Abstract
Free full text

The Integration of Negative Affect, Pain, and Cognitive Control in the Cingulate Cortex
Preface
It has been argued that emotion, pain, and cognitive control are functionally segregated in distinct subdivisions of the cingulate cortex. But recent observations encourage a fundamentally different view. Imaging studies indicate that negative affect, pain, and cognitive control activate an overlapping region of dorsal cingulate, the anterior midcingulate cortex (aMCC). Anatomical studies reveal that aMCC constitutes a hub where information about reinforcers can be linked to motor centers responsible for expressing affect and executing goal-directed behavior. Computational modeling and other kinds of evidence suggest that this intimacy reflects control processes that are common to all three domains. These observations compel a reconsideration of dorsal cingulate’s contribution to negative affect and pain.
Introduction
In humans and other primates, the cingulate, a thick belt of cortex encircling the corpus callosum, is one of the most prominent features on the mesial surface of the brain (Figure 1a). Early research suggested that the rostral cingulate gyrus (Brodmann’s ‘precingulate’1; architectonic areas 24, 25, 32, and 33) plays a key role in affect and motivation (Figure 1b)2. More recent research has enlarged the breadth of functions ascribed to this region; in addition to emotion3, the rostral cingulate plays a central role in contemporary models of pain4, 5 and cognitive control6, 7. Work in these three basic domains has, in turn, strongly influenced prominent models of social behavior8, psychopathology9-11, and neurological disorders12.
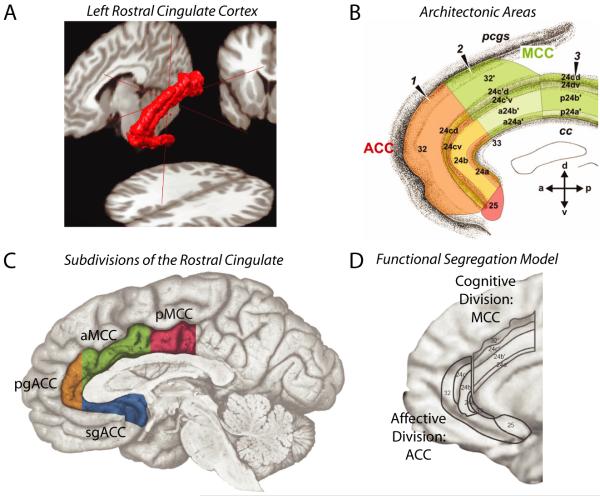
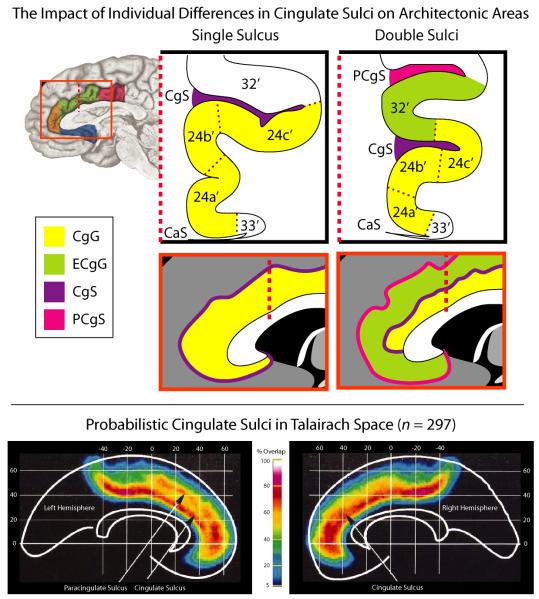
The rostral cingulate has been partitioned on physiological and anatomical grounds at spatial scales ranging from the macroscopic to the molecular. A. Three-dimensional rendering of the left rostral cingulate cortex. The cingulate, shown in red, was manually traced on a single subject’s magnetic resonance image (MRI). Much of the constituent cortical gray matter lies buried within the cingulate sulci, a fact not apparent from inspection of the mesial surface (for additional information, see Box 1 and the Supplement). B. Architectonic areas of the cingulate. Areas were defined209 on the basis of differences in microanatomy and neurotransmitter chemistry and, hence, differ somewhat from the classical descriptions of Brodmann and other pioneering neuroanatomists1, 210. Architectonic features provide one means of defining homologies across species183, 211. Adapted with permission from Ref. 209. C. The four major subdivisions of the rostral cingulate. Subdivisions were defined by Vogt and colleagues211 on the basis of regional differences in microanatomy, connectivity, and physiology. The supracallosal portion of the cingulate is designated the midcingulate cortex (MCC) and is divided into anterior (aMCC: green) and posterior (pMCC: magenta) subdivisions. The portion of the cingulate lying anterior and ventral to the corpus callosum is designated the anterior cingulate cortex (ACC) and is divided into pregenual (pgACC: orange) and subgenual (sgACC: cyan) subdivisions by the coronal plane at the anterior tip of the genu. Adapted with permission from Ref. 212 (for additional information, see the Supplement). D. The functional segregation model of Bush, Luu and Posner. On physiological and anatomical grounds, Bush et al.14 argued that the rostral cingulate consists of two functionally segregated regions: a rostroventral ‘affective’ division (ACC; originally termed ‘ventral ACC’) and a dorsal ‘cognitive’ division (MCC; originally termed ‘dorsal ACC’). Adapted with permission from Ref. 14.
Despite this progress, key questions about the functional organization and significance of activity in the rostral cingulate remain unresolved. Perhaps the most basic question is whether emotion, pain, and cognitive control are segregated into distinct subdivisions of the rostral cingulate or are instead integrated in a common region. In a pair of landmark reviews, Devinsky et al.13 and Bush et al.14 marshaled a broad range of functional imaging, electrophysiological, and anatomical data in support of functional segregation, arguing that the anterior cingulate cortex (ACC or ‘rostral’ ACC) is specialized for affective processes, whereas the midcingulate cortex (MCC or ‘dorsal’ ACC) is specialized for cognitive processes (Figure 1c, 1d). Subsequent meta-analyses of imaging studies have provided some support for this claim15.
Although the segregationist model remains highly influential, new data suggests that it is no longer tenable. For instance, recent imaging data implicate MCC in the regulation of autonomic activity16, 17 and the perception and production of emotion3, 18. Likewise, neuronal recordings demonstrate that MCC is responsive to emotionally-charged words in humans19. Especially robust links have been forged between activity in the anterior subdivision of MCC (aMCC; Figure 1c) and the experience of more intense states of negative affect, as with the anticipation20-22 and delivery23, 24 of pain and other kinds of aversive stimuli25, 26. A particularly dramatic example comes from a recent study showing that aMCC activation parametrically tracks the physical imminence of a live spider placed near the foot27. Importantly, meta-analyses that have examined imaging studies of negative affect21, pain23, or cognitive control28 in isolation suggest that each of these domains consistently activate aMCC. Based on such observations, there is a growing recognition that aMCC might implement a domain-general process that is integral to negative affect, pain, and cognitive control5, 29-34.
In this Review, we examine this integrative hypothesis about the functional organization of the rostral cingulate with a special focus on the contribution of aMCC to negative affect and pain. We neither attempt a comprehensive overview (see Ref.12) nor do we provide a detailed discussion of this region’s role in appetitively-motivated learning and behavior, phenomena that have been the subject of other recent reviews35-37. We first address the question of whether MCC should be conceptualized as a territory specialized for ‘cognitive’ processes, as segregationist models claim. We show how three largely independent lines of evidence—physiological, anatomical, and functional—challenge longstanding claims of functional segregation in rostral cingulate. We then explore the possibility of using ideas adopted from computational models of cognitive control and reinforcement learning to address the contribution of aMCC to negative affect and pain. Although these models are familiar to many cognitive neuroscientists, we believe that they provide a useful, if underappreciated, framework for generating mechanistic hypotheses about the role of aMCC in aversively-motivated behavior. This perspective, which we term the ‘adaptive control hypothesis,’ can account for a number of observations not readily accommodated by segregationist models. But it also raises a number of interesting new questions. We conclude by outlining several strategies for answering them.
Anatomical and physiological convergence
Functional imaging evidence of overlap in aMCC
As the size and scope of the imaging literature have burgeoned, it has become increasingly difficult to synthesize new data into existing models of functional organization. This problem is particularly acute when attempting to integrate observations from disparate domains, such as affect, pain and cognition. This challenge can be overcome using new techniques for performing voxel-by-voxel, or ‘coordinate-based’, meta-analysis (CBMA)38. Here we used CBMA to evaluate whether imaging studies of negative affect, pain, and cognitive control provide evidence for co-localization or segregation in the rostral cingulate. Given the observations described earlier, we anticipated that all three domains would consistently activate an overlapping region within aMCC.
To do so in an unbiased and replicable way, we identified 939 studies in the Brainmap database (http://brainmap.org) reporting activation in ACC or MCC. We then identified activation foci (‘peaks’) associated with manipulations of negative affect, pain, or cognitive control in healthy unmedicated adults (for additional details, see the Supplement). The negative affect database included foci associated with manipulations designed to induce negative emotions, including fear, anger, and disgust. To minimize potential overlap with studies of cognition, manipulations that were unlikely to produce clear-cut affect, such as the perception of facial expressions or the reading of ‘taboo’ words, were excluded. The pain database included foci associated with the delivery of physically painful stimuli, such as heat, cold or electric shock. The cognitive control database included foci associated with a number of tasks designed to isolate the need to overcome the reflexive allocation of attention or execution of action (e.g., Stroop, Go/No-Go, and Eriksen Flanker tasks). Collectively, the three databases included 380 activation foci from 192 studies involving nearly 3,000 participants (Figure 2).
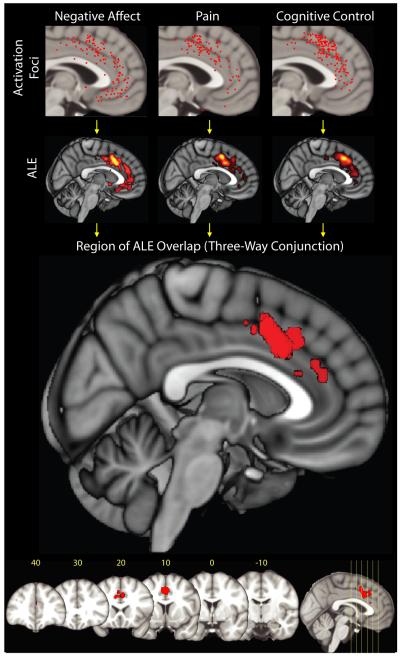
Map depicts the results of a coordinate-based meta-analysis (CBMA) of 380 activation foci derived from 192 experiments involving more than 3,000 participants. Uppermost row shows the spatially normalized foci for each domain. The next row shows thresholded activation likelihood estimate (ALE) 38, 213 maps for each domain considered in isolation. Bottom two rows depict the region of overlap across the three domains. Red cluster indicates the location of a three-way minimum significance conjunction214 of the three domains. The cluster lies in aMCC in the vicinity of areas 32′ and a24b’/c’ (Talairach coordinates: x=0, y=12, z=42; volume: 11680 mm3). No other cluster reached significance. Numbers indicate mm from the anterior commissure (for additional methodological details and results, see the Supplement).
We then used the activation likelihood estimate (ALE) algorithm38 to identify voxels within ACC or MCC that were consistently activated by negative affect, pain or cognitive control (for additional details, see the Supplement). Using these three maps, we created a single ‘conjunction map’39 showing voxels that were consistently activated across the three domains. If negative affect, pain, and cognitive control are strictly segregated, we would expect to see little or no overlap. Instead, the conjunction map revealed a sizable cluster in the dorsal portion of aMCC (Figure 2).
Another way to evaluate functional segregation is to test whether each domain differentially activated the ‘cognitive’ (MCC) compared to the ‘affective’ (ACC) division of the cingulate (Figure 1d). In the case of strict segregation, we would expect studies of cognitive control to activate MCC more frequently than ACC, and indeed, they did (odds=4.8, CI=3.2-7.1, p<.001). Conversely, we would expect studies of negative affect to activate MCC less frequently than ACC. But in fact they were equally likely to activate the two divisions (odds=1.1, CI=0.8-1.6, p=.64). It is less clear what to expect for studies of pain, but given the strong association between pain and negative affect40, we might expect pain to preferentially activate the ‘affective’ division (ACC). Instead, studies of pain were more likely to activate the ‘cognitive’ division (MCC) (odds=4.9, CI=2.9-8.3, p<.001).
Collectively, these observations refute claims that cognition and emotion are strictly segregated into different divisions of rostral cingulate, a conclusion that was itself largely based on an early meta-analysis of imaging studies14 (for a discussion of why our results differed from earlier analyses, see the Supplement). They instead show that aMCC is consistently activated by the elicitation of negative affect, pain, and cognitive control. Of course, these results do not preclude the possibility that this region plays a role in still other psychological processes, such as reward-motivated behavior. And they do not address whether segregation is present at finer levels of analysis, for instance, in individual participants or neurons. Likewise, segregation may be present on a finer timescale than that resolved by conventional imaging techniques30. Nevertheless, what these results do demonstrate is that conventional functional imaging studies of negative affect, pain, and cognitive control all consistently report activation in this subdivision of rostral cingulate.
Anatomical evidence of integration
It has often been claimed that MCC possesses few connections with regions of the brain implicated in affect, motivation and nociception14. But several recent tracing studies, along with a few older ones, indicate that this is not the case. In the remainder of this section, we focus largely on invasive tracing studies performed in monkeys—although rapid progress has been made in refining techniques for mapping structural connectivity in the living human brain, invasive studies are still considered the gold standard41. These data suggest that aMCC represents a hub, where information about pain and other, more abstract kinds of punishment and negative feedback could be linked to motor centers responsible for expressing emotion on the face and coordinating aversively-motivated instrumental behaviors.
aMCC harbors the rostral cingulate zone (RCZ), a somatotopically organized premotor area42. Originally identified on the basis of physiological and anatomical criteria in the monkey (where it is termed the rostral cingulate motor area), RCZ has been provisionally identified in humans with areas 32′/a24c’ in the vicinity of the cingulate sulcus (Figures (Figures1b,1b, ,3a3a and Box 1)43, 44. RCZ projects to the spinal cord, dorsal (‘sensorimotor’) striatum, and primary motor, premotor, and supplementary motor cortices. Physiological studies in humans and monkeys indicate that RCZ is sensitive to the more abstract aspects of action planning and inhibition42, 45. This stands in contrast to the caudal cingulate zone (CCZ), lying at the junction of aMCC and pMCC (Figures 3a, 3b), which has been linked to more specific parameters, such as the precise direction of movement42, 45.
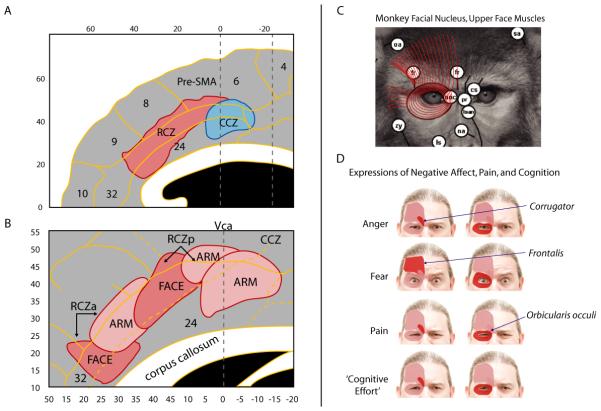
A. Locations of the rostral and caudal cingulate zones (RCZ and CCZ)6, 43. RCZ lies in aMCC, whereas CCZ lies at the junction of aMCC and posterior MCC (pMCC) (see Figure 1c). Zone borders are approximations (see also Ref. 44). Adapted with permission from Ref. 6 (for additional information, see the Supplement). B. Somatotopy in RCZ and CCZ based on human imaging studies43. Adapted with permission from Ref. 43 (for additional information, see the Supplement). C. Combined tracing and microstimulation work in macaques indicates that the monkey analogue of the human RCZ projects to the facial nucleus50, 215, allowing it to control the muscles of the upper face (shown in red for the macaque). The facial muscles are largely conserved across primate species216, 217. Adapted with permission from Ref. 216 (for additional information, see the Supplement). D. In humans, the muscles of the upper face have been associated with the elicitation of negative affect (e.g., anger, fear), pain, and consistent with Darwin’s suggestions218 perhaps ‘cognitive effort’ as well (for additional information, see the Supplement).
Other data suggest that RCZ can contribute to the expression of affect on the face (Figure 3c). In monkeys, RCZ sends heavy bilateral projections to neurons in the facial nucleus that, in turn, innervate the muscles of the upper face (e.g., corrugator, frontalis, and orbicularis oculi)46, the same muscles that underlie the expression of emotion and pain in monkeys47 and humans48, 49 (Figure 3d). Indeed, direct microstimulation of RCZ in monkeys can evoke facial displays classically associated with the fight-or-flight reaction47. The precise role of RCZ in the willful or spontaneous expression of emotion or the regulation of such expressions remains unknown.
For the remainder of this Review, we refer to the cluster of overlap obtained in our meta-analysis as aMCC (Figure 2). Nevertheless, the relatively dorsal position of the cluster within aMCC (approximately corresponding to architectonic areas 32′ and a24b’/c’; Figure 1b and the bottom panel of Figure 2) is consistent with the provisional location of RCZ44. This suggests that it is specifically RCZ which is commonly activated by imaging studies of negative affect, pain, and cognitive control.
aMCC is also characterized by substantial connections with subcortical regions involved in negative affect and pain (Figure 4). It is a primary cortical target of the spinothalamic system, the chief source of peripheral nociceptive information50. There is some evidence that it sends connections to the lateral column of the periaqueductal gray (lPAG), a region that is closely linked to vigilance, fight-or-flight and other defensive responses in rats and cats51. Robust reciprocal connections have also been found between aMCC and the lateral basal nucleus of the amygdala52, 53. Functional connectivity data from humans show a similar pattern54, 55. The basal nucleus is a convergence zone for information from the lateral nucleus of the amygdala (critical for the initial evaluation of motivationally significant stimuli) and OFC (a key source of inhibitory inputs to the amygdala)56. In rodents, the basal nucleus has also been implicated in the learning of aversively-motivated instrumental behaviors57.
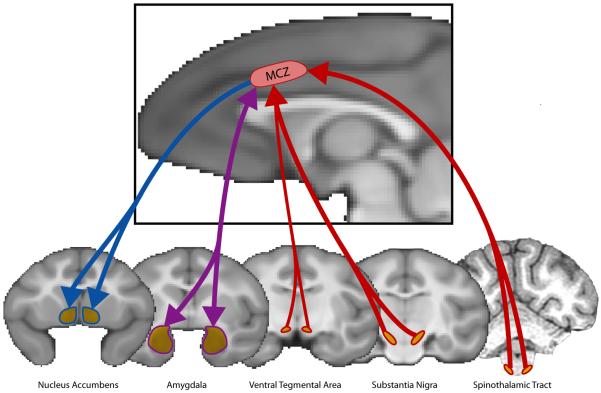
Afferents are depicted in red, efferents in blue, and reciprocal connections in purple. The monkey analogue to RCZ receives substantial inputs from the spinothalamic system, which relays nociceptive information from the periphery to RCZ via the mediodorsal nucleus of the thalamus. Dopaminergic inputs to RCZ arise from the substantia nigra and, to a lesser extent, the ventral tegmental area. RCZ projects to the ventral striatum, including the core region of nucleus accumbens, and has robust reciprocal connections with the lateral basal nucleus of the amygdala. For additional information, see the Supplement.
aMCC projects to the ventral striatum, including the core region of nucleus accumbens58. Although the ventral striatum is commonly associated with reward and appetitively-motivated behavior, it is also activated by the anticipation and avoidance of pain59, 60 and other aversive stimuli in humans59, 61, 62. Dopaminergic inputs to aMCC in the monkey are predominantly from the substantia nigra and retrorubrial area, with a weaker contribution from the ventral tegmental area63. Interestingly, some neurons in the primate substantia nigra are activated by aversive stimuli and cues predicting their occurrence64, suggesting that information about reinforcers, including punishment, could be passed to aMCC via ascending dopaminergic pathways.
In cortex, aMCC is reciprocally connected with frontoparietal regions implicated in cognitive control and the maintenance of goals (i.e., attentional set, rules), including dorsolateral prefrontal cortex (architectonic area 9/46)65, 66. But it is also connected with all major divisions of the insula67, 68, a region strongly implicated in affect69, pain70, 71, and cognitive control (including responses to errors and negative feedback)72-75.
Collectively, these data show that aMCC is well positioned to synthesize information about unlearned (pain, predators, threatening conspecifics) and learned reinforcers (aversive cues, negative feedback) with current goals. Via efferents targeting the facial nucleus, it could exploit this blend of information to drive or, more likely, to flexibly regulate76 the expressions needed to visually communicate with conspecifics and potential predators at close range. Such signals are a key element of many species’ defensive repertoire77, including that of our closest living relative, the chimpanzee78. The value of such expressions is not limited to communication; other evidence suggests that they serve to optimize perception, amplifying or attenuating the intake of sensory information79. Finally, the abundant connections linking aMCC to other motor centers would permit it to use information about reinforcers to plan or refine more complex, aversively-motivated instrumental behaviors. This stands in sharp contrast with other cortical regions implicated in affect and motivation, such as OFC and insula, that lack strong ties with motor centers35, 80.
Evidence of functional convergence
Our meta-analysis revealed that negative affect, pain, and cognitive control consistently activate an overlapping region of aMCC. This overlap suggests the possibility that aMCC performs a similar role across domains (for additional discussion of the logic underlying this inference, see the Supplement). The anatomical data reviewed in the prior section are consistent with this hypothesis. We next consider whether the three domains also exhibit convergent functional properties. The logic here is that if aMCC implements a single, domain-general function across the three domains, then measures of negative affect, pain, and cognitive control should covary. These measures should also respond similarly to particular experimental manipulations and covary with distinct individual differences.
Several lines of evidence indicate that negative affect, pain, and cognitive control exhibit a measure of functional convergence. First, individual differences in measures of MCC structure predict variation in trait negative affect (neuroticism)81, conditioned fear82, and cognitive control83. Broadly speaking, individuals with a larger MCC report that they are predisposed to experience greater negative affect, exhibit enhanced electrodermal activity (EDA) and neural activation in aMCC during aversive conditioning tasks, and show reduced interference when performing the Stroop task84. Moreover, individual differences in negative affect predict variation in the other two domains. Specifically, individuals characterized by greater negative affect show increased engagement of control processes (indexed by well-validated event-related potential (ERP) measures85 that are thought to be generated in MCC86) when performing prototypical cognitive control tasks (for additional information, see the Supplement). They also exhibit increased sensitivity to experimental pain, particularly the affective qualities of pain (pain ‘unpleasantness’)87-90.
Second, manipulations of all three domains have been shown to amplify measures of autonomic arousal and negative affect. In particular, pain91, 92 and cognitive control93, 94 have been shown to increase EDA and amplify the fear-potentiated startle reflex (Figure 3d). These findings are linked to MCC by the observation that individuals who exhibit larger startle reflexes in response to errors on a prototypical cognitive control task (Eriksen flanker) show ERP evidence of enhanced control-related activity in MCC93. Likewise, individuals showing increased EDA in response to pain exhibit greater activation in aMCC and amygdala91.
Third, manipulations of all three domains can produce distinct changes in the muscles of the upper face48, 49, 95-98 (Figure 3d). As noted earlier, tracing studies in monkeys suggest that these muscles can be modulated, via the facial nucleus, by aMCC.
Fourth, manipulations targeting one domain can alter measures of the others. Experimentally induced negative affect, for example, can selectively disrupt the performance of tasks that strongly engage cognitive control48, 99. Cognitive control tasks can attenuate the intensity of negative affect100 and pain101, 102. Indeed, concurrent performance of the Stroop task attenuates pain-evoked activation in MCC103. Analgesic placebos show evidence of ‘cross-domain transfer,’ that is, they attenuate negative affect elicited by aversive images in addition to decreasing pain104. Conversely, the administration of anxiolytic compounds (that are not directly analgesic) can reduce the experience of pain89 and reduce aMCC activation to cues predictive of imminent pain104. Evidence for cross-domain interactions is consistent with the idea that negative affect, pain, and cognitive control can compete for, or otherwise modulate, a common functional resource implemented in aMCC. Cross-domain disruption, in particular, indicates that this resource makes a necessary contribution across domains. It is important to emphasize, however, that such cross-domain influences are often complex and do not necessarily impair performance or attenuate the intensity of subjective experience48, 102.
Fifth, all three domains are similarly affected by manipulations of ‘certainty,’ variously described in terms of ambiguity (‘unknown uncertainty’ of an outcome), controllability, determinacy, predictability, risk (‘known uncertainty’ of an outcome), or volatility. For instance, reducing the predictability of a physical threat amplifies ratings of anxiety and peripheral measures of negative affect, such as fear-potentiated startle and EDA105, 106, and activates aMCC106. Likewise, uncertainty about the timing or magnitude of painful stimulation increases ratings of pain unpleasantness and can markedly alter the psychophysical function relating different ‘doses’ of painful stimulation to subjective perception105, 107-110. Reductions in perceived instrumental control have been shown to amplify pain-evoked activation in aMCC111 and increase preparatory MCC activity in aversively-motivated instrumental conditioning paradigms112. Moreover, ERP indices of cognitive control that are thought to be generated in MCC are amplified by response uncertainty86 and unexpected outcomes113. Along similar lines, greater response uncertainty during probabilistic learning114 and economic decision making tasks115, 116 activate aMCC. Taken together, these observations suggest that the common function implemented in aMCC is sensitive to certainty about ‘actions’ (which response to make) or ‘outcomes’ (the magnitude and likelihood of the reinforcers acquired or avoided by such actions).
Finally, the hypothesis that aMCC activation could reflect a common operation across domains is consistent with striking similarities in the functions that have been ascribed to negative affect, pain, and cognitive control by domain-specific theories. Cognitive control, for example, has been described as an ‘early warning system’ that allows organisms to proactively alter attention or behavior to avoid future errors117. A similar warning function has been ascribed to pain118 and to some kinds of negative affect, such as fear and anxiety119, 120. Like cognitive control, it has been suggested that negative affect (e.g., fear, anger) is goal-directed and flexibly coordinates anticipatory responses that decrease the likelihood of future punishment121, 122. Demands for cognitive control are also thought to motivate new learning34. Such demands may serve as a teaching signal that penalizes choices, strategies, or actions requiring greater control, promoting avoidance of cognitively taxing actions in the future. Indeed, it has been shown that variation in the error-related negativity (ERN; an ERP component that is sensitive to control demands and thought to be generated in MCC) predicts the degree to which individuals learn from the negative consequences of their actions: individuals with larger ERNs show enhanced avoidance learning for events associated with negative outcomes123, 124. Imaging studies have revealed broadly similar effects in MCC125. This teaching function is reminiscent of the role ascribed to pain and negative affect in reinforcing withdrawal-related behaviors and driving the acquisition of instrumental avoidance12, 119-121. Given these numerous parallels, Yeung and colleagues30 have speculated that the signals responsible for triggering cognitive control (for instance, the output of a response conflict monitor; Box 2) could represent the computational underpinning of negative affect. That is, the same computational machinery might be engaged when cognitive control or negative affect are elicited.
The Adaptive Control Hypothesis
aMCC uses information about punishment to control aversively-motivated action
The observations reviewed in the previous section suggest that aMCC makes a similar functional contribution to negative affect, pain, and cognitive control. But what is the nature of this ‘domain-general’ contribution? A plausible working hypothesis is that negative affect and pain tend to engage the same processes described by theories of cognitive control in order to solve conceptually similar problems. In the remainder of this Review, we refer to this process as ‘adaptive control’ rather than ‘cognitive control’ to underscore its broader contribution to negative affect and nociception. In the remainder of this section, we explore the utility of using computationally-inspired models of control and reinforcement learning (Box 2) to clarify the role of aMCC in negative affect and pain. Adapting such models to the study of negative affect and pain promises to enhance the mechanistic specificity of accounts describing this region‘s putative role in avoidance/defensive behaviors5, emotional appraisals21, emotional experience11, fear25, attention to pain26, pain expectancy126, 127, pain-related motor control5, 128, and so on.
Control processes are engaged when automatic or habitual responses are insufficient to support goal-directed behavior129. This occurs when there is uncertainty about the optimal course of action (as in situations involving probabilistic learning), when potential actions are associated with significant risks (e.g., of failure, punishment, or error), or when there is competition between plausible alternative actions or between action and inaction (e.g., flee or freeze, go or no-go). These features are hallmarks of environments where physical threat is genuine, as in many studies of fear, anxiety, and pain. Indeed, recent work in rodents demonstrates that physical threat can elicit competition between neural circuits mediating active (Go: avoidance) and passive (No-Go: freezing) defensive behaviors57. Not surprisingly, optimal instrumental behavior in threatening environments has long been thought to require cognitive control129, 130, which provides the biasing signal necessary to resolve response uncertainty or competition and avoid potentially catastrophic actions (Box 2).
On the basis of earlier suggestions5, 26, 29, 30, 33, 34 and more recent computational models of cognitive control and reinforcement learning (Boxes 2-3), we hypothesize that the core function common to negative affect, pain, and cognitive control is the need to determine an optimal course of action in the face of uncertainty, that is, to exert control. Based on the data reviewed in the prior section, we further hypothesize that aMCC implements adaptive control by integrating information about punishment (e.g., likelihood, magnitude) arriving from the amygdala, spinothalamic system, striatum, insula, and other regions (Figure 4) in order to bias responding in situations where the optimal course of action is uncertain or entails competition between alternative courses. Outgoing control signals would presumably be sent directly to subcortical and cortical motor centers. Alternatively, control signals generated in aMCC and directed at the amygdala or lPAG might serve to resolve conflict between passive and active defensive behaviors. Several other mechanisms are plausible and these are described more fully in the final section.
aMCC is responsive to control demands in threatening environments
To date, few studies have addressed whether aMCC is specifically involved in complex action planning or is sensitive to control demands (e.g., the number of response options, response inhibition) in response to perceived physical threat (although somewhat more is known about its role in reward-motivated learning, see Box 3). Nevertheless, the extant data are consistent with a role in modulating action in response to information about punishment. First, neuronal recordings in humans and monkeys show that pain-responsive MCC neurons are activated by both anticipation of pain131, 132 and instrumental escape from pain133. These data underscore the close connections between pain, negative affect elicited by imminent pain, and defensive action in MCC. Second, consistent with the work highlighted in the previous section, other research indicates that these neural signals are sensitive to uncertainty and conflict. For instance, source modeling analyses suggest that this MCC activity is amplified by uncertainty about the action associated with pain avoidance (action-outcome uncertainty)112. Likewise, the N2, an ERP signature of control thought to be generated in MCC, is amplified when pain delivery requires the inhibition of movement134 and attenuated when participants are allowed to move135, 136. Third, lesions of the cingulate sulcus in monkeys, which effectively destroys the monkey analogue to human RCZ, alter how threat modulates on-going behavior137. Specifically, lesioned monkeys are less reluctant to take food placed above a moving toy snake than controls, an effect reminiscent of that obtained following amygdala lesions138, 139 and consistent with our suggestion that aMCC exploits ascending punishment signals to modulate instrumental behavior. Fourth, recent imaging studies suggest that aMCC might play a more specific role in regulating defensive responses to threat, consistent with our emphasis on control. Across mammalian species, defensive behavior qualitatively varies with the psychological and physical imminence of threat—distal threats elicit risk assessment, vigilance, and the suppression of on-going appetitive and consummatory activities (such as foraging); as threat grows more imminent, these behaviors give way to affiliation and alarm calls, flight, or freezing (if escape is thwarted), and, ultimately, to active defensive displays or even defensive attack77, 140-142. In monkeys, the change of behavioral repertoire in response to increased threat imminence is associated with activation of aMCC143. Likewise, in humans aMCC is activated when escaping from a ‘virtual predator’ whose imminence dynamically varied in a game-like avoidance task (failure to escape the predator was paired with shocks)144, 145. These data are consistent with suggestions that aMCC plays a key role in regulating instrumental defensive behaviors34 or is involved in selecting ‘options,’ sequences of elementary actions aimed at accomplishing a goal146. Fifth, additional evidence for the adaptive control hypothesis comes from imaging studies showing that aMCC activity during aversively-motivated learning is predicted by formal computational models of control and reinforcement learning147-150. Schiller and colleagues147, for instance, recently showed that activation in aMCC encodes punishment prediction errors during the reversal of learned fear. These observations are broadly consistent with the hypothesis that aMCC uses such predictions to adopt the most adaptive response to threat. An alternative possibility is that this effect is a special case of this region‘s role in computing signals of reinforcer ‘salience’ during both appetitively- and aversively-motivated behavior31, 151-153 (Box 3).
A broader perspective on the rostral cingulate
The data that we have reviewed encourage a broader perspective on the functional significance of cingulate activity. The aMCC did not evolve to optimize performance on laboratory measures of ‘cold’ cognition, such as the Stroop task. Indeed, anthropological research suggests that the human brain, like that of our earlier ancestors, evolved in the context of significant pressure from physical threats, including predation and intraspecific aggression154, that demanded a neural system capable of flexibly controlling aversively-motivated behavior. The data we have surveyed are consistent with this speculation and suggest that the contribution of aMCC to laboratory measures of cognitive control might stem from its evolutionarily older role in regulating ‘hot’ behaviors25, 47, 155—such as expressive behavior on the face and aversively-motivated instrumental learning—that are elicited by stimuli and situations with affective and nociceptive significance (for a related perspective, see Refs. 34, 156). This view helps to explain why demands on cognitive control are associated with vestigial defensive reactions, such as brow furrowing and startle, and why individual differences in such measures predict the magnitude of control signals thought to be generated in aMCC (e.g., the N2). It also provides an explanation for why the anticipation and receipt of uncertain punishments, which place greater demands on control resources, produce greater activation of aMCC. Regardless of the evolutionary origins, observations such as these are not readily accommodated by accounts that emphasize a strict segregation of cognition from emotion and nociception in the cingulate.
Limitations of the Available Evidence
On the basis of a wide range of data and theory, we have suggested that activation of aMCC in studies of negative affect and pain reflects the engagement of control processes that help to optimize responses made in the face of uncertainties about instrumental actions and the outcomes they produce. We have further hypothesized that aMCC implements adaptive control by synthesizing information about punishment arriving from the amygdala, spinothalamic system, insula and other regions into a biasing signal that could modulate motor centers or subcortical regions, such as amygdala and lPAG, that more proximally influence active (fleeing) and passive (freezing) defensive behaviors. It is clear that these hypotheses reflect a number of indirect inferences, a limitation that reflects the state of the existing empirical record. Although much work remains, the adaptive control hypothesis provides a clear roadmap to the most profitable avenues for understanding the contribution of aMCC to negative affect and pain. Here, we outline several strategies for more directly testing and refining this account.
First, our meta-analysis demonstrates that aMCC is consistently activated at the subdivision level by manipulations of negative affect, pain, and cognitive control. High-resolution, single-subject imaging analyses and intracerebral recordings will be required to determine whether negative affect and pain are anatomically coincident with cognitive control at finer levels of resolution, are intermingled (as some early imaging studies suggest157-159), or are organized along overlapping gradients44, 160-162. To permit a more decisive test, such studies should employ a broad battery of well-matched tasks (matched on certainty and motor requirements, for instance). The use of single-subject conjunction analyses163 or single-subject spatial confidence intervals164 would provide a rigorous means of quantifying the degree of overlap. Studies of this kind will also prove useful for determining whether negative affect and pain differentially activate superficial (RCZ) versus deep regions of aMCC (Box 1) (cf. Ref. 137). Based on prior single-subject analyses of negative affect, pain, or cognitive control126, 158, 163, 165, we suspect that future work will reveal marked individual differences in the mapping of each domain to cingulate anatomy. Indeed, variation in the location of clusters across individuals within any one domain may well outweigh variation across domains.
Determining the source of such individual differences is a key challenge for future research. One promising way to tackle this problem is to acquire independent measures of affect, pain, or cognitive control (e.g., eye-tracking, facial action coding or electromyography, pupil dilation, and the startle reflex). Variation in such measures— across conditions, across individuals, and within individuals—can clarify the psychological processes that are probabilistically recruited by each domain166. For instance, individuals clearly differ in the intensity or likelihood of negative affect in response to physical threats48 or performance errors93. Although typically not measured, such differences likely play a key role in determining which subregions of the cingulate cortex are recruited in each individual. From a translational perspective, such variation may also help to account for differences in treatment response or other clinical features of disorders that are associated with MCC abnormalities, such as post-traumatic stress and bipolar disorders10, 11. Already some investigators have begun to use measures of pain experience (self-report) and expression (peripheral motor reflexes and autonomic activity) to map dimensions of the pain response onto the different subdivisions of the cingulate152, 167. Another, closely related strategy is to fit computational models to the data acquired from each participant and to use individual differences in the resulting parameter estimates to predict neural activity168.
Second, complex, multi-componential psychological processes—like negative affect, pain and cognitive control40, 169—are implemented in distributed neural networks. Although aMCC is involved in all three domains, it likely does so in combination with dissociable networks. Functional connectivity170, mediation152, or multivoxel pattern171 analyses (MVPA) would permit the identification and dissociation of such networks. MVPA may prove to be a particularly useful tool because it quantifies the degree to which distributed patterns of neural activity encode information about a domain. Using MVPA, one can ask, for instance, whether the pattern of neural activity corresponding to pain delivery is re-activated by performance errors or threat-of-shock in individual participants. MVPA would also potentially allow the discrimination of domain-specific processes that are intermingled at the sub-voxel level172. Ultimately, such multivariate analyses will be necessary to understand how negative affect, pain, and cognitive control emerge from the distributed activity of computationally specialized regions. They should also prove helpful for determining whether the function implemented by aMCC varies qualitatively across different patterns of regional coupling170.
Some of these regions may reside within rostral cingulate. We rejected claims of strict functional segregation because our CBMA demonstrated that imaging studies of negative affect, pain, and cognitive control consistently activate an overlapping region in aMCC (Figure 2) and because ACC (the putative ‘affective division’ of the cingulate) was not preferentially associated with negative affect or pain. Nevertheless, the results of the CBMA are consistent with a measure of functional specialization across rostral cingulate (Figure 1c). For instance, the CBMA indicated that only studies of negative affect consistently activated subgenual ACC (sgACC; see the Supplement). Likewise the elicitation of negative affect and pain consistently activated pregenual ACC (pgACC) and posterior MCC (pMCC), whereas cognitive control did not.
Third, thoughtful experimental design, combined with computational modeling and network analyses or more invasive manipulations in nonhuman animals, will be required to clarify how aMCC uses information about punishment to adaptively control complex instrumental behaviors. A key question is whether this region represents a monitor, a controller, or some combination of the two (Box 2). It is possible that incoming information about negative affect and pain reflects one of several kinds of inputs that are monitored by aMCC and used to trigger control signals30. Such control signals may be generated in distal regions of the brain, such as the striatum or lateral PFC, or may be generated locally in aMCC and conveyed directly to motor centers. Another possibility is that aMCC directly biases aversively-motivated actions through its connections with motor centers, but conveys the need for other kinds of control, such as the biasing of selective attention, to lateral PFC or parietal cortex173. Such a dissociation would help to reconcile the greater intimacy of aMCC with motor regions while acknowledging the well-documented role of lateral PFC in biasing activity in posterior sensory cortices174. A third possibility is that aMCC triggers or implements control in response to insular or amygdalar inputs. Consistent with this, more anxious individuals show aberrant coupling between aMCC and amygdala during the presentation of images known to elicit negative affect175. Amygdalar signals might reflect competition between passive and active responses to noxious stimuli57, punishment predictions or prediction errors176, or a more general source of information about errors177-182. Such signals could be conveyed directly to aMCC or indirectly, via connections from the amygdala to the striatum, insula, or pgACC. Why the amygdala generates such control signals and how this influences or is influenced by control processes implemented in aMCC are two crucial questions not addressed by any of the major computational models of control (Box 2).
Finally, studies of nonhuman primates and human lesion patients will be necessary to determine whether the contribution of aMCC to the adaptive control of punishment-motivated instrumental behavior is a necessary one. Nonhuman primate research will be particularly useful for bridging the gap between human imaging studies and invasive studies of threat, fear, and pain in rabbits and rodents25, 155, species that lack certain features of the primate cingulate183. Combining invasive techniques with imaging measures in primates should prove particularly useful in this regard. Functional imaging studies would be useful for more precisely identifying functionally homologous regions across species184. Nonhuman primate research will also be required to clarify the anatomical connectivity of aMCC, particularly of RCZ, and to develop a more detailed understanding of its role in planning complex actions42, 185. This will be particularly critical owing to the extreme rarity of circumscribed insults to aMCC in humans, a consequence of the wide ramifications of the arterial supply to this region13. Although the near absence of such data precludes strong inferences, extant neuropsychological studies are consistent with the idea that MCC makes a necessary contribution to adaptive control in humans186 (whether this is also true in monkeys remains contentious7). In particular, focal damage to left aMCC (including the probable location of RCZ) is associated with exaggerated response conflict and attenuation of the ERN ERP component187, 188 (but see Ref. 186). Studies employing neurofeedback techniques189 or microstimulation to directly manipulate activity in aMCC in humans would be a valuable adjunct to lesion studies. In particular, it would be useful to know whether these more direct manipulations exert similar effects on measures of negative affect, pain, and cognitive control.
Conclusions
In summary, a wide variety of evidence demonstrates that negative affect, pain, and cognitive control are anatomically and functionally integrated at the subdivision level in aMCC, likely within RCZ, the premotor area harbored in the dorsal portion of aMCC. On this basis, the claim that the cingulate cortex is strictly segregated into cognitive and affective divisions is no longer tenable. Computational models of cognitive control and reinforcement learning provide a foundation for integrating such observations into a mechanistic account of this region’s contribution to negative affect and pain. This framework leads to the adaptive control hypothesis, which suggests that aMCC uses information about punishment to bias responding when the most adaptive course of action is uncertain. This account is not a new theory of rostral cingulate function. Indeed, many of the ideas that we have reviewed are well known among certain groups of neuroscientists. It is instead a synthesis of earlier suggestions and new data into a clear working hypothesis about the contribution of aMCC to aversively-motivated behavior. As such, we have delineated the kinds of evidence that will be required to refine it. Perhaps one of the most important challenges is determining whether adaptive control is specific to punishment or, instead, extends to rewards and appetitively-motivated behavior. As we emphasized in Box 3, a direct test of this possibility using adequately potent, well-matched reinforcers has yet to be performed. To conclude, attempts to refine the adaptive control hypothesis or to adjudicate between it and narrower claims of segregation promise to enrich our understanding of this region’s contribution to negative affect and pain in health and disease.
Acknowledgements
The first two authors (A.J.S. and T.V.S.) contributed equally to this Review. Some of this work was presented at the 2008 annual meeting of the Society for Neuroscience. We thank the Laboratory for Affective Neuroscience and Waisman Laboratory for Brain Imaging and Behavior staff, UW—Madison Library Express, A. Dinndorf, M. Fox, L. Friedman, L. Hinsenkamp, A. Koppenhaver, A. Laird, B. Nacewicz, D. Rebedew and J.E. Shackman for assistance; M.X. Cohen, W. Irwin, S. Nieuwenhuis, J. Oler, T. Yarkoni and three anonymous reviewers for critical feedback; J. Coan and C. Thrasher for providing the face images; and G. Bush for generously providing unpublished details of the meta-analysis described by Bush, Luu and Posner. This work was financially supported by the European Commission (Marie Curie Reintegration Grant to H.A.S.), Fetzer Foundation (R.J.D.), National Institute of Mental Health (P50-MH069315, P50-MH084051 and R01-MH43454 (R.J.D.); A.J.S. was partially supported by R01-MH064498 (B.R. Postle); A.S.F. was supported by T32-MH018931 (R.J.D.)), and University of Toronto Centre for the Study of Pain (Clinician-Scientist award to T.V.S.). Author contributions: A.J.S., T.V.S., and R.J.D. designed the research; A.J.S., T.V.S., and J.J.W. prepared the meta-analysis databases; A.J.S., T.V.S., and A.S.F. performed the meta-analysis; A.J.S. and A.S.F. created the figures; A.J.S. wrote the paper; A.J.S., T.V.S., H.A.S., A.S.F., and R.J.D. commented on the paper.
Glossary
| Architectonic area | A region of the brain defined by its cellular and molecular neuroanatomy, including neuronal structure (‘cytoarchitecture’), myelin structure (‘myeloarchitecture’) and neurochemistry (‘chemoarchitecture’). |
| Attentional set | A template, rule, or goal held in memory to guide attention (e.g., search for angry faces in a crowded visual scene). |
| Cognitive control | A range of elementary processes (attention, inhibition, learning) that are engaged when automatic or habitual responses are insufficient to sustain goal-directed behavior. Control can be engaged proactively (in situations associated with a heightened risk of failure or decision uncertainty) or reactively (by actual failures, as with errors and negative feedback). |
| Computational model | A mathematically detailed simulation of a psychological construct that can afford quantitative predictions of trial-by-trial fluctuations in behavior and neurophysiology. |
| Electrodermal activity (EDA) | Changes in the electrical resistance of the dermis stemming from activity of the sweat glands. EDA reflects activation in the sympathetic nervous system and is used to index arousal, stress, and cognitive load |
| Electromyographic (EMG) activity | Electrical activity generated by the skeletal musculature. |
| Eriksen Flanker task | A task in which subjects rapidly respond to a centrally presented visual cue, such as an arrowhead, that is neighbored (‘flanked’) by cues that can potentially code an alternative response. |
| Event-related potential (ERP) | A scalp-recorded measure of the average brain electrical activity evoked by a particular stimulus or response. |
| Fear-potentiated startle reflex | Reflex evoked by the sudden onset of high intensity stimuli (e.g., a loud noise) and amplified by negative affect. In humans, this is measured using electrodes overlying orbicularis oculi, the muscle responsible for eye blinks. |
| Go/No-Go task | A task in which subjects must rapidly respond to one kind of cue (‘Go’) while withholding responses to another (‘No-Go’). |
| Instrumental Behavior | Behavior that is goal-directed insofar as it increases the likelihood of obtaining rewards or avoiding punishments. Instrumental behavior is distinguished from behaviors that are reflexively elicited independent of reinforcement, as in Pavlovian (‘classical’) conditioning. |
| Learning rate | In reinforcement learning models, the weight assigned to ‘prediction errors.’ New information (errors) is weighted more heavily when expectations are uncertain. |
| Neurofeedback | A kind of learning in which real-time neural activity is employed as feedback. |
| Pain psychophysics | Standardized techniques for relating the physical level of stimulation to variations in the subjective perception of pain (magnitude, intensity, or unpleasantness); used to determine the stimulus associated with a distinct perceptual experience. |
| Prediction error | In reinforcement learning models, an explicit description of the discrepancy between ‘reinforcer expectations’ and actual reinforcement. |
| Reinforcers | Stimuli that are capable (intrinsically or through learning) of eliciting instrumental behavior; rewards and punishments. |
| Reinforcement learning (RL) models | A class of computational models describing how organisms learn to maximize reinforcement based on experience. RL models assume that organisms update ‘reinforcer expectations’ on the basis of ‘prediction errors’ and the current ‘learning rate.’ |
| Reinforcer expectation | In reinforcement learning models, an explicit prediction about the amount and probability of contingent outcomes. |
| Response conflict | Competition elicited by stimuli associated with multiple, incompatible response tendencies, as in the Stroop task. |
| Stroop task | A task in which subjects rapidly respond to a color word, such as ‘BLUE,’ on the basis of the color in which the letters are displayed. The task is easy when the color and word are compatible (the word ‘BLUE’ is depicted in blue), but is more difficult when the two are incompatible (‘BLUE’ is depicted in red). |
Biography
Alexander J. Shackman
Alexander J. Shackman earned his Ph.D. working in the laboratory of R.J. Davidson (University of Wisconsin—Madison, USA), where he used peripheral and central physiological measures to study the impact of threat and threat-induced negative affect on visual processing, working memory and cognitive control. He is currently a postdoctoral fellow in the laboratory of B.R. Postle (University of Wisconsin—Madison, USA), where he is using transcranial magnetic stimulation and functional imaging techniques to understand how prefrontal cortex biases distal regions of the brain in support of goal-directed cognition and behavior.
Tim V. Salomons
Tim V. Salomons completed his Ph.D. in Clinical Psychology at the University of Wisconsin—Madison working in the laboratory of R. J. Davidson. He is currently a postdoctoral fellow at the Toronto Western Research Institute working under the supervision of K.D. Davis. His work uses structural and functional neuroimaging, peripheral psychophysiology, and various behavioral and self-report measures to examine the biological mechanisms through which cognition and emotion alter the experience of pain.
Heleen A. Slagter
Heleen A. Slagter is an assistant professor in the Brain and Cognition unit of the Department of Psychology at the University of Amsterdam, the Netherlands. She received her Ph.D. from the University of Amsterdam, where she was supervised by A. Kok and collaborated with M.G. Woldorff and G.R. Mangun (Duke University, USA). She subsequently performed postdoctoral research at the University of Wisconsin—Madison (USA) in the laboratory of R.J. Davidson. Her work aims to understand how the brain selects and coordinates information in accord with current goals and the amenability of these abilities to training. She was recently awarded a VIDI grant by the Dutch Science Foundation.
Andrew S. Fox
Andrew S. Fox is a graduate student working in the laboratories of R.J. Davidson and N.H. Kalin (University of Wisconsin—Madison, USA). His research uses behavioral economic tasks, as well as structural and functional brain imaging techniques, in humans and rhesus monkeys to understand how emotional brain systems influence temperament, guide decision-making, and sculpt motivated behavior.
Jameel J. Winter
Jameel J. Winter received his undergraduate degree at the University of Wisconsin—Madison (USA), where he worked in the laboratory of R.J. Davidson. He is currently a fourth year medical student (University of Minnesota Medical School, Minneapolis, MN, USA).
Richard J. Davidson
Richard J. Davidson is William James and Vilas Professor of Psychology and Psychiatry, University of Wisconsin—Madison (USA). He received his Ph.D. from Harvard University (USA) and has been at the University of Wisconsin—Madison since 1985. He directs the Laboratory for Affective Neuroscience, the Waisman Laboratory for Brain Imaging and Behavior, and the Center for Investigating Healthy Minds. He is a founding co-editor of the journal Emotion, past-president of the Society for Psychophysiological Research and the Society for Research in Psychopathology, and a recipient of the American Psychological Association’s Distinguished Scientific Contribution award and the Association for Psychological Science’s William James Fellow award. His life long research focus is on affective neuroscience.
Websites
Alexander Shackman’s homepage: http://psyphz.psych.wisc.edu/~shackman/
Heleen Slagter’s homepage: http://home.medewerker.uva.nl/h.a.slagter/
Richard Davidson’s homepages: http://brainimaging.waisman.wisc.edu/, http://www.healthemotions.org/, and http://www.investigatinghealthyminds.org/cihmLaboratory.html
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/nrn2994
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3044650?pdf=render
Citations & impact
Impact metrics
Article citations
Longitudinal evidence for a mutually reinforcing relationship between white matter hyperintensities and cortical thickness in cognitively unimpaired older adults.
Alzheimers Res Ther, 16(1):240, 28 Oct 2024
Cited by: 0 articles | PMID: 39465440 | PMCID: PMC11520063
Dissecting shared genetic architecture between depression and body mass index.
BMC Med, 22(1):455, 11 Oct 2024
Cited by: 0 articles | PMID: 39394142 | PMCID: PMC11481102
A Bayesian incorporated linear non-Gaussian acyclic model for multiple directed graph estimation to study brain emotion circuit development in adolescence.
Netw Neurosci, 8(3):791-807, 01 Oct 2024
Cited by: 1 article | PMID: 39355441 | PMCID: PMC11349030
Neural correlates of reward valuation in individuals with nonsuicidal self-injury under uncertainty.
Psychol Med, 1-10, 06 Sep 2024
Cited by: 0 articles | PMID: 39238080 | PMCID: PMC11496225
Brain plasticity following lumbar disc herniation treatment with spinal manipulation therapy based on resting-state functional magnetic resonance imaging.
Heliyon, 10(18):e37703, 11 Sep 2024
Cited by: 0 articles | PMID: 39315226 | PMCID: PMC11417269
Go to all (1,065) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Distinct Regions within Medial Prefrontal Cortex Process Pain and Cognition.
J Neurosci, 36(49):12385-12392, 02 Nov 2016
Cited by: 34 articles | PMID: 27807031 | PMCID: PMC5148227
Motor and emotional behaviours elicited by electrical stimulation of the human cingulate cortex.
Brain, 141(10):3035-3051, 01 Oct 2018
Cited by: 87 articles | PMID: 30107501
Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function.
Psychophysiology, 44(3):343-351, 01 May 2007
Cited by: 182 articles | PMID: 17433093
Contributions of anterior cingulate cortex to behaviour.
Brain, 118 ( Pt 1):279-306, 01 Feb 1995
Cited by: 1937 articles | PMID: 7895011
Review
Funding
Funders who supported this work.
NICHD NIH HHS (1)
Grant ID: P30 HD003352
NIMH NIH HHS (22)
Grant ID: P50-MH084051
Grant ID: P50 MH069315-04
Grant ID: R01 MH064498-06
Grant ID: T32 MH018931
Grant ID: T32 MH018931-19
Grant ID: P50 MH069315
Grant ID: R01 MH043454
Grant ID: R01 MH043454-19
Grant ID: P50 MH084051-03
Grant ID: P50 MH084051-04
Grant ID: P50 MH084051
Grant ID: P50-MH069315
Grant ID: R01 MH043454-20
Grant ID: R01 MH043454-21
Grant ID: R01 MH064498
Grant ID: R01 MH064498-07
Grant ID: R01-MH43454
Grant ID: T32 MH018931-18
Grant ID: R01-MH064498
Grant ID: P50 MH069315-05
Grant ID: R01 MH043454-22
Grant ID: T32-MH018931