Abstract
Free full text

The signalling mechanisms of syndecan heparan sulphate proteoglycans
Summary of recent advances
Syndecans are membrane proteins controlling cell proliferation, differentiation, adhesion and migration. Their extracellular domains bear versatile heparan sulphate chains that provide structural determinants for syndecans to function as co-receptors or activators for molecules like growth factors and constituents of the matrix. Syndecans also signal via their protein cores and their conserved transmembrane and cytoplasmic domains. The direct interactions and signalling cascades they support are becoming better characterized. These interactions are regulated by phosphorylation, induced clustering and shedding of the syndecan extracellular domain. Moreover evidence is emerging that syndecans concentrate in unconventional lipid domains and might govern novel vesicular trafficking pathways. Here we focus on recent findings that refine our understanding of the complex structure-function relationships of these cellular effectors.
Introduction
Syndecans are membrane proteins that are expressed at up to one million copies per cell in nearly every cell of the body. Mammals express four different syndecans. Each syndecan family member has a distinctive temporal and spatial pattern of expression. Syndecan-1 is primarily expressed in epithelial and plasma cells, syndecan-2 is mainly expressed in fibroblasts, endothelial cells, neurons and smooth muscle cells, syndecan-3 is the major syndecan of the nervous system but is also important for chondrocyte proliferation, and syndecan-4 is nearly ubiquitous.
In invertebrates like Drosophila melanogaster and C. elegans, there is only a single syndecan gene, which is essential for neuronal development and axon guidance. In mammals, knock-out animals are viable and fertile but show multiple defects following exposure to physiological stresses [1]. These include perturbed wound healing, angiogenesis and inflammation for syndecan-1-/- and -4-/-. Interestingly, syndecan-1 null mice are protected from tumour development possibly because of impaired pathogenic activation of progenitor/stem cells [2]. Syndecan-3 deficient mice have difficulties of learning and memory, develop muscular dystrophy and have an abnormal feeding behaviour after food deprivation [3]. To date, syndecan-2 null mice have not been reported, but studies in Xenopus and zebrafish point to a role in left-right asymmetry and VEGF induced vascular development [4]. In these same models, syndecan-4 is required for modulating migration processes during embryonic development that are regulated by the non-canonical Wnt pathway and for neural induction [5-7]. Xenopus syndecan-1 regulates dorso-ventral patterning of the ectoderm by modulating BMP signalling [8]. The severity of Xenopus/fish versus mice knock-out phenotypes may be because shorter gestational times are prohibitive of compensation mechanisms. The analysis of combined knock-outs should clarify how mammals accommodate the lack of multiple syndecans.
Structurally, all syndecans are composed of an extracellular domain (ED), a characteristic transmembrane region (TM) and a conserved short C-terminal cytoplasmic domain (CD). The ED is substituted with HS chains synthesized and modified in the Golgi-apparatus and edited post-synthetically by sulfatases and heparanase (Figure 1). The structural features of the HS chains are responsible for the interaction of syndecans with a number of soluble factors, cell-associated molecules and extracellular matrix components. In some cases HS engagement can promote the active conformation of the ligand. Generally, syndecans do not function as primary receptors but cooperate with these as co-receptors. Although in vitro experiments clearly indicated that specific HS-structures mediate specific high-affinity binding activities [9], loss-of-function studies of the different HS modifying enzymes in mice have established that compensation mechanisms can take place and that the ‘sugar code’ is quite degenerate [10-12]. Except for the HS attachment sites, the amino-acid sequence of the ED varies considerably between the different syndecan family members. Within a syndecan type there is usually over 70% homology between different mammals. A few studies now document that syndecan EDs contain non-HS intrinsic protein-binding structures such as the NXIP motif in syndecan-4 [13] and the ‘synstatin’ part of syndecan-1 that associates with alpha(v)beta(3) and alpha(v)beta(5) integrins and that can block angiogenesis and tumorigenesis [14]. The TM and CD domains harbour structural features that are unique to syndecans and support signal transduction across the membrane. Syndecans do not appear to encode any intrinsic catalytic activity, however multiple molecular interactions have been identified with kinases, GTPases, cytoskeletal molecules and other intracellular components (Table 1). These interactions are regulated by phosphorylation and clustering of the syndecans. Below we review how structural features of the TM and the CD of syndecans can contribute to their signalling, how heparanase and shedding can modulate their activity and how endocytic routes might support their function. Complementary information can be found in other recent reviews [1,15-17].
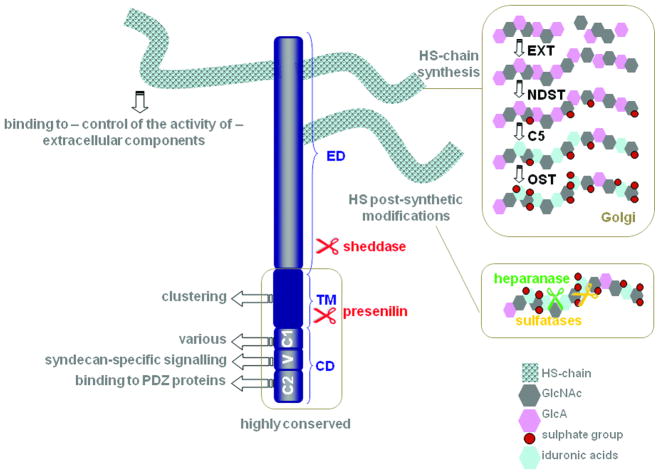
Syndecans consist of an extracellular (ED), transmembrane (TM) and cytoplasmic domain (CD). The CD includes the conserved regions C1 and C2 flanking the variable (V) region, specific for each syndecan. The CD binds to various intracellular proteins supporting signalling (see box 1), the TM supports syndecan oligomerization, and the HS-chains on the ED support interactions with various ligands (e.g. FGF, VEGF, TGF-beta, Wnt, BMP, chemokines, cytokines, proteases, L-selectin, N-CAM, fibronectin, laminin, vitronectin, collagen, thrombospondin-1). The ED can also be substituted by chondroitin sulphate (not shown). HS biosynthetic and post-synthetic modifications determine the strength and outcome of HS-ligand interactions. The HS chains vary with regard to disaccharide composition, domain arrangement and size; parameters that are cell type- and developmental stage-specific. HS-chain assembly is catalyzed by polymerases from the EXT glycosyltransferase family adding units of N-acetylglucosamine (GlcNAc) and glucuronic acid (GlcA). Next, N-deacetylase/N-sulfotransferases (NDST) replace the acetylgroups from GlcNac with sulphate groups, C5-epimerase (C5) modifies GlcA to iduronic acids and sulfotransferases (OST) introduce additional sulphate groups. These modifications are incomplete and not uniformly distributed over the length of the polymer (fine structure) creating highly sulphated or so-called S-domains, transition zones and non-sulphated or NA-domains (not shown). At the cell surface, HS-chain structure is further refined by sulfatases that selectively remove sulphates and heparanase that cleaves the HS-chains (see text). Sulfatase activity modulates Wnt, FGF2 and BMP signalling. The syndecan ED can be separated from the TM and the CD by sheddases that cleave the core protein. The remaining C-terminal fragments are processed by presenilin, with further cytosolic release of the CD.
Table 1
| neurofibromin (TM+C1) | Syndecan-1, -2, -3, -4 interaction was demonstrated by Y2H, biochemical fractionation of rat brain extracts, co-IP of endogenous proteins from rat brain and co-localization of endogenous proteins in neuronal cultures; syndecan-2 interaction is important for filopodia formation in neuronal cells (1-2) |
| syndecan (TM) | Syndecan-1, -2, -3, -4 interaction was demonstrated by in vitro binding and the use of TM peptides in cell free in vitro system (3-5) |
| Rab5 (CD) | Syndecan-1 interaction demonstrated by in vitro binding and co-IP of endogenous proteins; the interaction regulates syndecan-1shedding (6) |
| ezrin (C1) | Syndecan-2 interaction demonstrated by in vitro binding, co-IP and co-localization of endogenous proteins (7-8) |
| Src family, cortactin, tubulin complex (C1) | Syndecan-3 interaction demonstrated by affinity purification, biochemical fractionation of rat brain extracts and co-IP of endogenous proteins; a role in neurite outgrowth was proposed (9) |
| dynamin II (C1) | Syndecan-4 interaction demonstrated by Y2H, in vitro binding, co-IP and co-localization of endogenous proteins; a role in focal adhesion formation was proposed (10) |
| alfa-actinin (V) | Syndecan-4 interaction demonstrated by in vitro binding, co-IP and co-localization of endogenous proteins (11-12) |
| syndesmos (C1+V) | Syndecan-4 interaction demonstrated by Y2H, in vitro binding, co-IP of endogenous proteins and co-localization of overexpressed proteins; syndesmos is important for cell spreading (13) |
| PIP2 (V) | Syndecan-4 interaction demonstrated by NMR spectroscopy; the interaction is important for the super-activation of PKCalpha (14-17) |
| PKCalpha (V) | Syndecan-4 interaction demonstrated by Y2H, in vitro binding and co-IP of endogenous proteins; the interaction is important for cell spreading, focal adhesion formation, polarized migration, Rac1 and Rho regulation, neural induction, tube formation and mTOR Complex2 subcellular recruitment (14, 16, 18-31) |
| synectin (C2) | Syndecan-4 interaction demonstrated by Y2H and co-IP of endogenous proteins; the interaction is important for cell migration (32-33) |
| synbindin (C2) | Syndecan-2 interaction demonstrated by Y2H, in vitro binding, co-localization of overexpressed proteins in neuronal cells and co-IP of endogenous proteins in mouse brain; the interaction has a possible role in dendritic spine formation (34) |
| CASK (C2) | Syndecan-1, -2, -3, -4 interaction demonstrated by Y2H, in vitro binding, biochemical fractionation of rat brain for syndecan-3, co-IP of overexpressed syndecan-2 and CASK, co-IP of endogenous syndecan-3 and CASK from rat brain and co-localization of endogenous proteins in rat epithelial tissues and brain (35-37) |
| syntenin (C2) | Syndecan-1, -2, -3, -4 interaction demonstrated by Y2H, in vitro binding, co-IP for syndecan-1 and co-localization of endogenous proteins; the interaction is important for syndecan endocytic recycling (38-40) |
Interaction mapped in the (TM), Transmembrane domain; (CD), Cytoplasmic domain; (C1), Conserved region 1; (C2), Conserved region 2; (V), Variable region; Y2H, Yeast two hybrid; co-IP, co -immunoprecipitation; NMR, Nuclear magnetic resonance
1) Hsueh et al., 2001; 2) Lin et al., 2007; 3) Bernfield et al., 1992; 4) Asundi and Carey 1995; 5) Dews and MacKenzie 2007; 6) Hayashida et al., 2008; 7) Granés et al., 2000; 8) Granés et al., 2003 ; 9) Kinnunen et al., 1998 ; 10) Yoo et al., 2005 ; 11) Greene et al., 2003; 12) Choi et al., 2008 ;13) Baciu et al., 2000 ; 14) Oh et al., 1998 ; 15) Lee et al., 1998; 16) Horowitz et al., 1998; 17) Couchman et al., 2002; 18) Oh et al., 1997 ; Oh et al., 1998 ; Horowitz et al., 1998; 19) Horowitz et al., 1999; 20) Lim et al., 2003; 21) Keum et al., 2004; 22) Thodeti et al., 2003; 23) Baciu and Goetinck, 1995; 24) Dovas et al., 2006 ; 25) Matthews et al., 2008 ; 26) Bass et al., 2007; 27) Bass et al., 2008 ; 28) Kuriyama et al., 2009; 29) Murakami et al., 2002; 30) Horowitz et al., 2002; 31) Partovian et al., 2008; 32) Gao et al., 2000; 33) Tkachenko et al., 2006; 34) Ethell et al., 2000; 35) Cohen et al., 1998; 36) Hsueh et al., 1998; 37) Hsueh et al., 1999; 38) Grootjans et al., 1997; 39) Zimmermann et al., 2001; 40) Zimmermann et al., 2005.
Structure-function relationships in syndecans
A growing number of studies document the importance of a given syndecan in the activation of a particular signalling cascade, but these properties may be context or cell-type specific. In some cases, the syndecan ED (membrane anchored or soluble) has proven to suffice for supporting some aspects of their biological function, while other study-cases have demonstrated that the CD, or subdomains thereof, are essential or sometimes even sufficient, when clustered, to mediate biological effects. Ligand-induced clustering or multimerization of syndecans appears to be a key event during transmembrane signalling and is possibly linked to the property of syndecans to form detergent-resistant homo-multimers. Chimeric syndecan constructs in which the TM of syndecans is replaced by the TM of the PDGFR form very few detergent-resistant dimers and their specific signalling function is lost [18]. Multimerization requires the ubiquitously conserved GxxxG motif in the TM. Yet, the TM domains of the four different syndecans self-associate to very different degrees implicating residues outside the GxxxG motif in modulating the different associations [19]. Consistently, the minimal amino acid sequence that supports the ability of the syndecan-3 core protein to form multimeric complexes includes, in addition to the TM, the last four amino acids of the ED [20]. Although heteromeric interactions among full-length syndecans have not been reported in vivo, Dews and Mackenzie [19] recently showed that each syndecan TM is able to form stable heteromeric complexes with other paralogs. These interactions exhibit selectivity as, for example the TM of syndecan-1 forms a heterocomplex with syndecan-2 and syndecan-3 but not with syndecan-4. By extension, it would be interesting to know whether hetero-dimerization via the TM with non-syndecan receptors might support syndecan function as co-receptor.
The CD of the syndecans can be divided into two highly conserved regions (C1) and (C2) separated by a variable (V) region, unique for each syndecan (Figure 1). The importance of these domains was highlighted in studies using syndecan chimeras, deletion or point mutants and knock-out cells transfected with rescue constructs. The list of molecules directly interacting with the syndecan CD is growing and the functional relevance of these interactions has been clearly established in some cases (Table 1).
The identity of these interactions is significantly contributing to a progressive understanding of syndecan-specific signalling cascades such as the role of syndecan-2 in neuronal maturation. In neuronal cells, filopodia are recognized as one origin of dendritic spines, bulbous protrusions on the surface of dendrites which have been implicated as a cellular basis for learning and memory. In an elegant study, Lin et al. showed that neurofibromin, interacting with syndecan-2 C1, mediates the activation of PKA and consequent phosphorylation of Ena/VASP proteins that can then promote actin filament elongation by interacting with barbed ends [21]. This signalling cascade induces filopodia formation in cells and hippocampal neurons, but is not sufficient for the maturation of spines. In independent studies, Yamaguchi and co-workers showed that syndecan-2 clustering following tyrosine phosphorylation in the C1 and V region by EphB2 and the presence of the C2 domain are essential for the maturation of spines but not for filopodia formation [22,23]. These data demonstrate that syndecan-2 coordinates two sequential processes in neurons eventually resulting in the formation of dendritic spines.
Syndecan-4 has been the best characterized with respect to direct intracellular interactions of the CD (Table 1). Syndecan-4 localizes to cell attachment sites where together with the α5β1 integrin it generates stress fibers and focal adhesions [24] via activation of protein kinases (PKCalpha and Rho) and coordination of structural and adaptor proteins [16,25]. It has also been shown to transduce FGF-mediated signalling as part of a ternary complex via its protein core [17]. Although not completely defined, syndecan-4 has been shown to preferentially localize into lipid raft compartments following growth factor stimulation [17]. Redistribution of syndecan-4 to lipid rafts generates a potential means of controlling the initiation and duration of syndecan-4/FGF-2 signalling, as this complex is internalized in a Rac-dependent manner [17] and might be subsequently recycled to the cell surface via a syntenin-mediated mechanism (see below) [26]. Localization of syndecan-4 to the lamellipodium of migrating cells serves two purposes: to induce polarization by directing activated Rac to the leading edge [27,28] and to stimulate PKCalpha [29]. PKCalpha regulates cell spreading by activating Rho [30] while concurrently regulating that activation via targeted localization of phosphorylated p190RhoGAP [31]. Rho activation has also been shown to occur in a PKCalpha independent manner to promote phosphorylation of FAK tyrosine-397 leading to focal adhesion turnover [25]. This is in contrast to FGF-2 stimulation which diminishes FAK phosphorylation and leads to enhanced syndecan-4 mediated migration in M5 melanoma cells [32].
This divergence in outcome following syndecan-4 activation by either ECM or growth factor ligation, along with the selective localization of Rho and Rac, suggests that the proteoglycan serves to enact a balance between signalling pathways. In vivo studies, utilizing Xenopus and zebrafish models, have started to address this issue. Syndecan-4 mediated induction of parallel pathways (FGF/ERK and PKCalpha/Rac) promotes neural plate induction [5]. Syndecan-4 in complex with Frizzled 7 also supports the activation of the planar cell polarity (PCP) or non-canonical Wnt signalling pathway that is essential for appropriate directionality during convergence extension and neural crest cell (NC) migration, in response to adhesion to fibronectin [6,7]. Intriguingly, syntenin directly binds Frizzled 7 and syndecan-4 and also supports convergence extension [33] suggesting it might play a role in the formation or the activity of the complex, see also below.
Regulation of syndecan activity by heparanase and shedding
Heparanase cleaves HS-chains at a few specific sites producing fragments of 10-20 sugar units. HS-chain fragments generated by heparanase are biologically more active than the native HS-chain in FGF2 signalling. In addition and somewhat counter-intuitively, heparanase can transform syndecan into a highly selective interaction protein. Binding of lacritin, an epithelial proliferation factor, to the N-terminus of syndecan-1 is required for mitogenic signalling, but lacritin binding is only accomplished after partial or complete removal of the syndecan-1 HS-chains by heparanase [34]. Finally, heparanase does not only cleave HS-chains but is also able to induce syndecan-1 shedding and the functional consequence thereof (see below). Thus heparanase can modulate syndecan function in several ways.
Syndecan ED can be proteolytically cleaved in the juxtamembrane region, a process inducing the release of the ED called shedding (Figure 1). Proteolytic syndecan-1 cleavage is mediated by a variety of proteases of the matrix metalloproteinase (MMP) family. The MMPs involved are cell type specific yielding soluble proteoglycans (EDs) that in principle retain the binding properties of their cell surface precursors. All syndecan members are constitutively shed to some extent as part of their normal turn-over. Yet, shedding of the syndecan core proteins can be accelerated. A variety of extracellular stimuli like growth factors, chemokines, bacterial virulence factors and cell stress are known to induce syndecan-1 shedding. Recently heparanase has been shown to fine-tune the tumour micro-environment by controlling the shedding of syndecan-1. Knock-down of heparanase in myeloma cell lines results in a decrease of soluble syndecan-1 [35]. One possible mechanism to explain the effect of heparanase on syndecan shedding is that cleavage of the HS-chains by heparanase makes the syndecans more accessible for the proteases. How extracellular stimuli influence the sheddases to mediate syndecan cleavage is not yet clear. Chemical inhibitor studies suggest that diverse signal transduction cascades, such as protein kinase C, protein tyrosine kinase, mitogen-activated protein kinases and the nuclear factor kappaB pathways are involved. In addition, the CD seems to be essential for the regulation of agonist induced shedding. Hayashida et al. [36] showed that the CD of syndecan-1 binds to inactive Rab5 and dissociation is induced by agonist-mediated stimulation of shedding. Their data suggest that Rab-5 might serve as an intracellular on-off switch to control the onset of syndecan-1 shedding.
Release of syndecans from the cell surface by shedding clearly has functional consequences. Membrane-bound and soluble forms of syndecan-1 have opposite roles in breast carcinoma cells. Syndecan-1 overexpression stimulates proliferation of MCF-7 cells (adenocarcinoma), whereas overexpression of a constitutively shed syndecan-1 decreases the proliferation of these cells. Constitutively membrane-bound syndecan-1 inhibits invasiveness, whereas constitutively shed syndecan promotes invasion of these same cells [37]. Thus proteolytic conversion of syndecan-1 from a membrane-bound into a soluble molecule marks a switch from a proliferative to an invasive phenotype. Yet, the effect of the shed syndecan-1 ED on cell proliferation seems to be cell type specific, as exposure of the T47D human breast carcinoma cell line (ductal carcinoma) to shed syndecan-1 stimulates cell proliferation [38]. In Xenopus embryos, binding of FGF to syndecan-4 stimulates the shedding of syndecan-4 mediated by secreted serine protease xHtrA1. This releases soluble FGF-syndecan-4 complexes that serve as a long-range growth factor signalling complex for mesoderm induction and body plan establishment [39]. Finally it has been shown that, upon shedding of the ED, the remaining C-terminal fragment is further fragmented by presenilins (Figure 1). Such intramembrane proteolysis might be a mechanism for terminating signal transduction across the membrane and/or for sending soluble signals in the cytosol [40].
Endocytic routes and syndecan function
HSPGs and syndecans are not only essential for the internalization of physiological extracellular ligands, but also for that of viruses, bacteria and basic peptides or poly-cation-nucleic-acid complexes [41-43]. The early endocytic routes used by syndecans appear unconventional and characteristic (Figure 2) [17,44-52]. After clustering, syndecans are internalized by clathrin- and caveolin-independent routes. Several observations point to a role for membrane rafts and macropinocytosis. Requirement of dynamin, flotilin-1 or Rac activation might be context-dependent (i. e. cell-specific syndecan expression, nature of the ligand, presence of co-receptors and/or cell-type specific machineries). An intriguing observation is that dynamin interacts with syndecan-4 C1 region [53] suggesting syndecans might be mechanistically linked to endosome formation. Our knowledge of the fate of the syndecans after internalization is quite limited. Some traffic through late endosomes [49] and then to lysosomes to be degraded together with their cargo, which would support syndecan-dependent signal extinction. Interestingly the C2 domain, not important for syndecan internalization per se [26], supports alternative endocytic routes. This domain corresponds to a canonical PDZ-binding motif (PDZBM). PDZ interactions are important for cell polarization and support the targeting and organization of large signalling complexes. Syntenin was the first PDZ protein identified to bind the C2 domain of the syndecans. It functions as a rate limiting factor for syndecan plasma membrane recycling and likely for the recycling of HS associated cargo like FGF/FGFR. Mechanistically, this pathway relies on the ability of syntenin PDZ domains to interact with PIP2 and on the activation of ARF6 GTPase on perinuclear endosomes [26]. Intriguingly, another role for syntenin might be to support syndecan-dependent [7] non-canonical Wnt signalling (see above). Indeed syntenin directly interacts with Frizzled, Wnt receptors and can bridge them to syndecan. Moreover syntenin is necessary for convergent-extension movements and mediates PKCalpha membrane recruitment [33]. How this might be supported by syndecan-syntenin recycling has not been investigated. A second PDZ protein, the relevance of which has been more specifically established in the context of syndecan-4 biology, is synectin/GIPC. Synectin appears important for syndecan-4 dependant activation of Rac at the leading edge and thereby for syndecan-4 dependant endothelial cell migration [28]. It is worth noting that the mechanisms supporting synectin function might involve actin-dependent endocytic trafficking [54]. Synbindin, a third protein that interacts with the PDZBM of syndecan-2, is involved in membrane trafficking at post-synaptic sites and might support the maturation of dendritic spines that depend on syndecan-2 and their PDZBM (see also above) [55]. Finally, the PDZBM of syndecan-1 was shown to mediate polarized secretion to the basolateral membrane of epithelial cells [56]. None of the known PDZ interactors of syndecan PDZBM (Table 1) can account for this effect indicating that other PDZ proteins relevant for syndecan biology remain to be discovered.
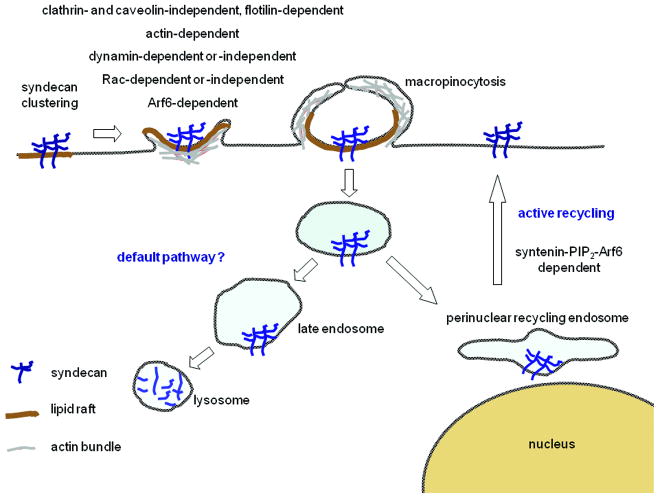
Upon ligation and clustering by LDL, syndecan-1, -2 and -4 become enriched in detergent-resistant membranes and mediate the internalization of ligand via a non-clathrin endocytic route [45,46]. Syndecan-1 overexpression can restore apoE-VLDL uptake in human fibroblastic cells that possess no LDL receptor activity by non-clathrin, but raft-dependent process [51]. Syndecan-4 mediates FGF2 endocytosis in endothelial cells in a non-clathrin dependent way initiated by syndecan-4 clustering. This process is inhibited by cholesterol depletion, but not by dominant-negative dynamins. It resembles macropinocytosis as it is accelerated by active Rac, consistent with a need for actin membrane protrusions, blocked by dominant-negative Rac and by amiloride that blocks Na+/H+ exchange required for macropinocytosis [17,50]. Interestingly, syndecan-1 and -2 might have different kinetics of internalization and have differential effects on post-endocytic gene delivery mediated by polyethyleneimines [57]. Recent studies corroborate a role for HSPGs in raft-dependent, non-clathrin macropinocytic uptake. Although they lack crucial controls with syndecan-specific siRNA and thereby potentially also reflect the biology of other HSPGs i.e. glypicans, one can nevertheless reasonably assume these studies are relevant for syndecans. (1) Arginine-rich peptides, including the R8 and HIV-1 Tat peptides, require cell surface HSPGs and Rac activation for their macropinocytic uptake [47]. (2) DNA uptake relies on HSPGs, lipid rafts and macropinocytosis but is independent of clathrin, dynamin and caveolin-1 [52]. (3) Eosinophil cationic protein is internalized by macropinosomes, uses HSPGs as major receptor and trafficks along a detergent resistant compartment in a clathrin- and caveolin-independent manner. This endocytic route is regulated by Rac and ARF6 and requires cholesterol and PI3kinase activity [44]. (4) The internalization of Herpes Simplex virus protein 22 depends on HSPGs through a lipid raft, actin and ARF6 endocytic pathway, independent of clathrin and caveolin. Yet this pathway appears different from the above mentioned by two criteria. It is independent of Rac and dependent on dynamin [48]. (5) Dependence on dynamin was also observed in another study with other HSPG ligands, including HSPG antibodies [49]. Yet these internalizations were invariably clathrin- and caveolin-independent. Moreover these HSPGs endosomes are decorated with flotillin-1 and can be inhibited by siRNA against this protein. The further fate of HSPGs-endosomes is less clear. Evidence suggests progression to late endosomes [49] and lysosomes unless recycling can take place in a syntenin-dependent manner [26].
In summary, syndecans might represent specific ‘trafficking machines’ for cargo to be secreted, degraded or reutilized. This function is influenced by their clustering and also depends on the interaction of their C2 domain with different PDZ proteins.
Concluding remarks
Syndecans have complex structure-function relationships. By means of their versatile HS chains they sense extracellular environments. As co-receptors for cell-adhesion, cell-proliferation and fate determination molecules and other extracellular signals, they collaborate with ‘primary’ receptors to control signalling. Their conserved TM and CD add specificity to their signalling, by supporting oligomerization and various intracellular connections in a regulated manner. Relatively little is known about the mechanisms by which extra and intracellular domain functions are integrated or disconnected by shedding. When does the CD function in concert with the ED, and does it function after ED cleavage? Does repulsion due to HS negative charges compromise self-assembly required for signalling? The investigation of syndecan endocytic routes has been neglected but emerging studies point to requisite and unanticipated functions in the control of signalling. Our knowledge of these mechanisms is still scarce. Yet, discovering the intricate and subtle dynamics of syndecan signalling might provide opportunities for the treatment of cancer, wound healing, inflammation, viral and bacterial pathogenesis as well as contribute greatly to stem cell biology and cell therapy.
Acknowledgments
We apologize to colleagues whose work we could not describe owing to the condensed format. We thank Prof. Guido David for his feedback during the redaction of the manuscript and Prof. Bassem Hassan and Dr. Aurélie Melchior for their suggestions and proofreading. The laboratory of PZ is supported by the Fund for Scientific Research-Flanders (FWO), the Interuniversity Attraction Poles of the Prime Ministers Services (IUAP), the Belgian Federation against Cancer (BFK), the Concerted Actions Program of the K.U.Leuven and the EMBO young investigator program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.ceb.2009.05.002
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2758656?pdf=render
Citations & impact
Impact metrics
Article citations
Mutual Inhibition of Antithrombin III and SARS-CoV-2 Cellular Attachment to Syndecans: Implications for COVID-19 Treatment and Vaccination.
Int J Mol Sci, 25(14):7534, 09 Jul 2024
Cited by: 0 articles | PMID: 39062776 | PMCID: PMC11277105
Proteoglycans of basement membranes: Crucial controllers of angiogenesis, neurogenesis, and autophagy.
Proteoglycan Res, 2(3):e22, 29 Jun 2024
Cited by: 0 articles | PMID: 39184370
Exploring Heparan Sulfate Proteoglycans as Mediators of Human Mesenchymal Stem Cell Neurogenesis.
Cell Mol Neurobiol, 44(1):30, 28 Mar 2024
Cited by: 1 article | PMID: 38546765 | PMCID: PMC10978659
Review Free full text in Europe PMC
Extracellular Microvesicles Modified with Arginine-Rich Peptides for Active Macropinocytosis Induction and Delivery of Therapeutic Molecules.
ACS Appl Mater Interfaces, 16(14):17069-17079, 02 Apr 2024
Cited by: 1 article | PMID: 38563247
Progressive Irreversible Proprioceptive Piezo2 Channelopathy-Induced Lost Forced Peripheral Oscillatory Synchronization to the Hippocampal Oscillator May Explain the Onset of Amyotrophic Lateral Sclerosis Pathomechanism.
Cells, 13(6):492, 12 Mar 2024
Cited by: 2 articles | PMID: 38534336 | PMCID: PMC10969524
Go to all (122) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Specific structural features of syndecans and heparan sulfate chains are needed for cell signaling.
Braz J Med Biol Res, 39(2):157-167, 02 Feb 2006
Cited by: 48 articles | PMID: 16470302
Review
Conformations, interactions and functions of intrinsically disordered syndecans.
Biochem Soc Trans, 51(3):1083-1096, 01 Jun 2023
Cited by: 3 articles | PMID: 37334846
Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences.
J Biol Chem, 275(13):9410-9417, 01 Mar 2000
Cited by: 77 articles | PMID: 10734086
Syndecans in heart fibrosis.
Cell Tissue Res, 365(3):539-552, 14 Jul 2016
Cited by: 35 articles | PMID: 27411689
Review
Funding
Funders who supported this work.
NICHD NIH HHS (2)
Grant ID: R01 HD037490-10
Grant ID: HD 037490





