Abstract
Free full text

Threshold size for optimal passive pulmonary targeting and retention of rigid microparticles in rats
Abstract
The relationship between microparticle (MP) size and lung targeting efficiency, intra-lung distribution and retention time was systematically studied after intravenous administration of rigid fluorescent polystyrene MPs of various sizes (2, 3, 6 and 10μm) to Sprague-Dawley rats. Total fluorescence was assessed and it was found that 2μm and 3μm MPs readily passed through the lung to the liver and spleen while 10μm MPs were completely entrapped in the lung for the one-week duration of the study. Approximately 84% of 6μm MPs that were initially entrapped in the lung were cleared over the next 2 days and 15% were cleared over the remaining 5 days. A Caliper IVIS® 100 small animal imaging system confirmed that 3μm MPs were not retained in the lung but that 6μm and 10μm MPs were widely distributed throughout the lung. Moreover, histologic examination showed MP entrapment in capillaries but not arterioles. These studies suggest that for rigid MPs the optimal size range required to achieve transient but highly efficiently targeting to pulmonary capillaries after IV injection is >6μm but <10μm in rats and that systemic administration of optimally sized MPs may be an efficient alternative to currently used inhalation-based delivery to the lung.
1. Introduction
The standard route of administration for treating lung diseases is inhalation whereby drugs and delivery vehicles are applied to the mucosal surface of the lung. Inhaled drugs must first penetrate the protective mucus layer and then the epithelial cell layer in order to enter the lung tissues. Once a drug traverses the air-blood interface it is rapidly cleared from the lung into the systemic circulation. Pulmonary delivery by means of inhalation is typically an inefficient process with only 10-30% of administered drugs reaching the systemic circulation [1-8]. Total drug absorption after inhalation occurs by two pathways: directly through the lung mucosa and indirectly by the intestine (i.e., after deposition in the mouth and pharynx or after it is moved by the mucociliary escalator in the upper respiratory tract and swallowed). Exposure of the lung mucosal surface to the applied drug is also a function of the breathing capacity of the patient [9], particle size [1] and inhaler technique [10, 11]. A patient with compromised lung function resulting from impaired deep lung inhalation will not have extensive mucosal surface coverage in the alveolar sacs and the resulting drug bioavailability will be lower than the already low theoretical maximum.
The capillary beds of “filter” organs (i.e., the lung, liver and spleen) act as mechanical filters that efficiently entrap MPs. In fact, over the years a number of reports on the gross biodistribution of rigid particulates such as silica dust [12, 13], glass [14-16], carbon [17, 18] and polystyrene [19-26] have been published. Following intravenous (IV) administration, MPs larger than the diameter of capillaries become entrapped in the pulmonary circulation offering a unique opportunity for passive drug targeting. Although MP biodistribution and toxicity are generally thought to be dependent upon their size, rigidity and dose, a systematic study of the relationship between MP size and lung retention, intra-lung distribution or blood vessel entrapment has not been performed. Most recent studies have focused on rigid MPs. Several studies have found that smaller rigid particulates (<4 μm) pass through the lung and become entrapped in the reticuloendothelial system (RES) whereas the vast majority (80-90%) are found in the liver with the remainder in the spleen (5-8%) and bone marrow (1-2%) [24, 27-29]. Larger particulates (>10 μm) became entrapped in the lung.
There is also a body of work dating back to the 1960’s that focuses on MPs that are somewhat flexible. Radiolabeled macroaggregated albumin (MAA) particles are used for perfusion scanning of various organs in mice, rats, dogs, monkeys, rabbits and humans [30-32]. MAA is a non-spherical, somewhat flexible biodegradable MP that is administered by IV injection and is currently approved for determining lung perfusion in humans (Pulmolite®, Pharmalucence, Inc. Bedford, MA and DraxImage®, Draxis Health, Inc. Kirkland, Québec, Canada). These products adhere to guidelines set forth in the USP that state “>90% of MPs must have a size between 10-90 μm and no MPs may be larger than 150 μm” [33]. Interestingly, humans have over 280×109 capillary segments with an average diameter of 7-10 μm [34, 35]. The diameter of interior pulmonary capillaries in dogs and rats are 6.23 ± 1.5 μm and 5.15 ± 1.3 μm, respectively [36]. Given the wide array of factors that control particle retention and persistence in the lung, it is not clear if a species-independent optimal particle size can be determined.
Following a standard dose of MAA (1×106 MPs) and assuming that every MP is entrapped in an individual capillary, only 0.5-0.7% of a healthy lung becomes occluded [29]. However, Davis found that clearance was not just dependent upon size but also on deformability since the clearance rates of similarly-sized MAA were significantly shorter than more rigid human albumin microspheres (HAM) [29]. Human MAA dosages are much lower (<0.2 mg/kg or ~350,000 MPs) than the corresponding LD50 for mice, rats, dogs and monkeys (72.2 mg/kg, 43.8 mg/kg, 68.1 mg/kg and 82.6 mg/kg, respectively) [31].
The IV injection of MPs and passive lung targeting affords the opportunity to treat lung diseases from the vascular side eliminating the need to traverse the mucus/surfactant and mucosal layers. This would be particularly useful in situations where lung capacity is compromised and inhalation is not a viable option. Higher deposition efficiency and prolonged retention may translate into reduced doses, less frequent administration and lower bioavailability variability. In addition, it is possible to treat deep lung injury regardless of the patient’s ability to inhale the large volumes of air that are necessary for deep lung penetration. In the current report, the effect of size on the passive pulmonary targeting, lung distribution and persistence rigid MPs is reported in rats.
2. Materials and methods
2.1. Materials
Fluorescent, internally labeled, polystyrene MPs of various sizes (2, 3, 6 and 10 μm) were purchased from Polysciences Inc. (Warrington, PA). Polycarbonate filters with a 0.8 μm pore size were purchased from Osmonics Laboratory Products Inc. (Minnetonka, MN). 2-Ethoxyethyl acetate was purchased from Acros/Fisher Scientific (Fair Lawn, NJ). Male Sprague Dawley rats were purchased from Hilltop Lab Animals, Inc. (Scottdale, PA). Rats were fed a standard rat diet, had free access to water and were housed in a room with a 12-hour light-dark cycle for at least one week before the study. All rat studies were performed in AAALAC accredited animal facilities under approved protocols from the Rutgers University Animal Use and Care Committee.
2.2. Methods
2.2.1. Preparation of MPs for IV injection
Yellow-green fluorescent polystyrene MPs used in the fluorescent plate reader studies with various diameters (2, 3, 6 and 10 μm) and blue fluorescent polystyrene MPs used in the IVIS® 100 studies of various diameters (3, 6 and 10 μm) were washed several times using distilled water before being suspended in 0.9% NaCl containing 0.1% Tween 80 (20 mg MPs/mL). MPs were fully suspended in solution by sonicating and vortexing immediately prior to IV administration.
2.2.2. Particle size analysis
The size distribution of the polystyrene MPs were determined using a Multisizer™ 3 Coulter Counter (Beckman Coulter, Inc. Miami, FL) with a 70 μm aperture tube, which is able to detect particles from 1.4–42 μm. MPs were added drop wise to 20 mL of ISOTON® II dispersed phase until the concentration of particles was acceptable (<10%). 1 mL of dispersed phase was counted.
2.2.3. Scanning electron microscopy (SEM)
MPs were washed twice with methanol and dried using a CentriVap concentrator (Labconco Corp., Kansas City, MO) for 30 minutes. The MPs were fixed on aluminum stubs with conductive tape, and coated with gold-palladium for 2 minutes at 30 mA and 5×10−2 mbar in an argon atmosphere, using a Balzer SCD 004 Sputter Coater. The MP coated stubs were then examined using a SEM (AMRAY 1830 I) with an EDX 9800 X-ray system and a Robinson backscatter detector.
2.2.4. Biodistribution studies quantitated using a fluorescence plate reader
Groups of three male Sprague Dawley rats weighing 300 ± 50 g were used for this study. Yellow-green fluorescence MPs (4 mg) of various diameters (2, 3, 6 and 10 μm) were suspended in 0.9% NaCl containing 0.1% Tween 80. MPs were administered intravenously into the lateral tail vein of conscious rats. The animals were kept under close observation for any signs or symptoms of embolism for 6 hours or until euthanized. At predetermined time points, animals were euthanized by CO2 asphyxiation. The lung, right kidney, heart, spleen and a portion of the right lobe of the liver were collected for further processing. The rest of the liver was removed and placed in a separate tube for the calculation of the proportion of the MPs in the whole liver.
2.2.4.1. MP recovery from organs
The analytical method for quantification of polystyrene MPs in organs has been described elsewhere and modified as follows [37]. Briefly, the organ samples were digested using freshly prepared 4N KOH in 2% Tween 80. An internal standard of 6 μm bright blue fluorescent polystyrene MPs (0.5 mL, 0.025 w/v%) was added to each sample. The digested samples were then filtered through 0.8 μm polycarbonate filters, washed with 2% Tween 80 (2 × 10 mL), and finally washed once with phosphate buffer (10 mL, 43.2mM KH2PO4 and 131mM K2HPO4). After lightly vacuum drying the MPs, the filter with fluorescent MPs was carefully transferred to a polypropylene tube. 2-ethoxyethyl acetate (2 mL) was added to each sample and the samples were mixed thoroughly before being stored in the dark at room temperature for further analysis.
2.2.4.2. Quantitation of MPs using a fluorescent plate reader
Samples containing fluorescent MPs were analyzed in triplicate using a GENios fluorescence plate reader (Tecan U.S. Inc., Research Triangle Park, NC). Sample aliquots were diluted to a total volume of 100 μL in 96-well polypropylene microplates. The MPs (yellow-green fluorescence) were measured at λex = 430 ± 35 nm and λem = 510 ± 20 nm. The internal standard MPs (bright blue fluorescence) used during tissue processing were measured at λex = 360 ± 42 nm and λem = 465 ± 35 nm.
2.2.5. Fluorescence imaging
Fluorescence imaging of rats was performed using an IVIS® 100 small animal imaging system (Caliper Life Sciences, Hopkinton, MA). CY5.5 excitation (λex = 615-665 nm) and ICG emission (λem = 810-875 nm) filters were used. Identical illumination settings, including exposure time (5 s), binning factor (4), f-stop (2), and fields of view (15 × 15 cm), were used for all image acquisition. Fluorescent and photographic images were acquired and overlaid. The pseudocolor image represents the spatial distribution of photon counts within the organs. Background fluorescence was subtracted prior to analysis. Images were acquired and analyzed using Living Image 2.5 software (Caliper Life Sciences, Hopkinton, MA).
Internally dyed, bright blue fluorescent polystyrene MPs of various diameters (3, 6 and 10 μm) were washed twice in 0.1% Tween 80 in phosphate buffered saline (PBS, pH = 7.4). MPs were volumetrically dispensed and weighed to yield a 4 mg dose of MPs and resuspended in sterile PBS (200 μL) containing 0.1% Tween 80.
Groups of three male Sprague Dawley rats weighing 200 ± 50 g were used for in vivo imaging studies and fed a standard rat diet (AIN-93G). Rats were de-epilated immediately prior to reduce any auto-fluorescence effects due to diet or hair. Rats were anesthetized by isoflurane using an EZ-3500 Multi-Animal Anesthesia System (Euthanex Corp., Palmer, PA). A tail vein catheter was then inserted and used as the injection site. MPs (4 mg) in 0.1% Tween 80 in PBS (200 μL) were injected through the tail vein catheter. The tail vein catheter was flushed with up to 0.8 mL of 0.1% Tween 80 in PBS.
Three animals were euthanized at each time point and the heart, lung, liver, spleen, and kidney were removed and imaged intact. Imaging time points were as follows: No injection, 1 hour, 12 hours, 24 hours, 168 hours. Rats were euthanized by CO2 asphyxiation.
2.2.6. Histology studies
Upon euthanasia after the administration of MPs, organs were collected for gross and histological inspection. The lung was inflated via tracheal cannulas with 10% buffered formalin solution (Fisher Scientific, Fair Lawn, NJ) till the pleura were smooth. The inflated lung along with the liver, kidney, heart and spleen were fixed by immersion in fixative for at least 24 hours before being further processed. Sections (2×2×2 cm3) of the lung were dissected and used to prepare the tissue section slides. Tissue sections were then embedded in glycol methacrylate-based polymer (GMA) and tissue slices (6 μm thick) were cut and mounted onto glass slides. The tissue slides were stained using hematoxylin and eosin (H&E) dye, and observed histopathologically under a microscope to check for any possible tissue damage.
2.2.7. Statistical analysis
Experimental values are expressed as mean ± standard deviation. Differences between experimental groups were tested using a Student’s t-test at α = 0.05 using GraphPad Prism v.4 (GraphPad Software, San Diego, CA). The regression analysis of the standard curves was performed using least squares linear regression (Microsoft Excel v.9.0). Figure 3 was generated by GraphPad Prism v.4.
3. Results
3.1. Polystyrene MP characterization
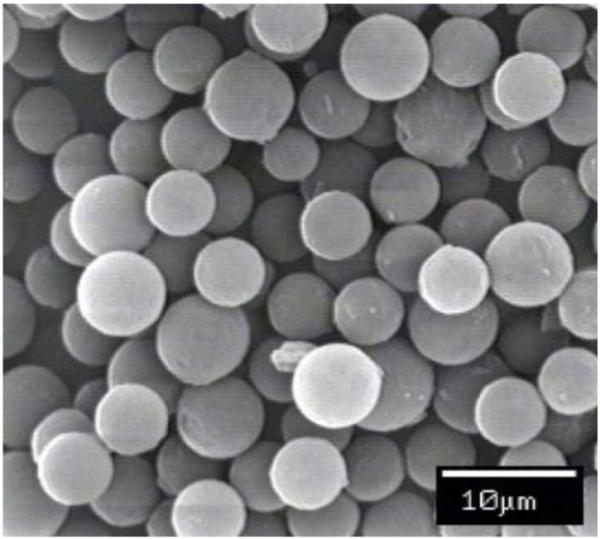
Polystyrene MPs were characterized for size and polydispersity. The manufacturer’s reported values of the polystyrene MPs differ from the experimental results obtained by Coulter Counter measurements, and the results are shown in Fig. 1. However, the measured values were statistically similar to their reported values by Student’s t-test. There is no overlap between 3 and 6 μm MPs; however, the amount of overlap between the 6 and 10 μm MPs is 4.86%. All MPs were further characterized by SEM to determine their size, shape and surface morphology. A representative scanning electron micrograph of 6 μm polystyrene MPs is shown in Fig. 2. The polystyrene MPs appear spherical with a smooth surface, are discrete, and have low polydispersity.
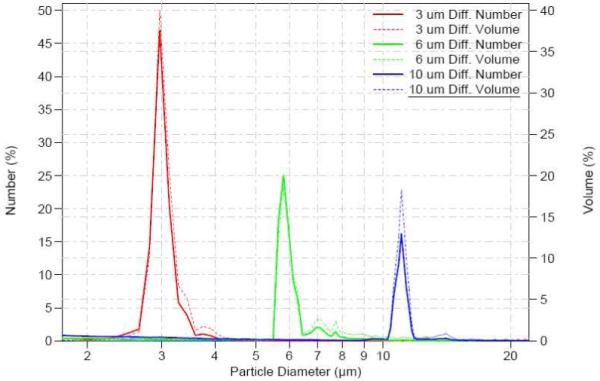
Numeric (solid) and Volumetric (dashed) distribution of polystyrene MPs: 3 μm (red); 6 μm (green), 10 μm (blue). The size of the 3 μm, 6 μm and 10 μm polystyrene MPs as reported by the manufacturer were: 3.005 ± 0.112 μm, 6.098 ± 0.573 μm, 10.94 ± 0.396 μm, respectively; the volumetrically measured size of polystyrene MPs were: 3.066 ± 0.236 μm, 5.915 ± 0.205 μm, and 11.06 ± 0.309 μm, respectively.
3.2. Biodistribution of polystyrene MPs using the fluorescence plate reader
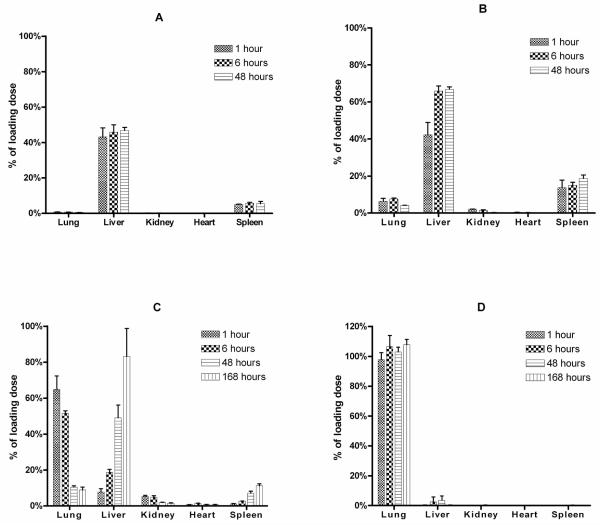
The biodistribution of fluorescently labeled polystyrene MPs of four well defined size distributions was examined after IV injection in rats at 1, 6, 48 and 168 hours post injection. The biodistribution of these MPs in the lung, liver, spleen and kidney was highly dependent on the size injected. Small MPs (2, 3 μm) were found to distribute quickly (within about 1 hour) to the liver (43.12 ± 5.05 and 42.2 ± 6.74% of administered 2 and 3 μm MPs, respectively) and spleen (5.05 ± 0.23 and 13.64 ± 4.18%, respectively) (Fig. 3A, B). Recovery of the 2 μm MPs slightly increased after 48 hours in the liver and spleen (46.73 ± 1.86 and 5.37 ± 1.3%, respectively). Interestingly, the majority of the 3 μm MPs were detected in the liver and spleen (66.81 ± 1.4 and 18.57 ± 1.89%, respectively) at 48 hours. Large MPs (10 μm) distribute quickly (within 1 hour) to the lung (97.77 ± 4.62%) and remain there for at least one week (107.75 ± 3.51%) (Fig. 3D). However, 6 μm MPs were found to quickly distribute to the lung (64.86 ± 7.46%) and liver (7.84 ± 1.85%) after 1 hour but then migrated from the lung (8.92 ± 1.6%, 168 hours) to the liver (83.2 ± 15.59%, 168 hours) over time (Fig. 3C).
3.3. Distribution of polystyrene MPs in the lung using the IVIS® 100 small animal imaging system
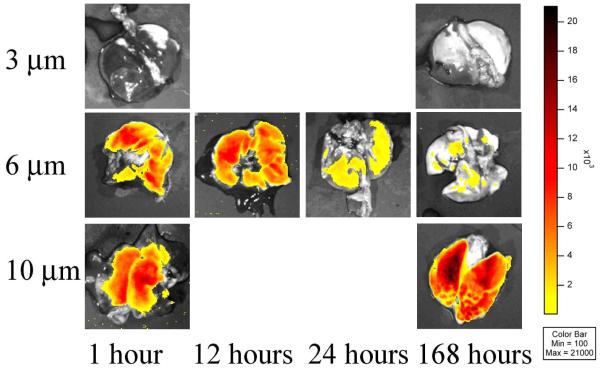
Since standard gross biodistribution studies only give an indication of the total number of MPs associated with an organ, a IVIS® 100 small animal imaging system was used to determine MP distribution in the lung of rats after IV injection. Rats were euthanized at 1, 12, 24 and 168 hours post injection and the organs were removed and imaged. MP distribution pharmacokinetics were highly size dependent (Fig. 4). Consistent with gross MP biodistribution (Fig. 3), 3 μm MPs did not become entrapped in the lung (Fig. 4, top row) and 10 μm MPs quickly distributed (1 hour) to the lung, remaining there for at least 168 hours (Fig. 4, third row). However, 6 μm MPs were found to distribute quickly to the lung (Fig. 4, second row) and then migrate from the lung over time. As seen in Fig. 4, the perfusion distribution shows that the MPs are spaced in a fairly uniform manner when observed from an anterior-posterior view. While the IVIS® 100 was successfully used to demonstrate lung distribution of the MPs ex vivo, it was unable to detect the fluorescent signal from the internally labeled MPs in whole body animals and in organs such as the liver.
3.4. Histology studies
It was observed that the injected 10 μm MPs deposited throughout the lung (Fig. 5C) and upon closer inspection in the pulmonary capillaries, especially at the septal microvessel area (junctions among alveoli), but not in small arterioles (Fig. 5D). Few 10 μm MPs were found in the liver and spleen through microscopic observation. Emboli or tissue infarction were not observed macro- and/or microscopically in the tissue samples.
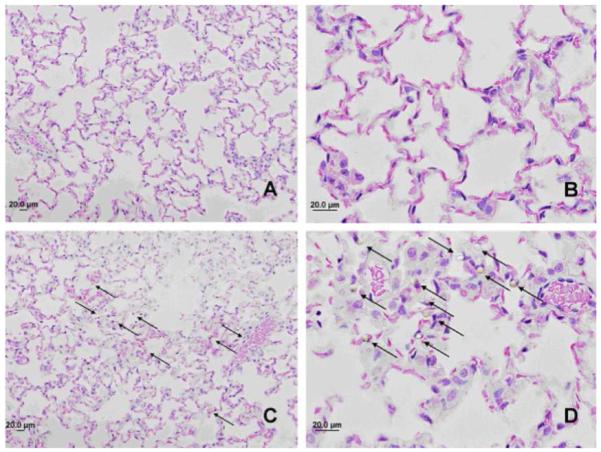
H & E stained healthy rat lung in GMA polymer matrix: control rat lung (Panel A & B); rat lung 48 hours after administration of 10 μm MPs (Panel C & D). MPs are indicated by arrows; showing they were entrapped in the pulmonary septal microvessels. Magnification of Panels A & C: 20x; Panels B & D: 60x.
4. Discussion
One of the lung’s main functions is to filter out cellular debris and clots on a daily basis [38]. In fact, the lung at rest only uses ~30% of its capacity and is therefore able to recruit other capillaries to avoid massive increases in the arterial pressure when blockages do occur [38]. At rest, the bulk of blood passes through the base of the lung due to gravimetric forces [38]. However, as blood pressure increases during obstruction or exercise, the level or height to which blood flows through the lung also increases thereby recruiting minimally used capillaries [38]. The objective of this study was to evaluate the potential of achieving high lung targeting using a parenterally administered delivery system that exploits the body’s natural filtering system.
The current study explores the threshold size necessary for optimal passive entrapment of MPs in the pulmonary capillaries of rats. Small MPs (≤3 μm) were quickly entrapped in the liver and spleen with a very low percentage in the lung. The relatively low recovery of ≤ 3 μm MPs is most likely due to their entrapment in other capillary beds, excretion through the feces or sequestration by macrophages in other organ systems. Larger MPs (10 μm) were nearly completely entrapped in the lung and remained there for a prolonged period of time. The current results are consistent with a series of studies reported in rats and dogs by DeLuca and colleagues. They examined the biodistribution of 141Ce-labeled polystyrene MPs of various sizes (3, 5, 7 and 12 μm and 3, 8, 15 and 25 μm) in dogs [25, 26] and 3 μm in rats [39]. In dogs, MPs <7 μm were transiently retained in the lung eventually passing into the liver while MPs >8 μm became entrapped in the lung. In both dogs and rats, 3 μm particles were found to distribute to the liver and spleen. Although this suggests that the rat model could be used as an alternate to dogs as the lung delivery strategy is further optimized, additional studies would be required to determine the inter-species correlation.
There is no doubt that larger (>10 μm) MPs can be delivered to and filtered by the lung where the MPs can serve as a depot for drug release. Several groups have tried to exploit this mechanism for the sustained delivery of anticancer drugs such as carboplatin [40], doxorubicin [41] and adriamycin [42]. Our group has shown that a camptothecin (CPT) prodrug immobilized on 6 μm MPs was more effective in reducing the amount of cancerous areas than a 10-fold higher dose of free CPT in an orthotopic lung cancer model in nude rats [43]. However, since larger MPs occlude larger vessels, any potential toxic effect is multiplied since many smaller downstream vessels would also be blocked. As such, it would be advantageous to lodge MPs in as small a blood vessel as is practical since this would limit adverse downstream effects. Therefore, the goal was to determine the ideal MP size that transiently targets the rat lung with high efficiency and entrapment in the pulmonary capillaries. In the current studies, the 6 μm MPs were predominately and very quickly entrapped in the rat lung (<1 hour) after which they slowly eluted over time and accumulated in the liver. Our 6 μm MP results in rats are consistent with the biphasic elimination of 5 μm MP in dogs. The first elimination phase decreased 79% between 6 and 48 hours with a more sustained second phase that only decreased 15% between 48 and 168 hours. The slow terminal elimination of the 6 μm MPs is possibly due to the redistribution of the 6 μm particles after their first escape from the lung. However, the biphasic elimination pattern of the 6 μm MPs from the lung is compounded by the fact that these MPs are not as monodisperse as the 3 and 10 μm MPs (as characterized by Coulter Counter). These >6 μm MPs found in the 6 μm group may be retained longer in the lung as their size approaches 10 μm; similar results were found in dogs [25, 26]. These results suggest that a mixture of 6 μm and larger MPs may be useful for delivering short-term loading doses followed by longer-term slow delivery. Such treatment strategies may be useful for diseases like cancer, asthma, emphysema or interstitial pulmonary disease.
Use of the IVIS® 100 provided for the first time evidence of the spatial distribution of various sized MPs in rat lung. Rigid MPs measuring 10 μm and smaller are widely distributed throughout the lung (Fig. 4) and become entrapped in pulmonary capillaries but not arterioles (Fig. 5). Targeting the septal microvessel area (i.e., junctions among alveoli) allows for a greater lung distribution pattern, more consistent drug gradients throughout lung tissues and a lower fraction of lung branch occlusions. Moreover, the 6 μm MPs appear to first sequester in the lung and then after 48 hours sequester in the liver, suggesting an intriguing pathway for potentially treating metastatic cancer.
Interestingly, the current results are also in good agreement with the transit times of stimulated neutrophils (mean diameter 8 μm) and leukocytes (6-8 μm), which are more rigid than erythrocytes (mean diameter 8 μm) [44, 45]. Neutrophils are larger than the pulmonary capillaries (mean diameter 5.5 μm) and generally take a few seconds to >20 minutes to cross the pulmonary capillary bed [46, 47]. In comparison, erythrocytes are able to deform more easily than neutrophils and transit through the pulmonary capillary bed in less than one second [48]. Nevertheless, the ability of neutrophils to deform allows them to transit through the pulmonary capillary bed, even if it occurs slowly. Rigid MPs larger than the capillary bed most likely rely on the ability of the capillaries to deform due to pressure changes in both the pulmonary and cardiovascular system. However, at some critical size and dose load, occlusion would occur due to the inadequate clearance rate. In an attempt to model erythrocytes and neutrophil transit through pulmonary capillaries, the passage of non-deformable MPs (≥8 μm) was simulated [49] and they found that only 12% were predicted to pass through the lung capillary beds at increased pressures. This prediction was consistent with studies performed in dogs [50]. These simulations also confirmed the findings of Wiggs et al. that only 15% of 5.85 μm polystyrene MPs were able to pass through the lung of rabbits whereas 86% of 3.08 μm MPs pass through the lung after less than 10 seconds [49, 51].
Standard gross biodistribution studies involve destructive sampling of tissues in order to quantify compounds of interest. One of the purposes of the current study was to evaluate the IVIS® 100 for studying biodistribution, intra-organ distribution and to reduce the number of animals required for future studies. Unfortunately, in vivo detection of fluorescently labeled MPs was unsuccessful. There are two possible reasons for this. First, the dye in this commercially available MP was optimized for laser excitation wavelengths available in flow cytometry rather than for the available IVIS® 100 filter set. This means that the signal may not have been strong enough due to inadequate excitation. Second, the amount of dye encapsulated in the MP might not have been optimal for these imaging studies leading to poor signal strength. However, the IVIS® 100 was able to give an indication of the intra-lung distribution of MPs. This represents the first time that fluorescence has been used to demonstrate the widespread distribution of MPs in lung ex vivo after IV administration. In order to demonstrate the distribution patterns in vivo, dyes with stronger signals (e.g., near IR dyes) would have to be used.
In the current studies, neither emboli nor tissue infarction was observed either macro- and/or microscopically in any of the tissue samples. This is remarkable considering some of the MP dose loads that were administered (i.e., 2.6×108, 3 μm MPs; 3.3×107, 6 μm MPs; and 7.3×106, 10 μm MPs). Previously, it was shown that rats subjected to a very high dose of large polystyrene MPs (8×106, 40 μm MPs) died acutely, and upon further investigation all the rats had MPs in the lung, half of the rats had MPs in the heart and only 2 animals had MPs in the liver [23]. However, doses of fewer MPs (4×105, 40 μm MPs) or smaller sizes (8×106, 10 μm MPs) did not result in adverse events. In several studies, rats were subjected to high doses of polystyrene MPs resulting in pulmonary embolism. In one case, a very high dose of large polystyrene MPs (2.1×107, 15 ± 5 μm MPs) was administered by saphenous vein injection in rats under anesthesia and the animals died in less than 15 hours. However, lower doses (1.6×107, 15 ± 5 μm MPs) did not result in deaths [52]. Another study involving the induction of pulmonary embolism in rats used a dose similar to the non-lethal dog dose of polystyrene MPs (~8×106, 24 ± 1 μm MPs) and the 16 hour survival rate was 60% (n = 15) [53]. These observations support the conclusion that larger rigid MPs (>10 μm) block larger upstream vessels effectively blocking larger areas of the pulmonary bed and strongly suggest that MP size should be minimized with the ultimate target being pulmonary capillaries. The lung has a variety of ways to compensate for natural MP exposure. Besides recruiting unused vessels, arteriovenous shunts can be created allowing pathways for MPs, which are larger than capillaries, to pass relatively unhindered into the venous circulation. In fact, MPs that are >25 μm have been found in the pulmonary venous blood after injection possibly due to arteriovenous shunts in the lung [15, 16, 54-57].
5. Conclusion
In the current study, the effect of particle size on the lung distribution and retention of rigid MPs was assessed in rats. Taken as a whole, the current results suggest that the threshold size for optimal lung entrapment and transient retention of rigid polystyrene MPs is >6 μm but <10 μm in Sprague Dawley rats. Given the observed lung targeting and retention properties of injectable MPs, it is entirely likely that once per week administration is possible with highly potent therapeutics, a potentially significant alternative to multiple daily administrations using inhalation. Entrapping MPs with long residence times in the pulmonary vasculature may have advantages in primary lung diseases over inhaled medications that may be more readily removed through exhalation or absorption and clearance into the systemic circulation. Finally, close proximity of the MPs to the alveolar sacs and subsequent wide distribution throughout the bronchial tree itself suggests that MPs have the potential for treating asthma, emphysema, interstitial pulmonary disease and disseminated lung cancer. We are now in the process of extending these studies by systematically altering the deformability/rigidity of MPs and examining lung targeting, distribution and retention.
Acknowledgements
Financial support from the Parke-Davis Chair in Pharmaceutics and Controlled Drug Delivery and National Institutes of Health CounterACT Program through the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Award #U54AR055073) is gratefully acknowledged. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. The NSF Integrative Graduate Education and Research Traineeship (IGERT) #0504497 and American Foundation for Pharmaceutical Education (AFPE) are acknowledged for providing graduate fellowships to Hilliard Kutscher. We would like to thank Drs. Carol Gardner, Robert Harris, and Ms. Theresa Choi for their assistance and fruitful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jconrel.2009.12.019
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc2840186?pdf=render
Citations & impact
Impact metrics
Article citations
Advanced nanomedicines and immunotherapeutics to treat respiratory diseases especially COVID-19 induced thrombosis.
World J Clin Cases, 12(16):2704-2712, 01 Jun 2024
Cited by: 0 articles | PMID: 38899301 | PMCID: PMC11185334
Review Free full text in Europe PMC
mRNA vaccine designs for optimal adjuvanticity and delivery.
RNA Biol, 21(1):1-27, 01 Jan 2024
Cited by: 2 articles | PMID: 38528828 | PMCID: PMC10968337
Review Free full text in Europe PMC
Quantitative Pharmacokinetics Reveal Impact of Lipid Composition on Microbubble and Nanoprogeny Shell Fate.
Adv Sci (Weinh), 11(4):e2304453, 30 Nov 2023
Cited by: 2 articles | PMID: 38032129 | PMCID: PMC10811482
Immunosuppressive dead cell as lung-targeting vehicle and cytokine absorption material for cytokine storm attenuation of pneumonia.
Mater Today Bio, 20:100684, 27 May 2023
Cited by: 0 articles | PMID: 37304577 | PMCID: PMC10250915
Calibrating and Validating the MFI-UF Method to Measure Particulate Fouling in Reverse Osmosis.
Membranes (Basel), 13(5):535, 22 May 2023
Cited by: 0 articles | PMID: 37233598 | PMCID: PMC10222867
Go to all (51) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Enhanced passive pulmonary targeting and retention of PEGylated rigid microparticles in rats.
Int J Pharm, 402(1-2):64-71, 29 Sep 2010
Cited by: 24 articles | PMID: 20883756 | PMCID: PMC3912554
Pulmonary targeting microparticulate camptothecin delivery system: anticancer evaluation in a rat orthotopic lung cancer model.
Anticancer Drugs, 21(1):65-76, 01 Jan 2010
Cited by: 32 articles | PMID: 19966540 | PMCID: PMC3859198
Synthesis and characterization of silk fibroin microparticles for intra-articular drug delivery.
Int J Pharm, 485(1-2):7-14, 24 Feb 2015
Cited by: 17 articles | PMID: 25724134 | PMCID: PMC4422162
Tissue distribution of 20 nm, 100 nm and 1000 nm fluorescent polystyrene latex nanospheres following acute systemic or acute and repeat airway exposure in the rat.
Toxicology, 263(2-3):117-126, 15 Jul 2009
Cited by: 44 articles | PMID: 19615422
Funding
Funders who supported this work.
NCI NIH HHS (1)
Grant ID: R01 CA155061
NIAMS NIH HHS (6)
Grant ID: U54 AR055073
Grant ID: U54 AR055073-039003
Grant ID: U54 AR055073-049003
Grant ID: U54 AR055073-019003
Grant ID: U54 AR055073-029003
Grant ID: U54AR055073