Abstract
Free full text

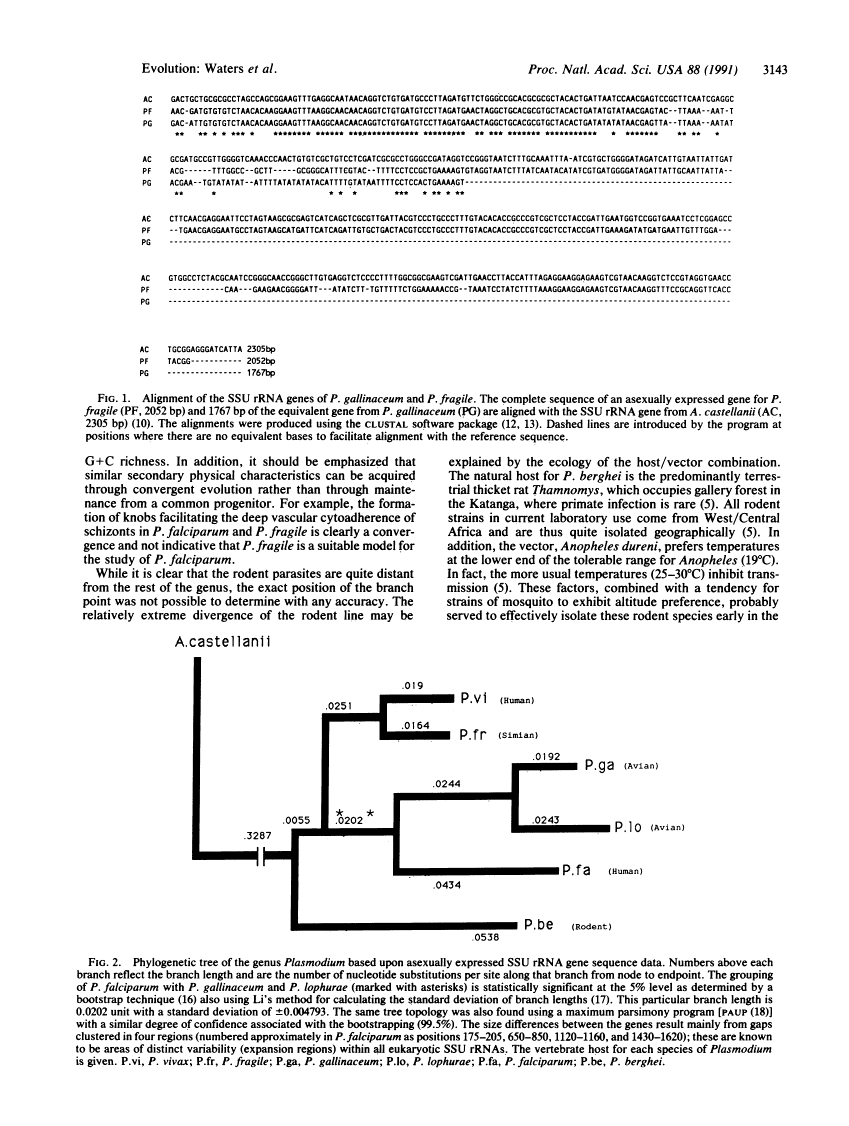
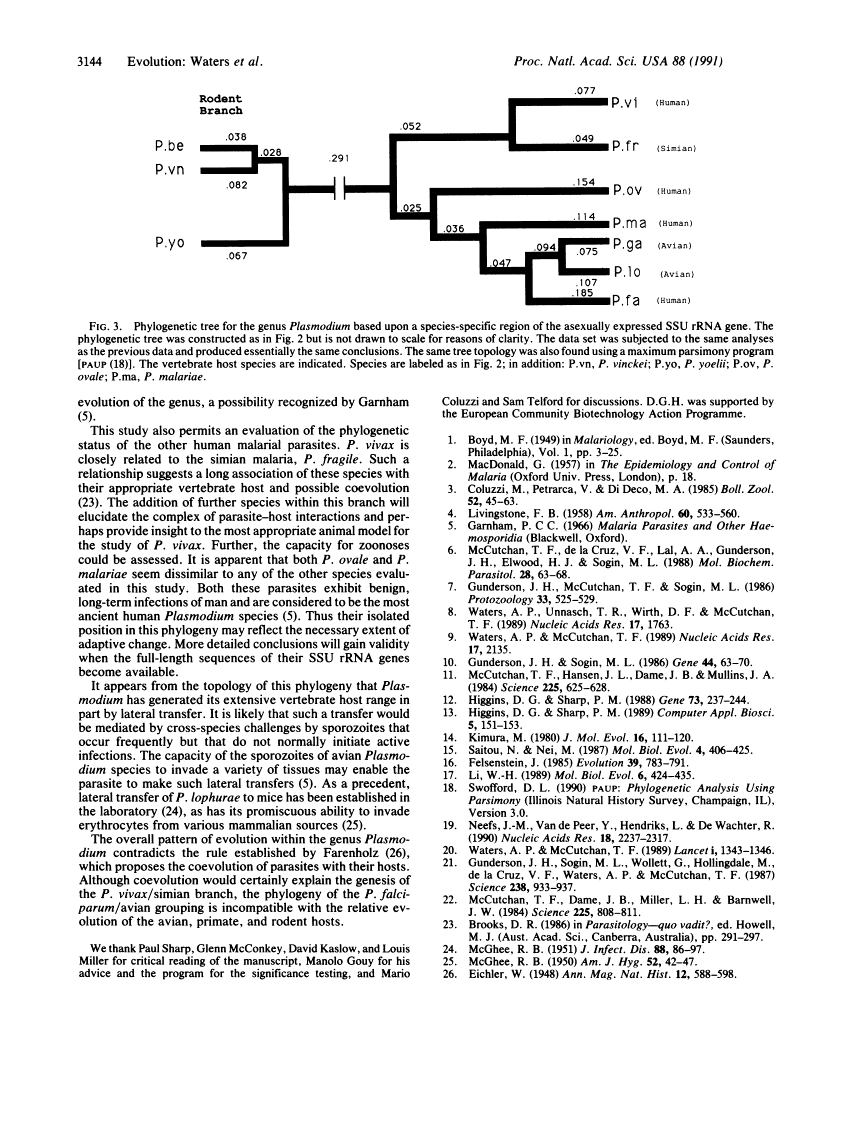
Plasmodium falciparum appears to have arisen as a result of lateral transfer between avian and human hosts.
Abstract
It has been proposed that the acquisition of Plasmodium falciparum by man is a relatively recent event and that the sustained presence of this disease in man is unlikely to have been possible prior to the establishment of agriculture. To establish phylogenetic relationships among the Plasmodium species and to unravel the mystery of the origin of P. falciparum, we have analyzed and compared phylogenetically the small-subunit ribosomal RNA gene sequences of the species of malaria that infect humans as well as a number of those sequences from species that infect animals. Although this comparison confirmed the three established major subgroups, broadly classed as avian, simian, and rodent, we find that the human pathogen P. falciparum is monophyletic with the avian subgroup, indicating that P. falciparum and avian parasites share a relatively recent avian progenitor. The other important human pathogen, P. vivax, is very similar to a representative of the simian group of Plasmodium. The relationship between P. falciparum and the avian parasites, and the overall phylogeny of the genus, provides evidence of an exception to Farenholz's rule, which propounds synchronous speciation between host and parasite.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.0M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- McCutchan TF, de la Cruz VF, Lal AA, Gunderson JH, Elwood HJ, Sogin ML. Primary sequences of two small subunit ribosomal RNA genes from Plasmodium falciparum. Mol Biochem Parasitol. 1988 Feb;28(1):63–68. [Abstract] [Google Scholar]
- Gunderson JH, McCutchan TF, Sogin ML. Sequence of the small subunit ribosomal RNA gene expressed in the bloodstream stages of Plasmodium berghei: evolutionary implications. J Protozool. 1986 Nov;33(4):525–529. [Abstract] [Google Scholar]
- Waters AP, Unnasch TR, Wirth DF, McCutchan TF. Sequence of a small ribosomal RNA gene from Plasmodium lophurae. Nucleic Acids Res. 1989 Feb 25;17(4):1763–1763. [Europe PMC free article] [Abstract] [Google Scholar]
- Waters AP, McCutchan TF. Partial sequence of the asexually expressed SU rRNA gene of Plasmodium vivax. Nucleic Acids Res. 1989 Mar 11;17(5):2135–2135. [Europe PMC free article] [Abstract] [Google Scholar]
- Gunderson JH, Sogin ML. Length variation in eukaryotic rRNAs: small subunit rRNAs from the protists Acanthamoeba castellanii and Euglena gracilis. Gene. 1986;44(1):63–70. [Abstract] [Google Scholar]
- McCutchan TF, Hansen JL, Dame JB, Mullins JA. Mung bean nuclease cleaves Plasmodium genomic DNA at sites before and after genes. Science. 1984 Aug 10;225(4662):625–628. [Abstract] [Google Scholar]
- Higgins DG, Sharp PM. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. [Abstract] [Google Scholar]
- Higgins DG, Sharp PM. Fast and sensitive multiple sequence alignments on a microcomputer. Comput Appl Biosci. 1989 Apr;5(2):151–153. [Abstract] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. [Abstract] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. [Abstract] [Google Scholar]
- Li WH. A statistical test of phylogenies estimated from sequence data. Mol Biol Evol. 1989 Jul;6(4):424–435. [Abstract] [Google Scholar]
- Neefs JM, Van de Peer Y, Hendriks L, De Wachter R. Compilation of small ribosomal subunit RNA sequences. Nucleic Acids Res. 1990 Apr 25;18 (Suppl):2237–2317. [Europe PMC free article] [Abstract] [Google Scholar]
- Waters AP, McCutchan TF. Rapid, sensitive diagnosis of malaria based on ribosomal RNA. Lancet. 1989 Jun 17;1(8651):1343–1346. [Abstract] [Google Scholar]
- Gunderson JH, Sogin ML, Wollett G, Hollingdale M, de la Cruz VF, Waters AP, McCutchan TF. Structurally distinct, stage-specific ribosomes occur in Plasmodium. Science. 1987 Nov 13;238(4829):933–937. [Abstract] [Google Scholar]
- McCutchan TF, Dame JB, Miller LH, Barnwell J. Evolutionary relatedness of Plasmodium species as determined by the structure of DNA. Science. 1984 Aug 24;225(4664):808–811. [Abstract] [Google Scholar]
- McGHEE B. The adaptation of the avian malaria parasite Plasmodium lophurae to a continuous existence in infant mice. J Infect Dis. 1951 Jan-Feb;88(1):86–97. [Abstract] [Google Scholar]
- McGHEE RB. The ability of the avian malaria parasite, Plasmodium lophurae, to infect erythrocytes of distantly related species of animals. Am J Hyg. 1950 Jul;52(1):42–47. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.88.8.3140
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc51401?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/101882703
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1073/pnas.88.8.3140
Article citations
Malaria disrupts the rhesus macaque gut microbiome.
Front Cell Infect Microbiol, 12:1058926, 13 Jan 2023
Cited by: 4 articles | PMID: 36710962 | PMCID: PMC9880479
Why Plasmodium vivax and Plasmodium falciparum are so different? A tale of two clades and their species diversities.
Malar J, 21(1):139, 03 May 2022
Cited by: 11 articles | PMID: 35505356 | PMCID: PMC9066883
Review Free full text in Europe PMC
Zoonotic Transmissions and Host Switches of Malaria Parasites.
Zoonoses (Burlingt), 1(1):11, 02 Nov 2021
Cited by: 5 articles | PMID: 35282332 | PMCID: PMC8909814
Evolutionary Insights into the Microneme-Secreted, Chitinase-Containing High-Molecular-Weight Protein Complexes Involved in Plasmodium Invasion of the Mosquito Midgut.
Infect Immun, 90(1):e0031421, 04 Oct 2021
Cited by: 4 articles | PMID: 34606368 | PMCID: PMC8788677
Review Free full text in Europe PMC
An Ecologically Framed Comparison of The Potential for Zoonotic Transmission of Non-Human and Human-Infecting Species of Malaria Parasite.
Yale J Biol Med, 94(2):361-373, 30 Jun 2021
Cited by: 1 article | PMID: 34211355 | PMCID: PMC8223545
Review Free full text in Europe PMC
Go to all (107) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Bayesian analysis of new and old malaria parasite DNA sequence data demonstrates the need for more phylogenetic signal to clarify the descent of Plasmodium falciparum.
Parasitol Res, 101(3):493-503, 29 Mar 2007
Cited by: 22 articles | PMID: 17393186
Plasmodium (Haemamoeba) cathemerium gene sequences for phylogenetic analysis of malaria parasites.
Parasitol Res, 96(2):90-94, 06 Apr 2005
Cited by: 14 articles | PMID: 15812672
Phylogeny of the malarial genus Plasmodium, derived from rRNA gene sequences.
Proc Natl Acad Sci U S A, 91(24):11373-11377, 01 Nov 1994
Cited by: 179 articles | PMID: 7972067 | PMCID: PMC45233
Why Plasmodium vivax and Plasmodium falciparum are so different? A tale of two clades and their species diversities.
Malar J, 21(1):139, 03 May 2022
Cited by: 11 articles | PMID: 35505356 | PMCID: PMC9066883
Review Free full text in Europe PMC