Abstract
Free full text

Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media.
Abstract
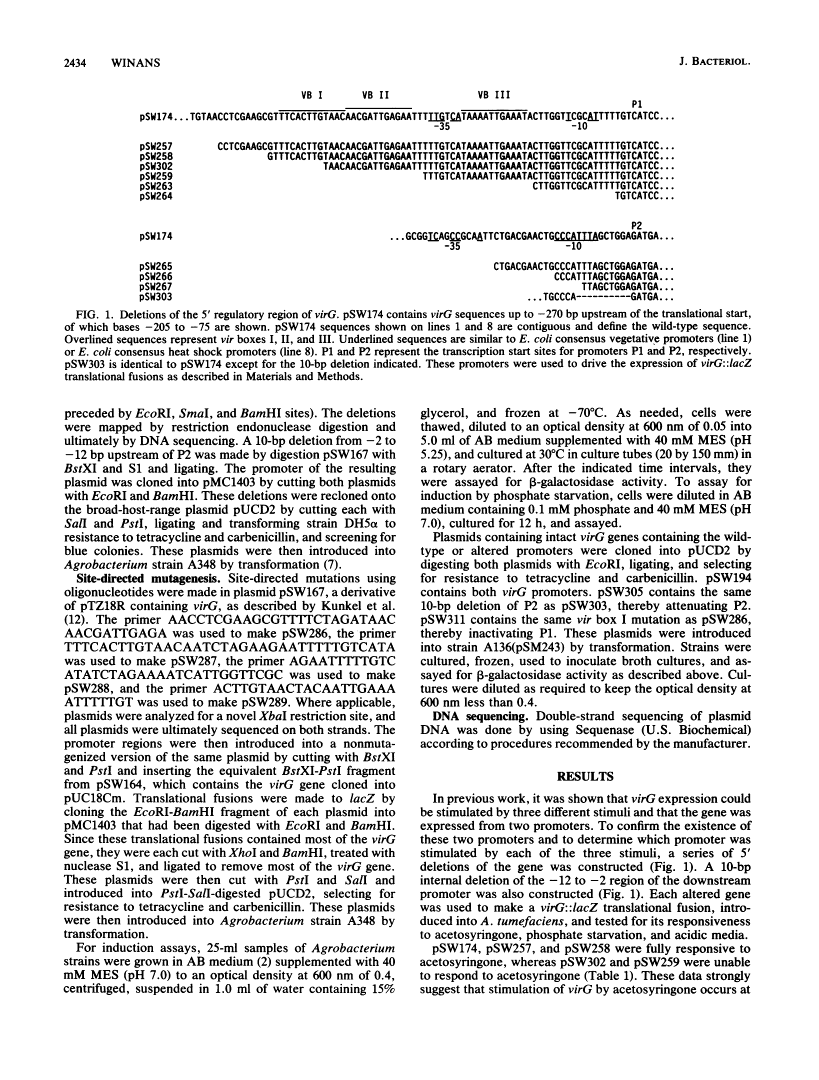
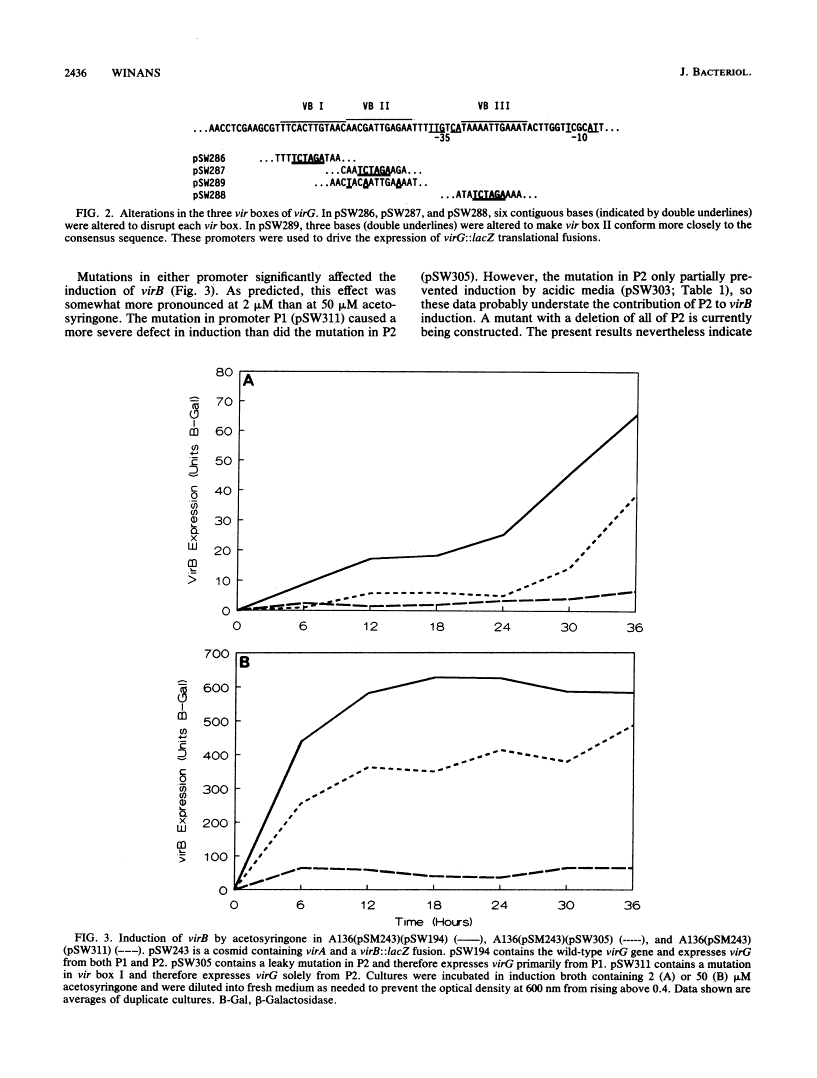
Transcription of the virG gene of Agrobacterium tumefaciens was previously shown to be expressed from two tandem promoters and to be responsive to three stimuli: plant-released phenolic compounds, phosphate starvation, and acidic media. In this report, I describe a set of deletions and other alterations of the 5' end of virG that show that the upstream promoter (P1) is necessary for induction by phenolic compounds and by phosphate starvation, whereas the downstream promoter (P2) is induced by acidic media. Upstream of promoter P1 there are three copies of a family of sequences (vir boxes) found near all VirA, VirG-inducible promoters. Site-directed mutagenesis of these sequences showed that vir box I and vir box III but not vir box II are needed for induction of P1 by acetosyringone. Induction of P1 by phosphate starvation requires vir box III (or an overlapping site), whereas vir box I and vir box II are not needed. The relative importance of promoters P1 and P2 in vir gene induction was tested by measuring the expression of a virB::lacZ fusion in strains containing mutations at either promoter P1 or P2. Mutations in either promoter significantly attenuated the expression of virB, indicating that both promoters play important roles in vir gene induction.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casadaban MJ, Chou J, Cohen SN. In vitro gene fusions that join an enzymatically active beta-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J Bacteriol. 1980 Aug;143(2):971–980. [Europe PMC free article] [Abstract] [Google Scholar]
- Chilton MD, Currier TC, Farrand SK, Bendich AJ, Gordon MP, Nester EW. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3672–3676. [Europe PMC free article] [Abstract] [Google Scholar]
- Close TJ, Zaitlin D, Kado CI. Design and development of amplifiable broad-host-range cloning vectors: analysis of the vir region of Agrobacterium tumefaciens plasmid pTiC58. Plasmid. 1984 Sep;12(2):111–118. [Abstract] [Google Scholar]
- Das A, Stachel S, Ebert P, Allenza P, Montoya A, Nester E. Promoters of Agrobacterium tumefaciens Ti-plasmid virulence genes. Nucleic Acids Res. 1986 Feb 11;14(3):1355–1364. [Europe PMC free article] [Abstract] [Google Scholar]
- Heltzel A, Gambill D, Jackson WJ, Totis PA, Summers AO. Overexpression and DNA-binding properties of the mer-encoded regulatory protein from plasmid NR1 (Tn21). J Bacteriol. 1987 Jul;169(7):3379–3384. [Europe PMC free article] [Abstract] [Google Scholar]
- Holsters M, de Waele D, Depicker A, Messens E, van Montagu M, Schell J. Transfection and transformation of Agrobacterium tumefaciens. Mol Gen Genet. 1978 Jul 11;163(2):181–187. [Abstract] [Google Scholar]
- Huang Y, Morel P, Powell B, Kado CI. VirA, a coregulator of Ti-specified virulence genes, is phosphorylated in vitro. J Bacteriol. 1990 Feb;172(2):1142–1144. [Europe PMC free article] [Abstract] [Google Scholar]
- Jin SG, Komari T, Gordon MP, Nester EW. Genes responsible for the supervirulence phenotype of Agrobacterium tumefaciens A281. J Bacteriol. 1987 Oct;169(10):4417–4425. [Europe PMC free article] [Abstract] [Google Scholar]
- Jin S, Roitsch T, Ankenbauer RG, Gordon MP, Nester EW. The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation. J Bacteriol. 1990 Feb;172(2):525–530. [Europe PMC free article] [Abstract] [Google Scholar]
- Jin SG, Roitsch T, Christie PJ, Nester EW. The regulatory VirG protein specifically binds to a cis-acting regulatory sequence involved in transcriptional activation of Agrobacterium tumefaciens virulence genes. J Bacteriol. 1990 Feb;172(2):531–537. [Europe PMC free article] [Abstract] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. [Abstract] [Google Scholar]
- Leroux B, Yanofsky MF, Winans SC, Ward JE, Ziegler SF, Nester EW. Characterization of the virA locus of Agrobacterium tumefaciens: a transcriptional regulator and host range determinant. EMBO J. 1987 Apr;6(4):849–856. [Europe PMC free article] [Abstract] [Google Scholar]
- Makino K, Shinagawa H, Amemura M, Kimura S, Nakata A, Ishihama A. Regulation of the phosphate regulon of Escherichia coli. Activation of pstS transcription by PhoB protein in vitro. J Mol Biol. 1988 Sep 5;203(1):85–95. [Abstract] [Google Scholar]
- Morel P, Powell BS, Rogowsky PM, Kado CI. Characterization of the virA virulence gene of the nopaline plasmid, pTiC58, of Agrobacterium tumefaciens. Mol Microbiol. 1989 Sep;3(9):1237–1246. [Abstract] [Google Scholar]
- Ronson CW, Nixon BT, Ausubel FM. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. [Abstract] [Google Scholar]
- Sciaky D, Montoya AL, Chilton MD. Fingerprints of Agrobacterium Ti plasmids. Plasmid. 1978 Feb;1(2):238–253. [Abstract] [Google Scholar]
- Stachel SE, Nester EW. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. [Europe PMC free article] [Abstract] [Google Scholar]
- Stachel SE, Zambryski PC. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. [Abstract] [Google Scholar]
- VanBogelen RA, Kelley PM, Neidhardt FC. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. [Europe PMC free article] [Abstract] [Google Scholar]
- Veluthambi K, Jayaswal RK, Gelvin SB. Virulence genes A, G, and D mediate the double-stranded border cleavage of T-DNA from the Agrobacterium Ti plasmid. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1881–1885. [Europe PMC free article] [Abstract] [Google Scholar]
- Wang QP, Kaguni JM. A novel sigma factor is involved in expression of the rpoH gene of Escherichia coli. J Bacteriol. 1989 Aug;171(8):4248–4253. [Europe PMC free article] [Abstract] [Google Scholar]
- Winans SC, Ebert PR, Stachel SE, Gordon MP, Nester EW. A gene essential for Agrobacterium virulence is homologous to a family of positive regulatory loci. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8278–8282. [Europe PMC free article] [Abstract] [Google Scholar]
- Winans SC, Kerstetter RA, Nester EW. Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens. J Bacteriol. 1988 Sep;170(9):4047–4054. [Europe PMC free article] [Abstract] [Google Scholar]
- Winans SC, Kerstetter RA, Ward JE, Nester EW. A protein required for transcriptional regulation of Agrobacterium virulence genes spans the cytoplasmic membrane. J Bacteriol. 1989 Mar;171(3):1616–1622. [Europe PMC free article] [Abstract] [Google Scholar]
- Yanofsky MF, Porter SG, Young C, Albright LM, Gordon MP, Nester EW. The virD operon of Agrobacterium tumefaciens encodes a site-specific endonuclease. Cell. 1986 Nov 7;47(3):471–477. [Abstract] [Google Scholar]
- Zambryski P, Tempe J, Schell J. Transfer and function of T-DNA genes from agrobacterium Ti and Ri plasmids in plants. Cell. 1989 Jan 27;56(2):193–201. [Abstract] [Google Scholar]
Associated Data
Articles from Journal of Bacteriology are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/jb.172.5.2433-2438.1990
Read article for free, from open access legal sources, via Unpaywall:
https://jb.asm.org/content/172/5/2433.full.pdf
Free to read at jb.asm.org
http://jb.asm.org/cgi/content/abstract/172/5/2433
Free after 4 months at jb.asm.org
http://jb.asm.org/cgi/reprint/172/5/2433
Citations & impact
Impact metrics
Article citations
Exploring Agrobacterium-mediated genetic transformation methods and its applications in Lilium.
Plant Methods, 20(1):120, 09 Aug 2024
Cited by: 0 articles | PMID: 39123215 | PMCID: PMC11313100
Review Free full text in Europe PMC
Acinetobacter baumannii coordinates central metabolism, plasmid dissemination, and virulence by sensing nutrient availability.
mBio, 14(6):e0227623, 19 Oct 2023
Cited by: 3 articles | PMID: 37855599 | PMCID: PMC10746170
Functional characterization of VirB/VirD4 and Icm/Dot type IV secretion systems from the plant-pathogenic bacterium Xanthomonas euvesicatoria.
Front Cell Infect Microbiol, 13:1203159, 01 Aug 2023
Cited by: 2 articles | PMID: 37593760 | PMCID: PMC10432156
Cryo-EM structure of the Agrobacterium tumefaciens T4SS-associated T-pilus reveals stoichiometric protein-phospholipid assembly.
Structure, 31(4):385-394.e4, 03 Mar 2023
Cited by: 8 articles | PMID: 36870333 | PMCID: PMC10168017
Improvement of a Genetic Transformation System and Preliminary Study on the Function of LpABCB21 and LpPILS7 Based on Somatic Embryogenesis in Lilium pumilum DC. Fisch.
Int J Mol Sci, 21(18):E6784, 16 Sep 2020
Cited by: 8 articles | PMID: 32947885 | PMCID: PMC7554901
Go to all (83) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The regulatory VirG protein specifically binds to a cis-acting regulatory sequence involved in transcriptional activation of Agrobacterium tumefaciens virulence genes.
J Bacteriol, 172(2):531-537, 01 Feb 1990
Cited by: 73 articles | PMID: 2404941 | PMCID: PMC208474
Transcriptional regulation of the virA and virG genes of Agrobacterium tumefaciens.
J Bacteriol, 170(9):4047-4054, 01 Sep 1988
Cited by: 133 articles | PMID: 2842300 | PMCID: PMC211408
Controlled expression of the transcriptional activator gene virG in Agrobacterium tumefaciens by using the Escherichia coli lac promoter.
J Bacteriol, 173(3):1139-1144, 01 Feb 1991
Cited by: 91 articles | PMID: 1991713 | PMCID: PMC207234
The sensing of plant signal molecules by Agrobacterium: genetic evidence for direct recognition of phenolic inducers by the VirA protein.
Gene, 179(1):83-88, 01 Nov 1996
Cited by: 25 articles | PMID: 8955632
Review
Funding
Funders who supported this work.
NIGMS NIH HHS (1)
Grant ID: 1 R29 GM2893-01