Abstract
Free full text

16S ribosomal DNA amplification for phylogenetic study.
Abstract
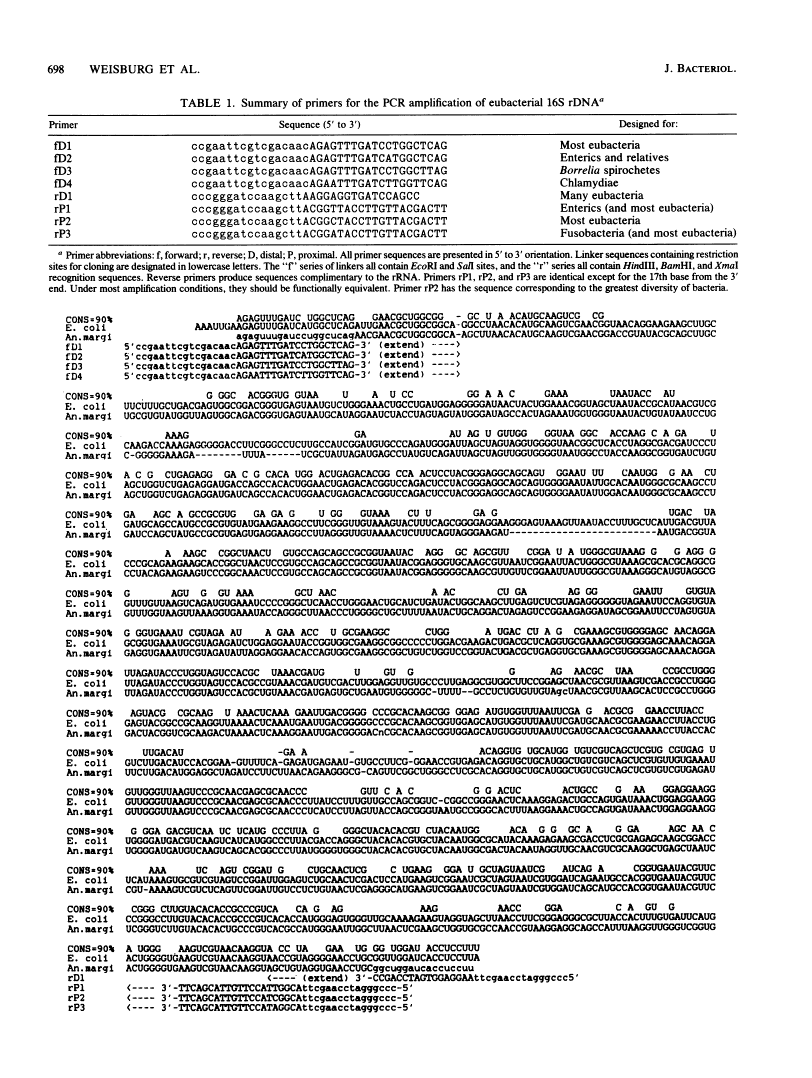
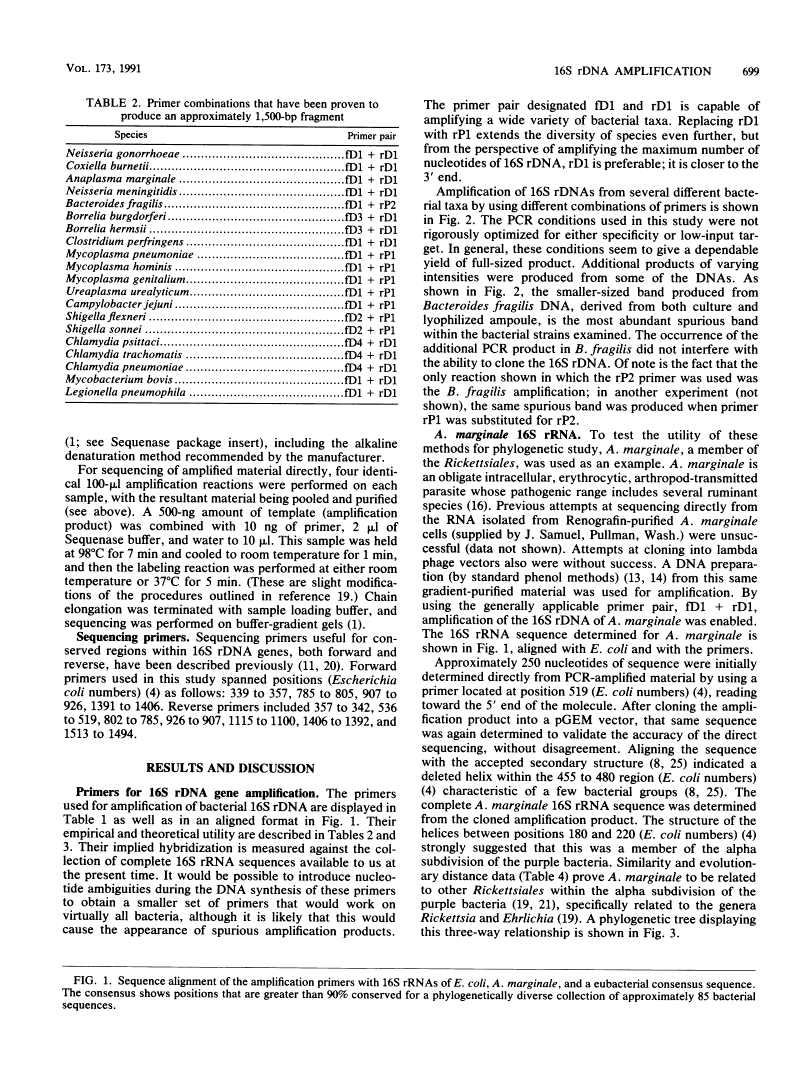
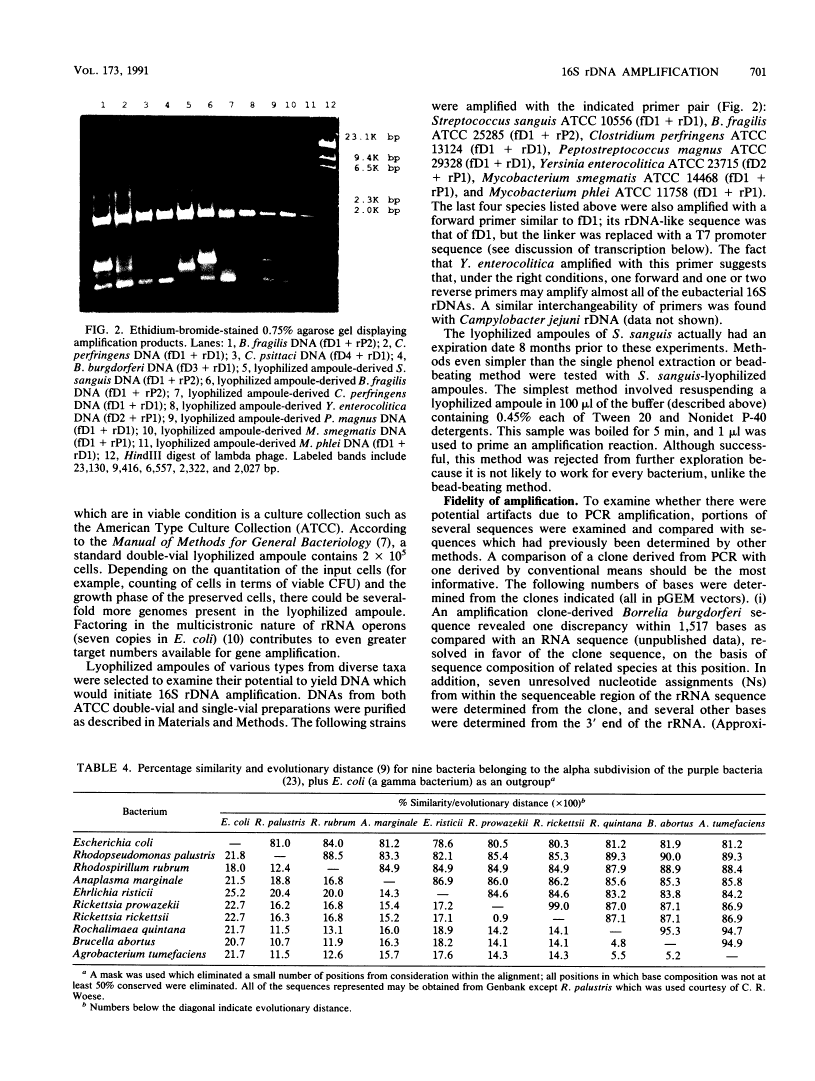
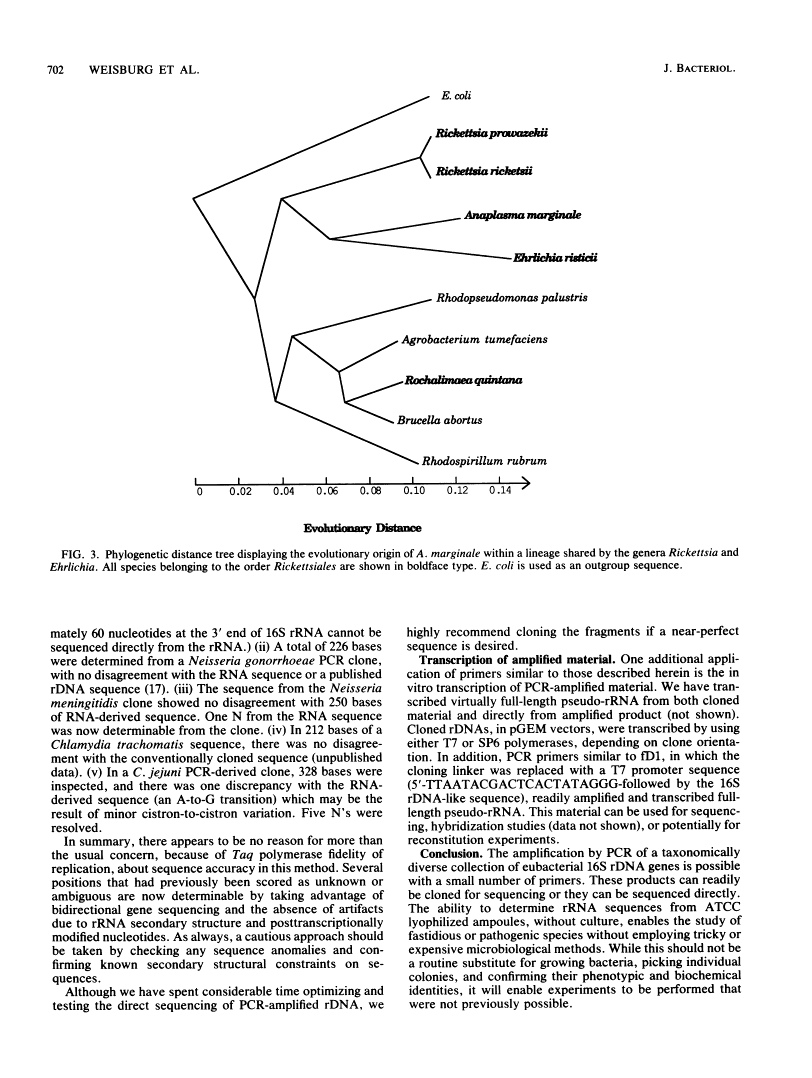
A set of oligonucleotide primers capable of initiating enzymatic amplification (polymerase chain reaction) on a phylogenetically and taxonomically wide range of bacteria is described along with methods for their use and examples. One pair of primers is capable of amplifying nearly full-length 16S ribosomal DNA (rDNA) from many bacterial genera; the additional primers are useful for various exceptional sequences. Methods for purification of amplified material, direct sequencing, cloning, sequencing, and transcription are outlined. An obligate intracellular parasite of bovine erythrocytes, Anaplasma marginale, is used as an example; its 16S rDNA was amplified, cloned, sequenced, and phylogenetically placed. Anaplasmas are related to the genera Rickettsia and Ehrlichia. In addition, 16S rDNAs from several species were readily amplified from material found in lyophilized ampoules from the American Type Culture Collection. By use of this method, the phylogenetic study of extremely fastidious or highly pathogenic bacterial species can be carried out without the need to culture them. In theory, any gene segment for which polymerase chain reaction primer design is possible can be derived from a readily obtainable lyophilized bacterial culture.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.3M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin MD, Gibson TJ, Hong GF. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. [Europe PMC free article] [Abstract] [Google Scholar]
- Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. [Europe PMC free article] [Abstract] [Google Scholar]
- Brosius J, Palmer ML, Kennedy PJ, Noller HF. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. [Europe PMC free article] [Abstract] [Google Scholar]
- Edwards U, Rogall T, Blöcker H, Emde M, Böttger EC. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 1989 Oct 11;17(19):7843–7853. [Europe PMC free article] [Abstract] [Google Scholar]
- Fox GE, Stackebrandt E, Hespell RB, Gibson J, Maniloff J, Dyer TA, Wolfe RS, Balch WE, Tanner RS, Magrum LJ, et al. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. [Abstract] [Google Scholar]
- Gutell RR, Weiser B, Woese CR, Noller HF. Comparative anatomy of 16-S-like ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1985;32:155–216. [Abstract] [Google Scholar]
- Kenerley ME, Morgan EA, Post L, Lindahl L, Nomura M. Characterization of hybrid plasmids carrying individual ribosomal ribonucleic acid transcription units of Escherichia coli. J Bacteriol. 1977 Dec;132(3):931–949. [Europe PMC free article] [Abstract] [Google Scholar]
- Lane DJ, Pace B, Olsen GJ, Stahl DA, Sogin ML, Pace NR. Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6955–6959. [Europe PMC free article] [Abstract] [Google Scholar]
- Medlin L, Elwood HJ, Stickel S, Sogin ML. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988 Nov 30;71(2):491–499. [Abstract] [Google Scholar]
- Rossau R, Heyndrickx L, Van Heuverswyn H. Nucleotide sequence of a 16S ribosomal RNA gene from Neisseria gonorrhoeae. Nucleic Acids Res. 1988 Jul 11;16(13):6227–6227. [Europe PMC free article] [Abstract] [Google Scholar]
- Weisburg WG, Dobson ME, Samuel JE, Dasch GA, Mallavia LP, Baca O, Mandelco L, Sechrest JE, Weiss E, Woese CR. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989 Aug;171(8):4202–4206. [Europe PMC free article] [Abstract] [Google Scholar]
- Weisburg WG, Oyaizu Y, Oyaizu H, Woese CR. Natural relationship between bacteroides and flavobacteria. J Bacteriol. 1985 Oct;164(1):230–236. [Europe PMC free article] [Abstract] [Google Scholar]
- Weisburg WG, Woese CR, Dobson ME, Weiss E. A common origin of rickettsiae and certain plant pathogens. Science. 1985 Nov 1;230(4725):556–558. [Abstract] [Google Scholar]
- Weiss E, Williams JC, Dasch GA, Kang YH. Energy metabolism of monocytic Ehrlichia. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1674–1678. [Europe PMC free article] [Abstract] [Google Scholar]
- Woese CR. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. [Europe PMC free article] [Abstract] [Google Scholar]
- Woese CR, Gutell R, Gupta R, Noller HF. Detailed analysis of the higher-order structure of 16S-like ribosomal ribonucleic acids. Microbiol Rev. 1983 Dec;47(4):621–669. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.173.2.697-703.1991
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc207061?pdf=render
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/173/2/697
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/173/2/697
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/jb.173.2.697-703.1991
Article citations
Characteristics and in vitro properties of potential probiotic strain Fructobacillus tropaeoli KKP 3032 isolated from orange juice.
Folia Microbiol (Praha), 14 Nov 2024
Cited by: 0 articles | PMID: 39541067
In vitro antimicrobial and antioxidant activities of bioactive compounds extracted from Streptomyces africanus strain E2 isolated from Moroccan soil.
Sci Rep, 14(1):27372, 09 Nov 2024
Cited by: 0 articles | PMID: 39521814 | PMCID: PMC11550811
In-vitro and in-vivo assessment of the bactericidal potential of peracetic acid and hydrogen peroxide disinfectants against A. hydrophila infection in Nile tilapia and their effect on water quality indices and fish stress biomarkers.
Sci Rep, 14(1):25715, 28 Oct 2024
Cited by: 0 articles | PMID: 39468161 | PMCID: PMC11519942
Comparative genomic analysis of two putative novel Idiomarina species from hypersaline miocene deposits.
BMC Genomics, 25(1):1007, 28 Oct 2024
Cited by: 0 articles | PMID: 39468450 | PMCID: PMC11514770
Development of a Multiplex PCR Assay to Detect Neofusicoccum parvum and Botryosphaeria dothidea in Walnut.
Curr Microbiol, 81(12):432, 29 Oct 2024
Cited by: 0 articles | PMID: 39472323
Go to all (5,144) article citations
Other citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Amplification of bacterial 16S ribosomal DNA with polymerase chain reaction.
J Clin Microbiol, 28(9):1942-1946, 01 Sep 1990
Cited by: 356 articles | PMID: 2095137 | PMCID: PMC268083
Rapid determination of bacterial ribosomal RNA sequences by direct sequencing of enzymatically amplified DNA.
FEMS Microbiol Lett, 53(1-2):171-176, 01 Nov 1989
Cited by: 55 articles | PMID: 2482222
Application of broad-range 16S rRNA PCR amplification and DGGE fingerprinting for detection of tick-infecting bacteria.
J Microbiol Methods, 52(2):251-260, 01 Feb 2003
Cited by: 69 articles | PMID: 12459246