Abstract
Free full text

Role of adenovirus E1B proteins in transformation: altered organization of intermediate filaments in transformed cells that express the 19-kilodalton protein.
Abstract
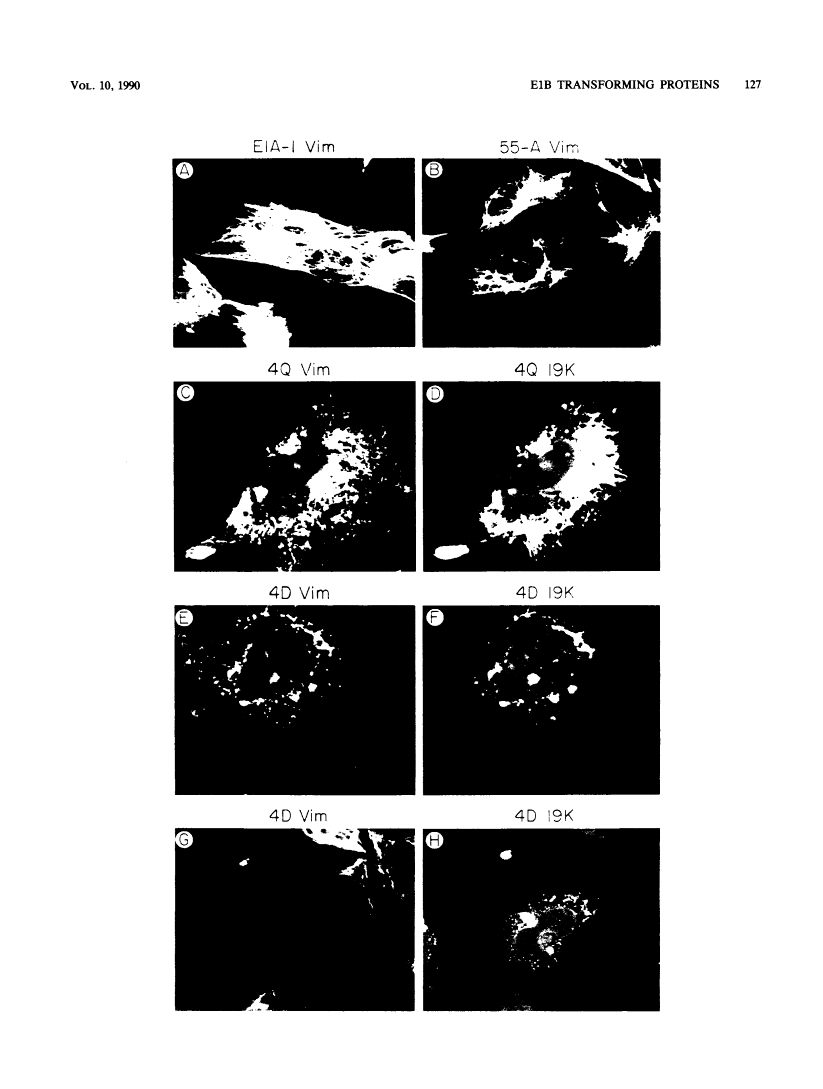
Cooperation of the nuclear oncogene E1A with the E1B oncogene is required for transformation of primary cells. Expression vectors were constructed to produce the 19-kilodalton (19K) and 55K E1B proteins under the direction of heterologous promoters in order to investigate the role of individual E1B proteins in transformation. Coexpression of E1A and either the 19K or 55K E1B gene products was sufficient for the formation of transformed foci in primary rat cells at half the frequency of an intact E1B gene, suggesting that the 19K and 55K proteins function via independent pathways in transformation. Furthermore, the effects of Ha-ras and the E1B 19K gene product were additive when cotransfected with E1A, suggesting that the 19K protein functions in transformation by a mechanism independent from that of ras as well. Although expression of E1A and either E1B protein was sufficient for the subsequent growth of cells in long-term culture, the 19K protein was required to support growth in semisolid media. As the 19K protein has been shown to associate with and disrupt intermediate filaments (IFs) when transiently expressed with plasmid vectors (E. White and R. Cipriani, Proc. Natl. Acad. Sci. USA, 86:9886-9890, 1989), the organization of IFs in transformed cells was investigated. Primary rat cells transformed by plasmids encoding E1A plus the E1B 19K protein showed gross perturbations of IFs, whereas cell lines transformed by plasmids encoding E1A plus the E1B 55K protein or E1A plus Ha-ras did not. These results suggest that an intact IF cytoskeleton may inhibit anchorage-independent growth and that the E1B 19K protein can overcome this inhibition by disrupting the IF cytoskeleton.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (3.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babiss LE, Fisher PB, Ginsberg HS. Effect on transformation of mutations in the early region 1b-encoded 21- and 55-kilodalton proteins of adenovirus 5. J Virol. 1984 Nov;52(2):389–395. [Europe PMC free article] [Abstract] [Google Scholar]
- Ball EH, Singer SJ. Association of microtubules and intermediate filaments in normal fibroblasts and its disruption upon transformation by a temperature-sensitive mutant of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6986–6990. [Europe PMC free article] [Abstract] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. [Abstract] [Google Scholar]
- Barker DD, Berk AJ. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987 Jan;156(1):107–121. [Abstract] [Google Scholar]
- Berk AJ. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. [Abstract] [Google Scholar]
- Bernards R, de Leeuw MG, Houweling A, van der Eb AJ. Role of the adenovirus early region 1B tumor antigens in transformation and lytic infection. Virology. 1986 Apr 15;150(1):126–139. [Abstract] [Google Scholar]
- Bernards R, Schrier PI, Bos JL, Van der Eb AJ. Role of adenovirus types 5 and 12 early region 1b tumor antigens in oncogenic transformation. Virology. 1983 May;127(1):45–53. [Abstract] [Google Scholar]
- Bishop JM. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. [Abstract] [Google Scholar]
- Blair Zajdel ME, Blair GE. The intracellular distribution of the transformation-associated protein p53 in adenovirus-transformed rodent cells. Oncogene. 1988 Jun;2(6):579–584. [Abstract] [Google Scholar]
- Bolen JB, Thiele CJ, Israel MA, Yonemoto W, Lipsich LA, Brugge JS. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. [Abstract] [Google Scholar]
- Boshart M, Weber F, Jahn G, Dorsch-Häsler K, Fleckenstein B, Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. [Abstract] [Google Scholar]
- Branton PE, Bayley ST, Graham FL. Transformation by human adenoviruses. Biochim Biophys Acta. 1985;780(1):67–94. [Abstract] [Google Scholar]
- Chan D, Goate A, Puck TT. Involvement of vimentin in the reverse transformation reaction. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2747–2751. [Europe PMC free article] [Abstract] [Google Scholar]
- Chinnadurai G. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983 Jul;33(3):759–766. [Abstract] [Google Scholar]
- Cone RD, Grodzicker T, Jaramillo M. A retrovirus expressing the 12S adenoviral E1A gene product can immortalize epithelial cells from a broad range of rat tissues. Mol Cell Biol. 1988 Mar;8(3):1036–1044. [Europe PMC free article] [Abstract] [Google Scholar]
- Cooper HL, Feuerstein N, Noda M, Bassin RH. Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol. 1985 May;5(5):972–983. [Europe PMC free article] [Abstract] [Google Scholar]
- Courtneidge SA, Smith AE. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. [Europe PMC free article] [Abstract] [Google Scholar]
- Flint SJ. Cellular transformation by adenoviruses. Pharmacol Ther. 1984;26(1):59–88. [Abstract] [Google Scholar]
- Fukui Y, Saito I, Shiroki K, Shimojo H. Isolation of transformation-defective, replication-nondefective early region 1B mutants of adenovirus 12. J Virol. 1984 Jan;49(1):154–161. [Europe PMC free article] [Abstract] [Google Scholar]
- Gallimore PH. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. [Abstract] [Google Scholar]
- Garrels JI, Franza BR., Jr Transformation-sensitive and growth-related changes of protein synthesis in REF52 cells. A two-dimensional gel analysis of SV40-, adenovirus-, and Kirsten murine sarcoma virus-transformed rat cells using the REF52 protein database. J Biol Chem. 1989 Mar 25;264(9):5299–5312. [Abstract] [Google Scholar]
- Glenney JR, Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989 Jun;108(6):2401–2408. [Europe PMC free article] [Abstract] [Google Scholar]
- Graham FL, van der Eb AJ. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. [Abstract] [Google Scholar]
- Graham FL, van der Eb AJ, Heijneker HL. Size and location of the transforming region in human adenovirus type 5 DNA. Nature. 1974 Oct 25;251(5477):687–691. [Abstract] [Google Scholar]
- Hendricks M, Weintraub H. Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5633–5637. [Europe PMC free article] [Abstract] [Google Scholar]
- Houweling A, van den Elsen PJ, van der Eb AJ. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. [Abstract] [Google Scholar]
- Hynes RO, Destree AT. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. [Abstract] [Google Scholar]
- Jochemsen AG, de Wit CM, Bos JL, van der Eb AJ. Transforming properties of a 15-kDa truncated Ad12 E1A gene product. Virology. 1986 Jul 30;152(2):375–383. [Abstract] [Google Scholar]
- Jochemsen AG, Peltenburg LT, te Pas MF, de Wit CM, Bos JL, van der Eb AJ. Activation of adenovirus 5 E1A transcription by region E1B in transformed primary rat cells. EMBO J. 1987 Nov;6(11):3399–3405. [Europe PMC free article] [Abstract] [Google Scholar]
- Jones N, Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. [Abstract] [Google Scholar]
- Kaczmarek L, Ferguson B, Rosenberg M, Baserga R. Induction of cellular DNA synthesis by purified adenovirus E1A proteins. Virology. 1986 Jul 15;152(1):1–10. [Abstract] [Google Scholar]
- Leonardi CL, Warren RH, Rubin RW. Lack of tropomyosin correlates with the absence of stress fibers in transformed rat kidney cells. Biochim Biophys Acta. 1982 Apr 29;720(2):154–162. [Abstract] [Google Scholar]
- Liebowitz D, Kopan R, Fuchs E, Sample J, Kieff E. An Epstein-Barr virus transforming protein associates with vimentin in lymphocytes. Mol Cell Biol. 1987 Jul;7(7):2299–2308. [Europe PMC free article] [Abstract] [Google Scholar]
- Matsumura F, Lin JJ, Yamashiro-Matsumura S, Thomas GP, Topp WC. Differential expression of tropomyosin forms in the microfilaments isolated from normal and transformed rat cultured cells. J Biol Chem. 1983 Nov 25;258(22):13954–13964. [Abstract] [Google Scholar]
- McKinnon RD, Bacchetti S, Graham FL. Tn5 mutagenesis of the transforming genes of human adenovirus type 5. Gene. 1982 Jul-Aug;19(1):33–42. [Abstract] [Google Scholar]
- McNutt NS, Culp LA, Black PH. Contact-inhibited revertant cell lines isolated from SV 40-transformed cells. IV. Microfilament distribution and cell shape in untransformed, transformed, and revertant Balb-c 3T3 cells. J Cell Biol. 1973 Feb;56(2):412–428. [Europe PMC free article] [Abstract] [Google Scholar]
- Persson H, Katze MG, Philipson L. Purification of a native membrane-associated adenovirus tumor antigen. J Virol. 1982 Jun;42(3):905–917. [Europe PMC free article] [Abstract] [Google Scholar]
- Pilder S, Logan J, Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984 Nov;52(2):664–671. [Europe PMC free article] [Abstract] [Google Scholar]
- Pollack R, Osborn M, Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. [Europe PMC free article] [Abstract] [Google Scholar]
- Quinlan MP, Grodzicker T. Adenovirus E1A 12S protein induces DNA synthesis and proliferation in primary epithelial cells in both the presence and absence of serum. J Virol. 1987 Mar;61(3):673–682. [Europe PMC free article] [Abstract] [Google Scholar]
- Rowe DT, Branton PE, Yee SP, Bacchetti S, Graham FL. Establishment and characterization of hamster cell lines transformed by restriction endonuclease fragments of adenovirus 5. J Virol. 1984 Jan;49(1):162–170. [Europe PMC free article] [Abstract] [Google Scholar]
- Ruley HE. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. [Abstract] [Google Scholar]
- Sarnow P, Ho YS, Williams J, Levine AJ. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. [Abstract] [Google Scholar]
- Senear AW, Lewis JB. Morphological transformation of established rodent cell lines by high-level expression of the adenovirus type 2 E1a gene. Mol Cell Biol. 1986 Apr;6(4):1253–1260. [Europe PMC free article] [Abstract] [Google Scholar]
- Shalloway D, Johnson PJ, Freed EO, Coulter D, Flood WA., Jr Transformation of NIH 3T3 cells by cotransfection with c-src and nuclear oncogenes. Mol Cell Biol. 1987 Oct;7(10):3582–3590. [Europe PMC free article] [Abstract] [Google Scholar]
- Shiroki K, Maruyama K, Saito I, Fukui Y, Shimojo H. Incomplete transformation of rat cells by a deletion mutant of adenovirus type 5. J Virol. 1981 Jun;38(3):1048–1054. [Europe PMC free article] [Abstract] [Google Scholar]
- Shiroki K, Shimojo H, Sawada Y, Uemizu Y, Fujinaga K. Incomplete transformation of rat cells by a small fragment of adenovirus 12 DNA. Virology. 1979 May;95(1):127–136. [Abstract] [Google Scholar]
- Stabel S, Argos P, Philipson L. The release of growth arrest by microinjection of adenovirus E1A DNA. EMBO J. 1985 Sep;4(9):2329–2336. [Europe PMC free article] [Abstract] [Google Scholar]
- Steinert PM, Roop DR. Molecular and cellular biology of intermediate filaments. Annu Rev Biochem. 1988;57:593–625. [Abstract] [Google Scholar]
- Subramanian T, Kuppuswamy M, Chinnadurai G. An adenovirus 2-coded tumor antigen located on the endoplasmic reticulum and nuclear envelope is required for growth of transformed cells in Ca2+-deficient media. Mol Cell Biol. 1985 Nov;5(11):3297–3300. [Europe PMC free article] [Abstract] [Google Scholar]
- Subramanian T, Kuppuswamy M, Mak S, Chinnadurai G. Adenovirus cyt+ locus, which controls cell transformation and tumorigenicity, is an allele of lp+ locus, which codes for a 19-kilodalton tumor antigen. J Virol. 1984 Nov;52(2):336–343. [Europe PMC free article] [Abstract] [Google Scholar]
- Takemori N, Cladaras C, Bhat B, Conley AJ, Wold WS. cyt gene of adenoviruses 2 and 5 is an oncogene for transforming function in early region E1B and encodes the E1B 19,000-molecular-weight polypeptide. J Virol. 1984 Dec;52(3):793–805. [Europe PMC free article] [Abstract] [Google Scholar]
- Van den Elsen P, Houweling A, Van der Eb A. Expression of region E1b of human adenoviruses in the absence of region E1a is not sufficient for complete transformation. Virology. 1983 Jul 30;128(2):377–390. [Abstract] [Google Scholar]
- van den Elsen PJ, Houweling A, van der Eb AJ. Morphological transformation of human adenoviruses is determined to a large extent by gene products of region E1a. Virology. 1983 Nov;131(1):242–246. [Abstract] [Google Scholar]
- Van der Eb AJ, Mulder C, Graham FL, Houweling A. Transformation with specific fragments of adenovirus DNAs. I. Isolation of specific fragments with transforming activity of adenovirus 2 and 5 DNA. Gene. 1977;2(3-4):115–132. [Abstract] [Google Scholar]
- van der Eb AJ, van Ormondt H, Schrier PI, Lupker JH, Jochemsen H, van den Elsen PJ, DeLeys RJ, Maat J, van Beveren CP, Dijkema R, et al. Structure and function of the transforming genes of human adenoviruses and SV40. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):383–399. [Abstract] [Google Scholar]
- White E, Blose SH, Stillman BW. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol Cell Biol. 1984 Dec;4(12):2865–2875. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Cipriani R. Specific disruption of intermediate filaments and the nuclear lamina by the 19-kDa product of the adenovirus E1B oncogene. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9886–9890. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Denton A, Stillman B. Role of the adenovirus E1B 19,000-dalton tumor antigen in regulating early gene expression. J Virol. 1988 Sep;62(9):3445–3454. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Faha B, Stillman B. Regulation of adenovirus gene expression in human WI38 cells by an E1B-encoded tumor antigen. Mol Cell Biol. 1986 Nov;6(11):3763–3773. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Grodzicker T, Stillman BW. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984 Nov;52(2):410–419. [Europe PMC free article] [Abstract] [Google Scholar]
- White E, Spector D, Welch W. Differential distribution of the adenovirus E1A proteins and colocalization of E1A with the 70-kilodalton cellular heat shock protein in infected cells. J Virol. 1988 Nov;62(11):4153–4166. [Europe PMC free article] [Abstract] [Google Scholar]
- Whyte P, Buchkovich KJ, Horowitz JM, Friend SH, Raybuck M, Weinberg RA, Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. [Abstract] [Google Scholar]
- Whyte P, Williamson NM, Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. [Abstract] [Google Scholar]
- Zantema A, Fransen JA, Davis-Olivier A, Ramaekers FC, Vooijs GP, DeLeys B, Van der Eb AJ. Localization of the E1B proteins of adenovirus 5 in transformed cells, as revealed by interaction with monoclonal antibodies. Virology. 1985 Apr 15;142(1):44–58. [Abstract] [Google Scholar]
- Zantema A, Schrier PI, Davis-Olivier A, van Laar T, Vaessen RT, van der EB AJ. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985 Nov;5(11):3084–3091. [Europe PMC free article] [Abstract] [Google Scholar]
- Zerler B, Moran B, Maruyama K, Moomaw J, Grodzicker T, Ruley HE. Adenovirus E1A coding sequences that enable ras and pmt oncogenes to transform cultured primary cells. Mol Cell Biol. 1986 Mar;6(3):887–899. [Europe PMC free article] [Abstract] [Google Scholar]
- Zerler B, Roberts RJ, Mathews MB, Moran E. Different functional domains of the adenovirus E1A gene are involved in regulation of host cell cycle products. Mol Cell Biol. 1987 Feb;7(2):821–829. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.10.1.120-130.1990
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/10/1/120
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/10/1/120
Citations & impact
Impact metrics
Article citations
Almost famous: Human adenoviruses (and what they have taught us about cancer).
Tumour Virus Res, 12:200225, 06 Sep 2021
Cited by: 12 articles | PMID: 34500123 | PMCID: PMC8449131
Review Free full text in Europe PMC
Regulation of p53 by E3s.
Cancers (Basel), 13(4):745, 11 Feb 2021
Cited by: 9 articles | PMID: 33670160 | PMCID: PMC7916862
Review Free full text in Europe PMC
High V-PPase activity is beneficial under high salt loads, but detrimental without salinity.
New Phytol, 219(4):1421-1432, 25 Jun 2018
Cited by: 16 articles | PMID: 29938800 | PMCID: PMC6099232
Complete eradication of hepatomas using an oncolytic adenovirus containing AFP promoter controlling E1A and an E1B deletion to drive IL-24 expression.
Cancer Gene Ther, 19(9):619-629, 13 Jul 2012
Cited by: 12 articles | PMID: 22790965
Chronic viral infection and primary central nervous system malignancy.
J Neuroimmune Pharmacol, 5(3):387-403, 13 Apr 2010
Cited by: 18 articles | PMID: 20387126 | PMCID: PMC2914282
Review Free full text in Europe PMC
Go to all (85) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Functional complementation of the adenovirus E1B 19-kilodalton protein with Bcl-2 in the inhibition of apoptosis in infected cells.
J Virol, 68(10):6553-6566, 01 Oct 1994
Cited by: 127 articles | PMID: 8083992 | PMCID: PMC237076
The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha.
Mol Cell Biol, 12(6):2570-2580, 01 Jun 1992
Cited by: 230 articles | PMID: 1317006 | PMCID: PMC364450
Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins.
J Virol, 65(6):2968-2978, 01 Jun 1991
Cited by: 133 articles | PMID: 1851867 | PMCID: PMC240940
Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus.
Oncogene, 24(52):7673-7685, 01 Nov 2005
Cited by: 250 articles | PMID: 16299528
Review