Abstract
Free full text

MicroRNAs and cancer therapeutics
Abstract
MicroRNAs (miRNAs) are small physiological non-coding RNAs that regulate gene expression through an RNA interference (RNAi) mechanism. The expression of miRNAs is tightly controlled both spatially and temporally. Aberrant miRNA expression has been correlated with various cancers. Recent findings suggest that some miRNAs can function as tumor suppressors or oncogenes. In model experiments, the cancer phenotype of some cells can be reverted to normal when the cells are treated with miRNA mimics or inhibitors. Here, we discuss in brief the potential utility of miRNA-based cancer therapy as well as the current limitations thwarting their useful clinical application.
Introduction
RNA interference (RNAi) is a physiological mechanism that was initially reported as “post-transcriptional gene silencing” (PTGS) in plants. It plays important regulatory roles in diverse biological processes including apoptosis, developmental timing, signal transduction, cell proliferation and immune defenses [reviewed in (1–4)]. In eukaryotes, one form of gene specific silencing uses the incorporation of 18 – 25 nucleotides (nts) small RNAs, called miRNA, into the RNAi pathway. The biogenesis of miRNAs progresses through a series of processing steps. First, a highly-structured RNA (pri-miRNA), is transcribed by RNA polymerase II (RNAP II). Within the nucleus, the pri-miRNA is cleaved by the microprocessor, a protein complex that contains a ribonuclease (Drosha) and a RNA binding protein (DGCR8), to a ~ 70 nt stem-loop RNA intermediate called pre-miRNA. The pre-miRNA is exported by Exportin 5 into the cytoplasm. Once in the cytoplasm, the hairpin of the pre-miRNA is removed by another ribonuclease (Dicer) to generate a double-stranded mature miRNA. The mature miRNA is then bound to a double-strand RNA binding protein [TAR RNA-binding protein (TRBP)] (5) and organized into a large multi-protein complex called the RNA-induced silencing complex (RISC). One strand of the double-stranded miRNA (guide strand) is incorporated into the complex. The other strand (passenger strand) is degraded. The miRNA-loaded RISC can then silence target mRNAs through imperfect complementarity between the mRNA and the guide miRNA. Early observations suggested that perfect complementary of the 5′ ends of miRNAs nucleotides 2 – 7, termed seed sequence, is important for function (6). However, new findings reveal that the central region (7) and the 3′UTR (8, 9) of the miRNA also play important roles in target recognition. The miRNA-RISC targeted mRNAs is silenced through inhibition of translation (10, 11) or mRNA degradation (12, 13). The biological importance of the RNAi mechanism is supported by findings that the perturbed expression of miRNAs or the components of the RNAi machinery results in the development of various diseases (14–21).
Aberrant miRNA expression and oncogenesis
MiRNA expression is tightly regulated both spatially and temporally (Figure 1). A role for miRNA in tumorigenesis was first reported by Calin et al. in describing a chromosome region containing miR-15a and miR-16-1 which is frequently deleted or translocated in chronic lymphocytic leukemia (CLL) (22). Subsequently, the roles of miRNAs in various cancers have been investigated. Some investigators have found a global down-regulation of miRNA levels in tumor cells (23). This down-regulation is partially explained by the fact that some tumor suppressors and oncogenes can modulate the miRNA-promoters’ activity. Indeed, tumor suppressors and oncogenes, including p53 and c-Myc which are transcriptional activators, have been shown experimentally to modulate the activities of miRNA promoters (24, 25). A bioinformatics analysis estimated that over 46% of the deduced promoters for 326 human miRNAs were found to have potential p53 binding sites (24). Others have reported specific miRNA signature profiles in different tumor types (23) and have demonstrated the usefulness of miRNA changes for predicting cancer prognosis (26) and therapeutic responses (27). Moreover, the deletion, downregulation, and/or mutation of key RNAi components, including Dicer, Drosha, Exportin 5, TRBP and Ago2 have been correlated with various types of cancer (14–21). Collectively, the extant data support that miRNA changes can contribute to oncogenesis and that it is important to elucidate how individual miRNA changes predispose cells to transformation.
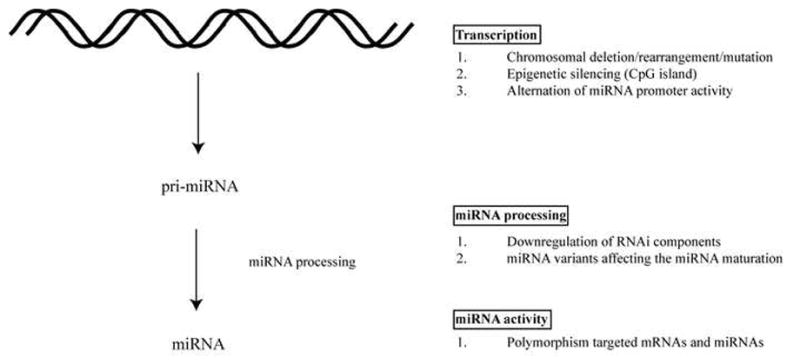
A) miRNAs are processed from precursor pri-, pre-, miRNA that contain stem-loop structure. The genes encoding miRNAs are regulated by their own promoters and regulatory elements (70, 71). The expression levels of miRNA precursor can be regulated at the transcriptional level by (1) Chromosomal deletion or translocation of regions containing miRNA genes. (2) Epigenetic silencing of regions containing miRNAs. (3) Alteration in the miRNA promoter activity. B) Functional miRNAs require a series of processing steps. (1) Downregulation of the RNAi components including Dicer, Drosha, Exportin 5, TRBP and Ago2 impairs the miRNA maturation process and has been correlated with cancer development (14–21). (2) miRNA structural variants with sequence changes at the processing-cleavage sites affect the ribonuclease activity of Drosha and Dicer (72). C) Mutations associated with sequence changes in miRNAs and miRNA target sites can affect the effectiveness of the gene silencing activity. (1) Polymorphisms, including SNP and potential deaminations of miRNAs and their targeted mRNAs have been detected in various cancers (73).
A well-characterized miRNA whose dysregulated expression correlates with tumorigenesis is miR-155. Elevated miR-155 expression was found associated with various cancers (28–32), and a role for miR-155 in modulating host immune responses has also been described (33). Interestingly, the oncogenic Kaposi’s sarcoma-associated herpesvirus (KSHV) encodes a miRNA called miR-k12-11 which shares an identical seed sequence with miR-155 (34, 35). Thus, it appears that some oncogenic cellular miRNAs and oncogenic virus-encoded miRNAs may employ common pathways for tumorigenesis. However, other transforming viruses such as human T-cell leukemia virus type 1 (HTLV-1) do not encode oncogenic miRNAs and instead seem to dysregulate cellular metabolism by modulating the promoter-expression of key cellular miRNAs (36, 37). HTLV-1 uses its viral oncoprotein, Tax, to induce miR-130b and miR-93 expression. Over-expression of miR-130b and miR-93 represses the expression of a cellular tumor suppressor protein, TP53INP1, and hence induces tumorigenicity (37, 38).
Many studies have shown that miRNAs can have oncogenic or tumor suppressing functions. For example, a well-characterized oncogenic miRNAs (oncomirs) is miR-21 which regulates the expression of the anti-apoptotic protein bcl-2 (39). It has been shown that the sequestration of miR-21 by anti-miR-21 in breast cancer MCF-7 cells enhanced apoptosis and suppressed cell growth. Replenishing miRNA expression in certain cancers has been found to be effective in controlling cell growth. A recent finding suggests that p53 can activate the expression of miR-34 (40) and that the transfection or transduction of miR-34 into p53-deficient gastric cancer cells was able to target bcl-2, Notch and HMGA2 expression and inhibit cell growth and/or induce apoptosis (41). Increasingly, it is recognized that miRNAs can target oncogenes and tumor suppressor genes (42). In animal models, modulation of certain miRNAs expression controls the size and the metastatic potential of specific tumors (43–52) (see Table 2). This recognition has led to nascent clinical trials using miRNA inhibitors to sequester cellular miRNAs such as miR-122 which is essential for Hepatitis C virus (HCV) replication with the hope of ameliorating the development of hepatomas (53–55). Additionally, efforts have been made to identify small molecules that suppress the function of oncogenic miRNAs possibly reversing their tumorigenesis phenotype (36). Time will tell whether miRNA-based therapy may represent a useful new way to treat cancer.
Table 2
The oncogenic and tumor suppressive roles of miRNA tested in animal models
| miRNA | Cancer type | Targets | Role | Delivery system | reference |
|---|---|---|---|---|---|
| miR-132 | Breast | p120RasGAP | Activator of Ras and inducer of neovascularization | Intraocular injection of antagomirs/Liposomes complex | (51) |
| miR-34a | Lung | Survivin and MAPK family | Induce apoptosis and reduce tumor load | Intravenous injections of mi-/si- RNAs/LPH complex | (52) |
| miR-17-3p | Prostate | Vimentin | Inhibit tumor growth | Subcutaneous injection of miRNA stably expressing cell lines | (46) |
| miR-221/222 | Prostate | p27 | Inducers of proliferation and tumorigenicity | Intratumoral injection of antagomirs | (47) |
| miR-16 | Prostate | CDK1 and CDK2 | Inhibit metastatic tumor growth in bone | Systemic injection of synthetic miRNA/atelocollagen complex | (48) |
| miR-26a | Liver | Cyclin D2 and Cyclin E2 | Inhibit cancer cell proliferation and induce tumor-specific apoptosis | Intravenous injection of adeno- associated virus (AAV) 8 serotype containing the self-complementary AAV vector expressing miRNA | (50) |
| miR-191 | Liver | SOX4, IL1A and TMC7 | Important for cell proliferation and cell survival | Systemic injection of antagomirs | (43) |
| Let-7 family | Lung | Ras family and HMG2 | Inhibit tumor growth, cell cycle arrest and induce cell death | Intratracheal infection of miRNA expressing lentiviruses | (44) |
| miR-10b | Breast | Hoxd10 | Important for the formation of lung metastases | Intravenous injection of antagomirs | (45) |
| miR-21 | Tongue | TPM1 and PTEN | Important for cell proliferation and cell survival | Intratumoral injection of antisense oligonucleotide | (49) |
| miR-122 | Liver | HCV | Essential for HCV replication | Subcutaneous injection of antagomirs | (53, 54, 55) |
In vivo delivery of small RNAs
Small RNA-based therapy can offer some advantage over conventional cancer drug treatment. Because a single miRNA can target many mRNAs, miRNA-based therapy could silence a broad array of genes, and by doing so, this method may potentially increase the efficiency of controlling the growth or spread of cancers. Also, the relatively low cost of synthesis, their small molecular size, and the ease of design, all make small RNA therapy appealing. However, similar to many other treatment modalities, the major hurdle to using small RNA-based therapy in vivo is the efficiency of focused delivery to specific cells.
Delivery of small RNA in vivo has been attempted using various methods (Table 1). Each has advantages and disadvantages. The earliest attempt to deliver small RNA in vivo involved direct hydrodynamic injection of siRNA intravenously (56). The injected siRNA accumulated in the hepatocytes of the mice silencing the expression of Fas. As a result, the injected mouse’s liver was successfully protected from the development of fulminant hepatitis and fibrosis. However, the hydrodynamic methodology is impractical clinically due to the requirement of injecting large quantities of small RNAs. Furthermore, naked siRNA could be relatively unstable due to the presence of endogenous RNases. Thus, high functional efficacy at desired sites remains a major challenge.
Table 1
Advantages and disadvantages of various small RNA delivery methods
| Methods | Advantages | Disadvantages |
|---|---|---|
| Hydrodynamic injection | Simple | Unstable; Require large amount |
| Viral delivery (Retroviruses; Adenoviruses; Adeno- associated viruses) | Long-term; High efficiency | Elicit immune response; Random integration |
| Lipid-based or polymer- based complex delivery | High efficiency | Highly immunogenic |
| Nanoparticle delivery | High efficiency; Targeted delivery; Low toxicity; Low immunogenic; Stable and relatively slow release rate | Complex formulation |
Lipid-based nucleic acids delivery has been widely used in vitro. The negatively charged nucleic acids are protected from the environment by interacting with the cationic lipids to form liposomes. The liposomes can fuse with the cell membrane, releasing the nucleic acids into the cells. Although cationic lipid-complexed siRNAs have been delivered effectively into cells, they are highly immunogenic (57). Other non-charged nanoparticles, such as biodegradable polymers which induce less inflammation could possibly reduce this problem. The application of in vivo delivery of mi-/si-RNAs will be discussed below.
Viral vectors represent another approach for small RNA delivery. Typically, viruses are engineered to be replication-defective, preventing them from propagating an undesired spread of infection. The precursor forms of mi-/sh-RNA are expressed and processed by the cellular machinery after transduction of cells by viral vectors carrying the appropriate mi-/sh-RNA expression cassettes. The advantages of this method include the ability to establish long-term knock down of targeted mRNAs and the high efficiency transduction using viral particles of many types of cell. However, an adverse effect can arise due to the integration of the viral vector into host cell DNA. Random insertion of the transduced viral vector could disrupt crucial genes and/or potentially activate harmful cellular proto-oncogenes (58). Lastly, undesired immune responses induced by the viral vectors may be harmful and should also be carefully evaluated.
The stability, toxicity, immunogenicity and durability of the above-mentioned delivery methods have hindered the progression of these approaches to clinical usage. The recent development of nanoparticles may address most of the problems. Generally, nanoparticles are positively-charged particles with diameters of ~ 50 – 70 nm. They elicit minimal inflammatory responses. The negatively charged mi-/si-RNAs are completely buried inside the electrolyte core formed by the nanoparticles. This significantly increases the stability of the embedded mi-/si-RNAs. Also, the protective core of the nanoparticles allows relatively slow release of small molecules for continuous knock-down of specific gene expression.
Recent clinical trials using the nanoparticles-siRNA delivery approach for treating solid cancers have shown promising results. The first nanoparticle formulation of siRNA underwent phase I clinic trial in 2008; it is called CALAA-01 (59). The trial employed several kinds of nanoparticles including cyclodextrin-containing polymer (CDP), polyethylene glycol (PEG) steric stabilization agent and human transferrin (hTf) as a targeting ligand for binding to transferrin receptors (TfR). The CALAA-01 agent was complexed with siRNAs designed to target ribonucleotide reductase M2 (RRM2), a gene involved in DNA replication that is commonly deregulated in many cancers. It has been shown that the in vivo delivery of a siRNA against RRM2 reduced the proliferative activity of a broad spectrum of human, mouse, rat and monkey cancers with minimal immune response or toxicity (60, 61).
CALAA-01 is the first siRNA nanoparticle to be therapeutically tried in humans (59). The promising results from this trial suggests that further clinical studies of the in vivo mi-/si-RNA nanoparticles delivery system including the new RNAi agents Atu027 and ALN-VSP may be warranted. For a more detailed review of preclinical and clinical development of RNAi drugs, the readers are referred to an excellent review article (62).
Dysregulated expression of miRNA correlates with the disease status of cancers. Indeed, a few recent studies have reported the successfully reversal of the cancer phenotype by nanoparticle-mediated delivery of miRNAs in animal models (see below and Table 2). It has been suggested that the tumor endothelium can upregulate miR-132 expression by activating cAMP response element-binding protein (CREB) through a vascular endothelial growth factor receptor 2 (VEGFR-2) dependent pathway (51). The upregulated miR-132 can act as an angiogenic switch suppressing endothelial p120RasGAP expression, leading to Ras activation and the induction of neovascularization (63). By using vessel-targeted nanoparticle delivery of anti-miR-132, investigators have successfully sequestered miR-132 in the tumor endothelium (51). As a result of this approach, p120RasGAP expression level was restored, and this was accompanied by suppressed angiogenesis and decreased tumor burden in an orthotopic xenograft mouse model of human breast carcinoma. In another study, the intravenous administration of a miR-16 mimic complexed with atelocollagen strongly inhibited the development of human prostate metastatic tumors in the bone of mice (48). The anti-tumor effect of the nanoparticle formulation can be enhanced when complexed with multiple small RNAs. A recent study using liposome-polycation-hyaluronic acid (LPH) nanoparticle formulation modified with tumor-targeting single-chain antibody fragment (scFv) nanoparticles delivered siRNAs and miR-34a into lung cancer metastases (52). The delivered siRNA and miRNA efficiently downregulated the targeted genes (c-Myc/MDM2/VEGF/survivin/MAPK family) and hence controlled the progressin of the lung tumor. The capacity to deliver siRNA and/or miRNA with a single formulation significantly increases the flexibility of therapeutic uses of mi-/si-RNA.
Other possible side effects and future challenges
Many concerns need to be addressed before miRNA-based therapy can be safely applied to clinical settings. First, one should be cautious regarding the specificity (or off-target effect) of miRNA-based gene silencing because a single miRNA can target many mRNAs (64). Choosing a miRNA which targets many genes in the same pathway may consolidate the silencing effect and diminish the magnitude of off-target effects. It should be noted that a single mRNA can also be targeted by multiple miRNAs. Utilizing a combination of miRNAs that converge on a single mRNA may lower the concentration of miRNAs required for silencing and enhance the specificity of silencing.
Second, double-stranded RNA can trigger the innate immune system through activation of double-stranded RNA (dsRNA)-dependent protein kinase R or retinoic acid inducible gene-I (57). The strength of activation of the innate response is determined by the amount, the length, and the sequence composition of the RNA. Minimal activation was observed with double-stranded RNA with size < 30 nucleotides. However, high amounts of uracil and guanine rich sequence content of small RNAs were reported to be able to elicit significant immune response (57). Future development of small RNA-based therapy requires careful design of sequence composition. Also, the use of modified nucleotides, such as LNA, will lower the amount needed for small RNA-based therapy and decrease the likelihood of innate immune activation.
Third, although viral delivery of small RNA is attractive because of its high efficiency, its local administration, and its persistence, elicit particular safety concerns. Efficient delivery into the host may also elicit unpredictable hyper immune reactions, and effective vector entry into cells also means abundant viral vector DNA integration into the host genome (65). An illustration of unwanted and unexpected side effects came from an early animal model using adeno-associated virus to deliver shRNA that targeted the expression of a human α-1 antitrypsin mRNA. In that study, overexpression of shRNA led to the oversaturation of the cell’s RNA-transporter protein, Exportin-5, and induced hepatotoxicity in the experimental animals (66). Hence, rather than using constitutive high efficiency expression of shRNAs, a modified approach using an inducible expression system for shRNA in the context of a viral vector could permit a more regulated method that uses just the needed amount of shRNA during a targeted timeframe for controlled knock down of specific genes (67–69).
Finally, the effectiveness of siRNA/miRNA-mediated silencing may be limited by several additional cellular factors including RNA-binding proteins that may act as RNAi suppressors and which may also be able to shield targeted RNAs from complementarity driven recognition by si-/mi-RNA. How intracellular RNA-binding proteins and RNA-modifying enzymes including RNA helicases may impede or enhance RNAi effects warrants further study.
Concluding remarks
RNAi represents a biological mechanism that in principle could be exploited to provide rapid and efficient down-modulation of gene expression. While the fundamental bases for siRNA- or miRNA- mediated gene silencing are becoming clearer, the challenges of applying these small RNA-based approaches to the therapy of diseases including cancers are non-trivial. Recent news that a large drug company Roche has retrenched or eliminated its commitment to RNAi research (http://pubs.acs.org/cen/news/88/i48/8848notw6.html) illustrates some of the real world obstacles to the application of miRNA and siRNA. As outlined above, the clinical uses of the fundamental principles behind RNAi require additional research breakthroughs that can allow investigators to deliver small therapeutic RNAs more efficiently, more specifically, and more durably for gene silencing in vivo.
Abbreviations
| miRNAs | MicroRNAs |
| RNAi | RNA interference |
| PTGS | Post-transcriptional gene silencing |
| nts | nucleotides |
| RNAP II | RNA polymerase II |
| TRBP | TAR RNA-binding protein |
| RISC | RNA-induced silencing complex |
| CLL | chronic lymphocytic leukemia |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| HTLV-1 | Human T-cell Leukemia Virus type 1 |
| oncomirs | oncogenic miRNAs |
| HCV | Hepatitis C virus |
| CDP | cyclodextrin-containing polymer |
| PEG | polyethylene glycol |
| hTf | human transferrin |
| TfR | targeting ligand for binding to transferrin receptors |
| RRM2 | ribonucleotide reductase M2 |
| CREB | cAMP response element-binding |
| VEGFR-2 | vascular endothelial growth factor receptor 2 |
| LPH | liposome-polycation-hyaluronic acid |
| scFv | single-chain antibody fragment |
| dsRNA | double-stranded RNA |
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s11095-011-0526-2
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3404888?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s11095-011-0526-2
Article citations
A critical review on modulators of Multidrug Resistance Protein 1 in cancer cells.
PeerJ, 10:e12594, 05 Jan 2022
Cited by: 7 articles | PMID: 35036084 | PMCID: PMC8742536
Review Free full text in Europe PMC
MicroRNAs in treatment-induced neuroendocrine differentiation in prostate cancer.
Cancer Drug Resist, 3(4):804-818, 12 Oct 2020
Cited by: 7 articles | PMID: 33426506 | PMCID: PMC7793563
Review Free full text in Europe PMC
MicroRNA-132 inhibits migration, invasion and epithelial-mesenchymal transition via TGFβ1/Smad2 signaling pathway in human bladder cancer.
Onco Targets Ther, 12:5937-5945, 23 Jul 2019
Cited by: 10 articles | PMID: 31413591 | PMCID: PMC6662166
Plant natural modulators in breast cancer prevention: status quo and future perspectives reinforced by predictive, preventive, and personalized medical approach.
EPMA J, 9(4):403-419, 12 Nov 2018
Cited by: 28 articles | PMID: 30538792 | PMCID: PMC6261910
Review Free full text in Europe PMC
MicroRNA-3619-5p suppresses bladder carcinoma progression by directly targeting β-catenin and CDK2 and activating p21.
Cell Death Dis, 9(10):960, 20 Sep 2018
Cited by: 29 articles | PMID: 30237499 | PMCID: PMC6147790
Go to all (19) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
MicroRNA-based therapeutics for cancer.
BioDrugs, 23(1):15-23, 01 Jan 2009
Cited by: 107 articles | PMID: 19344188
Review
MicroRNA: Promising Roles in Cancer Therapy.
Curr Pharm Biotechnol, 21(12):1186-1203, 01 Jan 2020
Cited by: 15 articles | PMID: 32310047
Review
microRNA Therapeutics in Cancer - An Emerging Concept.
EBioMedicine, 12:34-42, 20 Sep 2016
Cited by: 253 articles | PMID: 27720213 | PMCID: PMC5078622
Review Free full text in Europe PMC
Small non-coding RNAs as novel therapeutics.
Curr Mol Med, 10(4):361-368, 01 Jun 2010
Cited by: 44 articles | PMID: 20455856
Review
Funding
Funders who supported this work.
Intramural NIH HHS (2)
Grant ID: ZIA AI000547-23
Grant ID: Z99 AI999999
NIAID NIH HHS (1)
Grant ID: Y99 AI999999




