice transcript INS1b [73]. The INS1b transcript encoded a catalytically inactive protein and inhibited the telomerase activity leading to telomere shortening and an increased risk of genetic instability and tumorigenesis. This may explain the T allele is associated with an elevated risk of multiple cancers, which is in line with the results of our analyses for breast cancer, ovarian cancer, lung cancer, thyroid cancer, RCC and gastric cancer. However, it is estimated that activation of telomerase is crucial to cellular immortalization and malignant transformation of cancer cells [6]. In this regard, the T allele at rs10069690 would contribute to a decreased risk of cancer, which is in line with our findings for HCC, prostate cancer and pancreatic cancer. This could be explained by the high complexity of cancer etiology. Meanwhile, the interaction between environmental factors and genetic variants may be another important reason. For example, it has been shown that the expression of TERT and telomerase activity is regulated by estrogen in human ovary [74, 75]. In addition, a prior investigation identified a statistically significant interaction between postmenopausal estrogen-alone therapy and the T allele at rs10069690 with the risk of ovarian cancer [44]. Therefore, future research on potential effect of gene-environment interactions on the development of cancer is warranted.
The rs10069690 variant was previously identified in a GWAS study as a risk variant for ER-negative breast cancer and TNBC [40]. Several lines of evidence confirmed this finding [9, 26, 50]. Although some studies found that this variant was associated with the overall risk of breast cancer, the strength of the association with ER-negative cancer was stronger than ER-positive cancer [41]. Interestingly, one study showed that the rs10069690 variant was associated with a reduced risk for breast cancer in a Mexican population [22]. This might be due to the genetic heterogeneity among different populations. In addition, the relatively small sample size may also contribute to the contradictory result. In the present study, our pooled analysis demonstrated that the T allele of rs10069690 was significantly associated with an elevated risk of overall breast cancer. In the further stratified analyses, we found that there was significant association of this variant with risk of ER-negative breast cancer and TNBC. And the association was greater for TNBC than that for ER-negative breast cancer, in accord with prior findings [40, 46, 50]. ER-negative breast cancer and TNBC are aggressive subtypes of breast cancer, generally associated with a high risk of recurrence and poor prognosis. Further investigation of the relationship between rs10069690 and these tumor subtypes would shed light on the underlying mechanism of tumorigenesis and accelerate the identification of new targets for the therapy of breast cancer.
Lung cancer is the second common diagnosed cancer worldwide, and genetic factors play an important role in its pathogenesis [76]. Numerous genetic loci have been identified to be associated with the susceptibility of lung cancer, including the 5p15.33 locus (TERT- CLPTM1L region) [77, 78]. Some studies reported that the 5p15.33 locus was specifically associated with adenocarcinoma, the most common histologic type of lung cancer [43]. However, other reports suggested the TERT rs10069690 variant was associated with an increased risk of both adenocarcinoma and squamous cell carcinoma subtypes [13]. Our results also supported a significant association between this variant and the total lung cancer cohort. However, due to the lack of detailed clinical information of patients, we were not able to perform the meta-analysis stratified by lung cancer subtypes.
Thyroid cancer is the most common malignancy of endocrine system, which can be classified into four main histology groups: papillary (PTC, 80–85% prevalence), follicular (FTC, 10–15% prevalence), medullary (MTC) and undifferentiated or anaplastic thyroid carcinomas [79, 80]. Our results consisted with prior GWAS and association studies in European and Asian populations [39, 81, 82]. Interestingly, one study indicated that the rs10069690 variant was related to PTC risk, especially in females over 45 years of age [24]. Since sex and age have been identified as risk factors for thyroid cancer [83], further prospective investigations are warranted into the influence of these factors on the association of the rs10069690 variant with thyroid carcinoma.
There were three studies on gastric cancer included in the present study all of which were performed in Chinese population. Our result demonstrated that the rs10069690 T allele was significantly associated with an increased risk of gastric cancer. As for colorectal cancer, HCC, RCC, prostate cancer, pancreatic cancer and glioma, there were only two studies included in the pooled analysis, respectively. Our analysis demonstrated that there was significant association between the rs10069690 variant and an increased risk of RCC. On the contrary, the T allele of rs10069690 was found to associate with decreased risk of HCC, prostate cancer and pancreatic cancer. But due to the limited data and small sample size, the results should be interpreted with caution and further evaluation of the association among different races and ethnic groups is needed. Our result showed no significant association for colorectal cancer and glioma. However, it is worthy to note that this variant might have a significant interaction with lifestyle factors to influence the cancer risk. Individuals with body mass index (BMI) >
> 30 were found to have an elevated risk of colon cancer in the presence of the T allele of rs10069690 [52]. This finding once again highlights the important role of gene-environmental interaction in the cancer etiology.
30 were found to have an elevated risk of colon cancer in the presence of the T allele of rs10069690 [52]. This finding once again highlights the important role of gene-environmental interaction in the cancer etiology.
When stratified by solid cancer and hematological tumor, there was a significant association for solid cancer, but not for hematological tumor. Interestingly, in the subgroup analysis for leukemia, we found significant association after removing one study and reanalyzing the remaining three studies in European and Asian populations. This study by Bhat et al. investigated the role of the rs10069690 variant in the population of north India, and found a higher frequency of T allele in control group than in case group [20]. This discrepancy could be explained by different genetic background across populations. And our finding suggested that rs10069690 may be a risk factor for leukemia, while its effect in different ethnic groups still needs further research.
There are some limitations in our study. First, we performed this meta-analysis only based on allele model. Due to the lack of detailed genotype data in most studies, we were not able to calculate ORs under other genetic models. Second, substantial or moderate heterogeneity was observed in the pooled analyses. But after stratified by ethnicity and cancer type, most of heterogeneity reduced, indicating that these may be the main source of heterogeneity. Third, the interaction of gene-environment may affect the association of the TERT rs10069690 variant with cancer risk. However, there was no sufficient information, such as age, gender, BMI and status of smoking or drinking, available for us to correct for and perform a more refined analysis.
Conclusion
In conclusion, this comprehensive meta-analysis showed that the TERT rs10069690 variant is associated with cancer risk, especially in European population and Asians. Our results contributed to a better understanding of the effects of this variant on the susceptibility of different types of tumors. For example, this variant was identified to be significantly associated with the risk of gastric cancer, HCC and prostate cancer, which has not been found in previous meta-analysis. These new findings might be helpful to early clinical diagnosis and the selection of treatment strategy for these cancers. Larger studies with detailed clinical and histopathological data, as well as functional studies, are warranted to validate our finding and unveil the underlying mechanism in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
| TERT | Telomerase reverse transcriptase |
| NSCLC | Non-small cell lung carcinoma |
| RCC | Renal cell carcinoma |
| SNP | Single nucleotide polymorphism |
| TNBC | Triple-negative breast cancer |
| HCC | Hepatocellular carcinoma |
Author contributions
Conceptualization: XW; Data collection and extraction: CZ, YY, LS, LW and JZ; Data analysis and interpretation: CZ and XW; Manuscript writing: CZ and XW; Manuscript editing: XW. All authors have read and approved the final manuscript.
Funding
This work was supported by grant from the Natural Science Foundation of Shanghai (21ZR1433000), Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0674), Ministry of Education of the People’s Republic of China, University-Industry Collaborative Education Program of China (220604408010836), Nurture projects for basic research of Shanghai Chest Hospital (2021YNJCM01) and Laboratory of Ecological Security and Biodiversity Conservation of Cities on the Yangtze River Delta, Shanghai Science and Technology Museum.
Declarations
Not applicable.
Not applicable.
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Articles from BMC Cancer are provided here courtesy of BMC
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
SNPs
- (9 citations) dbSNP - rs10069690
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
TERT rs10069690 polymorphism and cancers risk: A meta-analysis.
Mol Genet Genomic Med, 7(10):e00903, 27 Aug 2019
Cited by: 9 articles | PMID: 31454181 | PMCID: PMC6785442
Association of the Telomerase Reverse Transcriptase rs10069690 Polymorphism with the Risk, Age at Onset and Prognosis of Triple Negative Breast Cancer.
Int J Mol Sci, 24(3):1825, 17 Jan 2023
Cited by: 4 articles | PMID: 36768147 | PMCID: PMC9916321
Polymorphisms in the telomerase reverse transcriptase promoter are associated with risk of breast cancer: A meta-analysis.
J Cancer Res Ther, 12(2):1040-1044, 01 Apr 2016
Cited by: 8 articles | PMID: 27461695
Telomerase reverse transcriptase locus polymorphisms and cancer risk: a field synopsis and meta-analysis.
J Natl Cancer Inst, 104(11):840-854, 20 Apr 2012
Cited by: 96 articles | PMID: 22523397 | PMCID: PMC3611810
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Basic Research Cultivation Program of Shanghai Chest Hospital (1)
Grant ID: 2021YNJCM01
Laboratory of Ecological Security and Biodiversity Conservation of Cities on the Yangtze River Delta, Shanghai Science and Technology Museum
Ministry of Education of the People's Republic of China, University-Industry Collaborative Education Program of China (1)
Grant ID: 220604408010836
Ministry of Education of the People’s Republic of China, University-Industry Collaborative Education Program of China (1)
Grant ID: 220604408010836
Natural Science Foundation of Chongqing (1)
Grant ID: cstc2021jcyj-msxmX0674
Natural Science Foundation of Shanghai (1)
Grant ID: 21ZR1433000

 2
2