Abstract
Purpose of review
Pulmonary arterial hypertension (PAH) is an important cause of morbidity and mortality in children. Approved medications for the treatment of adult PAH have been used to treat children, but evidence-based treatment algorithms for children are lacking.Recent findings
Pediatric PAH registries have begun to define the incidence and prevalence of idiopathic PAH and PAH associated with congenital heart disease. A pediatric-specific classification of pulmonary hypertensive vascular disease has been proposed. Furthermore, the first randomized placebo-controlled trial of type-5 phosphodiesterase therapy in treatment-naïve children with PAH has been completed and reported. This trial highlights the importance of the difficulties of performing clinical trials in children with targeted PAH therapy as well as the importance of long-term follow-up of adverse events.Summary
Classification, clinical trials, and therapy for children with PAH must take into account the unique aspects of PAH in children.Free full text

Advances in Pediatric Pulmonary Arterial Hypertension
Abstract
Purpose of Review
Pulmonary arterial hypertension (PAH) is an important cause of morbidity and mortality in children. Approved medications for the treatment of adult PAH have been used to treat children but evidence based treatment algorithms for children are lacking.
Recent Findings
Pediatric PAH registries have begun to define the incidence and prevalence of idiopathic PAH and PAH associated with congenital heart disease. A pediatric specific classification of pulmonary hypertensive vascular disease has been proposed. Furthermore, the first randomized placebo-controlled trial of type-5 phosphodiesterase therapy in treatment naïve children with PAH has been completed and reported. This trial highlights the importance of the difficulties of performing clinical trials children with targeted PAH therapy as well as the importance of long-term follow-up of adverse events.
Summary
Classification, clinical trials, and therapy for children with PAH must take into account the unique aspects of PAH in children.
Introduction
Diagnosis and treatment of children with pulmonary arterial hypertension (PAH) lags behind the understanding and treatment of this disorder in adults. Although advances in therapy have led to improved survival for many forms of PAH, there remains no cure for some, including Idiopathic PAH (IPAH). The off-label application of adult pulmonary hypertension specific therapies to children has broadened the available options for therapy. Selection of appropriate therapies for pulmonary hypertension is complex and must be carefully chosen according to the etiology and pulmonary vasoreactivity testing during cardiac catheterization. This review highlights the recent advances in pediatric PH classification and therapy.
Definition and Classification
PAH is defined as a mean pulmonary artery pressure (PAP) ≥ 25 mmHg at rest, with a normal pulmonary capillary wedge pressure (≤15 mmHg) and increased pulmonary vascular resistance index (≥3 Wood units x m2).(1) Classifying pediatric PH according to the WHO classification (Table 1) is problematic as many children with PH have associated co-morbidities and syndromes.(2) A working group of the Pulmonary Vascular Research Institute developed a classification specific to children (Table 2).(3) In particular, this classification recognized the concepts of the contribution of abnormalities of lung growth and development to pediatric PH. PH can develop in utero or can be superimposed on key periods of lung development leading to life-long airway and pulmonary vascular abnormalities. Pediatric pulmonary hypertensive vascular disease has been divided into 10 broad categories. Many children presenting with PH have heterogeneous disease consisting of varying predisposing factors, including prematurity, a chromosomal or genetic anomaly, congenital heart disease, and sleep disordered breathing. PH associated with bronchopulmonary dysplasia has been highlighted in this classification. For children with a biventricular circulation, the definition is similar to adults; however, the Panama classification specifically includes children with PH in the setting of single ventricle physiology after a cavopulmonary anastomosis. In this setting, pediatric pulmonary hypertensive vascular disease is defined as a pulmonary vascular resistance index >3.0 Wood units X m2 or a transpulmonary gradient >6 mmHg.(3) This classification was not designed to determine use of targeted PH therapy but rather an aid in classification and assessment of children with PH.
Table 1
Updated WHO clinical classification of pulmonary hypertension (Dana Point, 2008)
| 1 | Pulmonary arterial hypertension (PAH) |
1.1   Idiopathic Idiopathic | |
1.2   Heritable Heritable | |
1.2.1  BMPR2 BMPR2
| |
1.2.2  ALK1, endoglin (with or without hereditary hemorrhagic telangiectasia) ALK1, endoglin (with or without hereditary hemorrhagic telangiectasia) | |
1.2.3  Unknown Unknown | |
1.3   Drug- and toxin-induced Drug- and toxin-induced | |
1.4   Associated with (APAH) Associated with (APAH) | |
1.4.1  Connective tissue diseases Connective tissue diseases | |
1.4.2  HIV infection HIV infection | |
1.4.3  Portal hypertension Portal hypertension | |
1.4.4  Congenital heart disease Congenital heart disease | |
1.4.5  Schistosomiasis Schistosomiasis | |
1.4.6  Chronic hemolytic anemia Chronic hemolytic anemia | |
1.5   Persistent pulmonary hypertension of the newborn Persistent pulmonary hypertension of the newborn | |
|
| |
| 1′ | Pulmonary veno-occlusive disease and/or pulmonary capillary hemangiomatosis |
|
| |
| 2 | Pulmonary hypertension due to left heart disease |
2.1   Systolic dysfunction Systolic dysfunction | |
2.2    Diastolic dysfunction Diastolic dysfunction | |
2.3   Valvular disease Valvular disease | |
|
| |
| 3 | Pulmonary hypertension due to lung diseases and/or hypoxemia |
3.1   Chronic obstructive pulmonary disease Chronic obstructive pulmonary disease | |
3.2   Interstitial lung disease Interstitial lung disease | |
3.3   Other pulmonary diseases with mixed restrictive and obstructive pattern Other pulmonary diseases with mixed restrictive and obstructive pattern | |
3.4   Sleep-disordered breathing Sleep-disordered breathing | |
3.5   Alveolar hypoventilation disorders Alveolar hypoventilation disorders | |
3.6   Chronic exposure to high altitudes Chronic exposure to high altitudes | |
3.7   Developmental abnormalities Developmental abnormalities | |
|
| |
| 4 | Chronic thromboembolic pulmonary hypertension |
|
| |
| 5 | Pulmonary hypertension with unclear and/or multifactorial mechanisms |
5.1   Hematologic disorders: myeloproliferative disorders, splenectomy Hematologic disorders: myeloproliferative disorders, splenectomy | |
5.2   Systemic disorders: sarcoidosis, pulmonary Langerhans cell histiocytosis, Systemic disorders: sarcoidosis, pulmonary Langerhans cell histiocytosis,    lymphangioleiomyomatosis, neurofibromatosis, vasculitis lymphangioleiomyomatosis, neurofibromatosis, vasculitis | |
5.3   Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders | |
5.4   Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure on dialysis Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure on dialysis | |
ALK1 = activin receptor-like kinase 1 gene; APAH = associated pulmonary arterial hypertension; BMPR2 = bone morphogenetic protein receptor, type 2; HIV = human immunodeficiency virus; WHO = World Health Organization
Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54(suppl 1):S43-S54.
Table 2
The broad schema of 10 basic categories of Pediatric Pulmonary Hypertensive Vascular Disease
|
Cerro MJ, Abman S, Diaz G, Freudenthal AH, Freudenthal F, Harikrishnan S, et al. A consensus approach to the classification of pediatric pulmonary hypertensive vascular disease: Report from the PVRI Pediatric Taskforce, Panama 2011. Pulm Circ. 2011;1(2):286-98.
Epidemiology and Survival
Large registries of pediatric PH have recently been published, shedding light on the incidence and prevalence of PH in children as well as the general demographics. These registries include the Swiss Registry (4), the United Kingdom PH service (5), the French PH registry (6), the Netherlands PH registry (7), the TOPP registry (Tracking Outcomes in Pediatric Pulmonary Hypertension)(8), and finally the US REVEAL (Registry to EValuate Early And Long-Term PAH Disease Management) registry.(9)
The most common causes of transient PH the Netherlands registry was persistent pulmonary hypertension of the newborn and pulmonary hypertension associated with repair of “flow” CHD with resolution of the PH after surgery, with an incidence of 30.1 and 21.9 cases per million children, respectively (Figure 1).(7) IPAH and PAH associated with congenital heart disease (APAH-CHD) account for the majority of progressive forms of PH in the pediatric registries. IPAH accounts for 35-60% of patients in these registries with most studies having 50-60% of patients with IPAH.(4, 6, 7, 9) PAH associated with CHD is the second most cause of PAH in these registries accounting for 24-52% of patients. The incidence of IPAH in the national registries from the United Kingdom and Netherlands was 0.48 and 0.7 cases per million with a point prevalence of 2.1 and 4.4 cases per million, respectively. (7, 10) The incidence and point prevalence of APAH-CHD was 2.2 and 15.6 cases per million in the Netherlands registry.(7) Bronchopulmonary dysplasia is likely under-represented in these series and accounts for 12-13% of patients. (4, 8) Syndromes are common in pediatric PH practice, with Trisomy 21 being the most common syndrome.(7, 9)
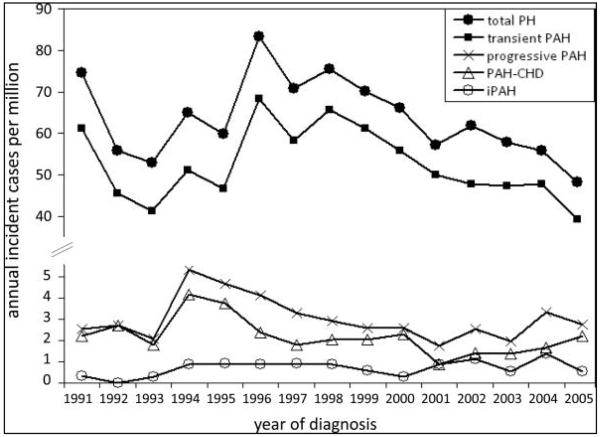
Annual incidence rates for pediatric pulmonary hypertension in the Netherlands. PH indicates pulmonary hypertension; PAH, pulmonary arterial hypertension; PAH-CHD, PAH associated with congenital heart defects; and iPAH, idiopathic PAH.
Source: [7] Van Loon RL, et al. Circulation. 2011 Sep 26;124:1755-64.
The most frequent presenting symptom of PH was dyspnea in most series (53% IPAH/familial pulmonary arterial hypertension (FPAH); 30% APAH-CHD). However, pre-syncope/syncope was more frequent in IPAH/FPAH (36%) than APAH-CHD (4%). Although survival in pediatric PH has markedly improved over the last 2 decades, data from these registries still show the need for improvement. The 1,3 and 5 year survival for 64 children with IPAH in the U.K. Service was 89%, 84% and 75%. (10) The U.S. REVEAL registry included 199 pediatric patients, of which 122 were diagnosed with IPAH/familial PAH and 77 with APAH-CHD. Five-year survival from diagnostic right heart catheterization was similar for IPAH/FPAH and APAH-CHD (75% ± 7% vs. 71% ± 14%, respectively).(9) Factors associated with a worse prognosis include WHO functional class III/IV, poor height and weight z-score at presentation, older age at presentation, specific diagnoses (pulmonary veno-occlusive disease, pulmonary capillary hemangiomatosis, FPAH), and hemodynamic variables such as lower mixed venous oxygen saturation and higher PVRI. (7, 9, 10) Survival of children with Eisenmenger syndrome appeared worse than reported in adults.(7) Although the overall survival in the UK service was similar for IPAH and APAH-CHD, patients with repaired CHD had the worst prognosis in 2 series (Figure 2).(5, 7)
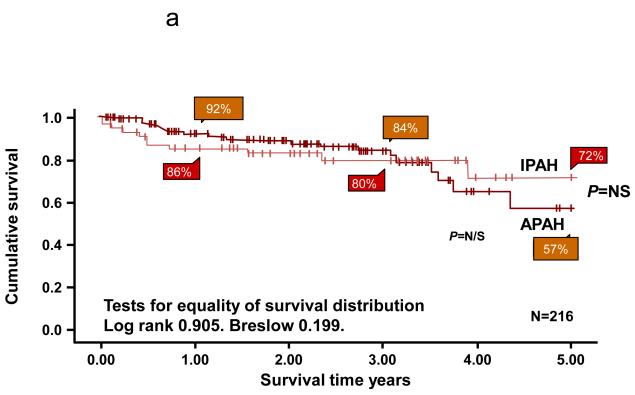
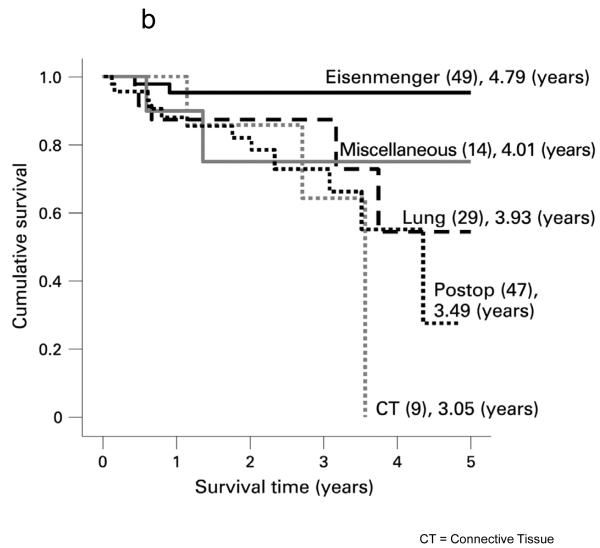
Survival curves for idiopathic pulmonary arterial hypertension (IPAH) and associated pulmonary arterial hypertension (APAH) cases censored for time in the study and for transplantation. There was no significant difference between the two groups. B. Survival curves for the subgroups within the associated pulmonary arterial hypertension (APAH) group. The number in each group (brackets) and the predicted survival out of a possible 5 years are shown. CT: Connective Tissue.
Source: [9] Haworth, SG et al. Heart 2009;95:312-317
Therapy of PAH
Based on understanding of abnormalities of the vascular endothelium, three classes of drugs have been studied for the treatment of PAH: prostanoids (epoprostenol, treprostinil, iloprost), endothelin receptor antagonists (bosentan, ambrisentan), and phosphodiesterase inhibitors (sildenafil, tadalafil) (11, 12) (13). Similarities in the pathobiology of PAH have led to similar treatment algorithms, in children (14, 15) (Figure 3) and adults (16)(Figure 4). However, as previously described, abnormalities of lung development are common in children with PAH, and infants and young children cannot be classified in the adult WHO functional classification. A pediatric classification has been developed, but PAH therapies have not been stratified to this classification (17).
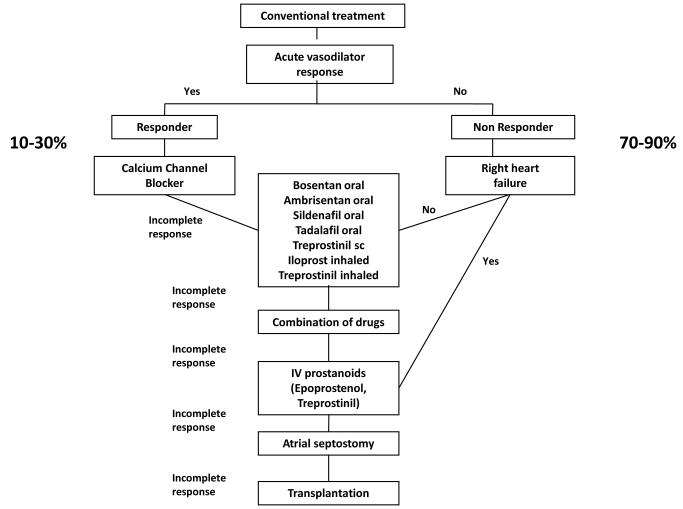
Treatment algorithm in children with severe pulmonary arterial hypertension.
Source: [14] Tissot C, et al. J Pediatr. 2010;157:528-532
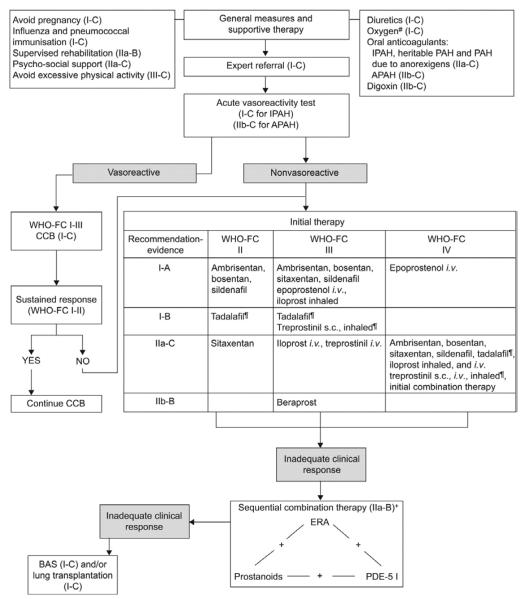
Evidence-based treatment algorithm for pulmonary arterial hypertension adults (for group 1 patients only). APAH: associated pulmonary arterial hypertension; BAS: balloon atrial septostomy; CCB: calcium channel blocker; ERA: endothelin receptor antagonist; IPAH: idiopathic pulmonary arterial hypertension; PAH: pulmonary arterial hypertension; PDE-5 I: phosphodiesterase type-5 inhibitor; s.c.: subcutaneously; WHO-FC: World Health Organization functional class. #: to maintain arterial blood O2 pressure >60 mmHg; ¶: under regulatory review in the European Union; +: IIa-C for WHO-FC II.
Source: [16] Galie N et al Eur Heart J. 2009;30(20):2493-2537.
Cardiac catheterization is an important initial step in the treatment of children with PAH, because it aids in ruling out associated disease states, such as pulmonary vein disease, and allows for determination of vasoreactivity, which is necessary to determine if calcium channel blocker therapy may be appropriate. The younger the child at the time of testing, the greater the likelihood of acute pulmonary vasodilation in response to vasoreactivity testing (18, 19). Many inhaled vasodilators have been used for vasoreactivity testing of vasodilator (20-23). The conventional pediatric definition of a positive response to vasodilators is a fall in mean PAP and PVRI by 20% with no significant change or an increase in cardiac index. The adult PH community has embraced a more strict definition as a fall in mean PAP of at least 10 mmHg to near normal levels to less than a mean PAP of 40 mmHg (11, 24). The most appropriate definition for children is not known. Catheterization in children is safer than previous decades using skilled cardiologists and anesthetists comfortable with these ill patients, but patients with suprasystemic PH pose particular risk. (25-27) Rarely cardiac catheterization may be briefly delayed for patient stabilization before catheterization is performed.
Calcium channel blockers
Treatment of PAH with calcium channel blockers is decreasing with the approval of more targeted therapy, and is limited to select patients with a positive acute vasodilator challenge.(12, 28) Elevated right atrial pressure and low cardiac output are contraindications to chronic calcium channel blockade. Although the exact definition of vasoreactivity in children to determine acceptability of calcium channel blockade is not known, marked reactivity with near normalization of pulmonary artery pressure is a common feature. Patients who do not have an acute vasodilatory response to short acting agents and who are then placed on calcium channel blocker therapy are likely to deteriorate (11). It is estimated that 60-80 % of children with severe PH are non-responsive to acute vasodilator testing, and therefore require therapy other than calcium channel antagonists.
Prostacyclins
Epoprostenol, synthetic prostacyclin, is the only therapy shown to improve survival in adults. (29, 30) Therapy with prostacyclin and its analogues has been extrapolated for use in children. Long-term IV epoprostenol has improved survival for children with PAH with a 4-year survival rate of 94% in IPAH (28), and a 10-year treatment success rate (freedom from death, transplantation, or atrial septostomy) of 37% (19). Epoprostenol has a short half-life rendering a continuous intravenous infusion with a permanent central venous catheter necessary. Complications such as line sepsis, local infection and catheter dislodgement are not unusual and can be responsible for life-threatening rebound PH (31). Recently, the use of specific closed hub systems has been shown to decrease the infection rate (32). Although uncommon, children with a dramatic sustained response to epoprostenol may be weaned to oral or inhaled therapy if hemodynamics return to near normal values. (33, 34) A room temperature formulation is FDA approved for use in adults, but has not been studied in children.
Iloprost is an inhaled prostacyclin analogue with a longer half-life (35). In children treated with iloprost, WHO functional class has been shown to be improved in 35%, remained unchanged in 50% and decreased in 15% (36). Lower-airway reactivity is a problem in some children, as well as poor compliance with the need for frequent aerosol administrations (6-8 times daily). Iloprost has been used in postoperative congenital heart disease associated with PH (37) and has been shown to be as efficacious in lowering mean PVR and improve systemic oxygen saturation compared to NO (22). Nevertheless, clinical deterioration, side effects, and poor compliance limit its chronic administration in children (36).
Treprostinil, a prostacyclin analogue, is approved by the FDA for subcutaneous use (2002), intravenous administration (2004) and inhalation (2009). Subcutaneous treprostinil has been shown to improve exercise tolerance and hemodynamics in adult patients with PAH (38). Discomfort at the infusion site is common and represents the most limiting factor. However, a recent study of subcutaneous treprostinil in young children showed promise with tolerable side effects.(39) Similar to epoprostenol, intravenous treprostinil requires central line access and continuous infusion, but is easier for families to mix, has a longer half-life, and may allow use of a smaller pump. Intravenous treprostinil has fewer side effects than intravenous epoprostenol, but there are no studies comparing efficacy. (40) Treprostinil in an inhaled form is increasing in usage. (41)
Endothelin
Blockade of the receptors of ET-1, a potent vasoconstrictor peptide, with bosentan lowers pulmonary artery pressure and resistance, and improves exercise tolerance in adults with PAH (42). Although bosentan is not approved for use in children with PAH in the US, several studies have suggested safety and a delay in disease progression (43-48). A retrospective study of 86 children on bosentan for a median exposure of 14 months with and without concomitant therapy found that bosentan provided a sustained clinical and hemodynamic improvement was overall well tolerated, and two year survival estimates were 91% (47). Follow-up of these patients at 4 years revealed the Kaplan-Meier estimate of disease progression in patients while on bosentan was 54% with a survival estimate of 82%.(49) In a cohort of children with IPAH and APAH-CHD, the Kaplan-Meier survival estimates for 101 patients treated with bosentan as part of an overall targeted PH strategy were 96, 89, 83 and 60% at 1, 2, 3 and 5 yrs., respectively. (48) In 146 children with PAH, elevated transaminase levels were reported in 2.7% of children compared with 7.8% of patients aged ≥12 years, and the overall discontinuation rate from bosentan was 14% in children compared with 28% in patients aged ≥12 years.(45) The prospective FUTURE-1 trial treated children with a pediatric specific water-dissolvable formulation at doses of 2 mg/kg/dose and 4 mg/kg/dose twice daily. There was no difference in drug exposure, and plasma concentrations did not reach adult levels with either dose. There were no abnormalities of liver function testing leading to a general recommendation of the 2 mg/kg/dose twice daily for children.(50) Bosentan has been studied in Eisenmenger syndrome in a placebo-controlled trial in patients. Bosentan was well tolerated and improved exercise capacity and hemodynamics without compromising peripheral oxygen saturation. (51) Bosentan, and other advanced therapies, have been associated with improved survival in adults with Eisenmenger syndrome.(52) (Figure 5) A specific pediatric formulation has been recently approved in Europe.
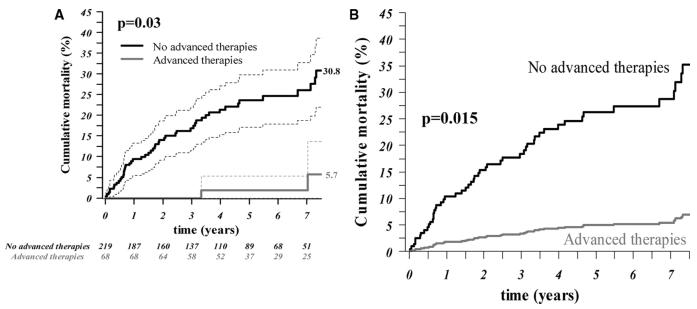
A. Unadjusted survival rate curves (with 95% CIs) by treatment with advanced therapies in adults with Eisenmenger syndrome (AT (n=287). P value refers to Cox model. B. Adjusted survival rate curves, based on the propensity score–adjusted Cox model, of patients within the third propensity score quartile, with and without advanced therapy.
Source: [52] Dimopoulos K et al; Circulation. 2010;121:20-25
Ambrisentan is a once-daily selective ETA endothelin receptor-antagonist that was FDA approved in 2007 for use in adults. In March 2011 ,monthly liver transaminase monitoring was no longer mandated, but many programs continue regular monitoring every few months. Adults showed significant improvements in 6-minute walk distance and significant delay in clinical worsening on ambrisentan.(53). Studies in children suggest that some patients treated with bosentan may show additional improvement on transition to ambrisentan. (54)
Phosphodiesterase-5 inhibitors
Sildenafil and tadalafil, are specific phosphodiesterase-5 inhibitors (5, 55-58),which promote an increase in cGMP levels and thus promote pulmonary vasodilation. Sildenafil may also be useful in the setting of inhaled nitric oxide therapy withdrawal (59) in post-operative PH (60), or in the presence of PH related to chronic lung disease.(57) In a 16-week, randomized, double-blind study of treatment naïve children, (STARTS-1), the effects of oral sildenafil in pediatric PAH were studied.(61) Children (n=234) with PAH (aged 1–17 yrs.; ≥8 kg) received low-, medium-, or high-dose sildenafil or placebo orally three times daily. The primary comparison was percent change in peak oxygen consumption (pVO2) for the three sildenafil doses combined from baseline to week 16; exercise testing was performed only in children able to exercise reliably. Secondary endpoints, including mean PAP, PVRI, and functional class, were assessed in all enrolled patients, including those unable to reliably exercise. The estimated mean ± standard error percentage change in pVO2 for the low-, medium- and high-doses combined versus placebo was 7.7% ± 4.0% (95% CI, – 0.2% to 15.6%; P=0.056)(Figure 6). Peak VO2, FC, mPAP, and PVRI improved with the medium- and high-dose groups versus placebo, whilst the low dose was ineffective. Upper respiratory tract infections, pyrexia, and vomiting occurred more often with sildenafil than placebo.(61) A long-term extension study included children continued on sildenafil monotherapy. For the first two years survival on sildenafil monotherapy was the same for all dosage groups. (Table 3) At three years, an increase in mortality was noted in the high-dose group compared to the low dose group, and the data safety monitoring board requested to decrease the dose of any child receiving high dose. Deaths in the extension study were related to etiology and baseline disease severity. The majority of deaths occurred in patients with IPAH/FPAH. Of patients who died, most had baseline values above median values for PVRI, mean pulmonary artery pressure, and right atrial pressure. Sildenafil is approved for use in children with PAH in Europe: 10 mg t.i.d. in patients up to 20 kg, and 20 mg t.i.d. in heavier patients, but sildenafil is not approved for children with PAH in the U.S.
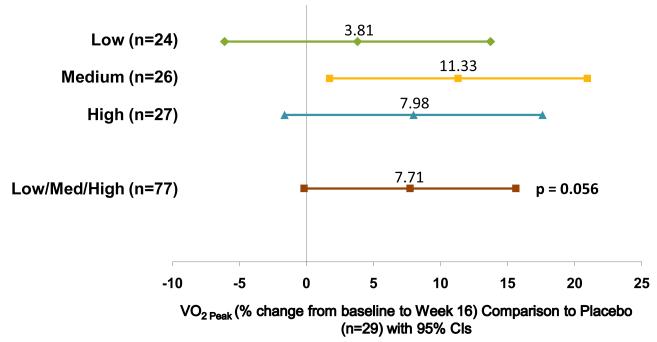
Placebo-adjusted percent change in peak VO2 by cardiopulmonary exercise after 16 weeks of sildenafil or placebo in a randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension.
Source: [9] Barst RJ, Ivy DD, et al. Circulation. 2011;In Print.
Table 3
Sildenafil Thrice Daily Dose in the STARTS-1 trial
| Sildenafil Dose (mg) | |||
|---|---|---|---|
| Body Weight (kg) | Low | Medium | High |
| ≥8 to 20 | NA† | 10† | 20 |
| >20 to 45 | 10 | 20 | 40 |
| >45 | 10 | 40 | 80 |
Barst RJ, Ivy DD, et al. Circulation. 2011;In Print.
Intravenous sildenafil has been shown to potentiate the increase in cGMP in response to NO in children with increased PVR related to CHD or in the post-operative state. Nevertheless, sildenafil infusion may be associated with increased intrapulmonary shunting and augmentation of hypoxemia related to V/Q mismatch in the postoperative CHD patient.(62, 63) However, a recent study of intravenous sildenafil has shown improvement in oxygenation index in persistent pulmonary hypertension of the newborn in patients treated with or without inhaled nitric oxide.(64) To evaluate the efficacy and safety of intravenous sildenafil for postoperative pulmonary hypertension in pediatric patients undergoing congenital heart surgery, a double-blind, multicenter, placebo-controlled, dose-ranging, parallel-group trial was conducted. Although the study was terminated after 15 months due to slow patient accrual, seventeen patients were randomized and treated, five with placebo and four each with low-, medium-, and high-dose sildenafil. In the first 24 h, 40% of placebo and 17% of sildenafil patients required additional therapy (p = 0.330). Median time to extubation (3 versus 8 days, p = 0.023) and intensive care unit stay (6 versus 15 days, p = 0.008) were shorter for sildenafil patients. No adverse events or systemic hypotension were attributed to sildenafil.(65)
Other PDE-5 inhibitors, such as tadalalfil, have been recently studied leading to US FDA approval in 2009. (66). Tadalafil, dosed daily may improve compliance and appears to have a similar hemodynamic profile to sildenafil in children. (67, 68) In 14 of 29 children transitioned from sildenafil to tadalafil, repeat cardiac catheterization showed statistically significant improvements in mean pulmonary arterial pressure (mmHg) (53.2+/−18.3versus 47.4+/−13.7, p<0.05) and pulmonary vascular resistance index (unitsxm2)(12.2+/−7.0 versus 10.6+/−7.2, p<0.05). (68)
Novel Therapies
Therapies targeting remodeling of the pulmonary vasculature are under study.(12) Imatinib, an antagonist of the platelet-derived growth factor (PDGF) receptor, is approved for the treatment of chronic myeloid leukemia. PDGF contributes to vascular remodeling and participates in mytogenic signaling and smooth muscle cell recruitment. Case reports of the use of imatinib for patients with severe PAH refractory to all available treatment showed clinical and hemodynamic improvement (69) with further promising results in a Phase II trial (70)
Bone-derived endothelial progenitor cells (EPCs) normally function to repair and regenerate blood vessels. Regeneration of lung vascular endothelium by injection of progenitor cells may represent a novel treatment paradigm for patients with PAH; trials are underway in Canada (71, 72). Other targets in the future may include blockade of stem cell populations contributing to the fibroproliferative process, such as fibrocytes.(73)
Novel agents leading to pulmonary vasodilation are being developed. Riociguat, a direct oral soluble guanylate cyclase (sGC) stimulator, increases cGMP directly in a non-NO dependent manner but also increases the sensitivity of sGC to NO; Phase III trials are currently underway. (74-77) Selexipag is an orally available prostacyclin receptor (IP receptor) agonist that is chemically distinct from PGI2 and is in clinical development for the treatment of pulmonary arterial hypertension in Phase III trials. Where prostacyclin and its analogs can activate prostanoid receptors other than the IP receptor, direct stimulation of these receptors may help minimize gastric side effects such as nausea and vomiting. (78) Novel endothelin receptor antagonists are currently under study in Phase III trials as well. Macitentan is a highly potent, tissue-targeting dual ETA, ETB receptor antagonist.(79)
Conclusions
Pulmonary arterial hypertension (PAH) is a life-threatening disease whose prognosis has changed dramatically over the past decade since the introduction of new therapeutic agents as well as the off-label application of adult pulmonary hypertension specific therapies to children. Therapy in adults is evidence based on randomized, placebo-controlled trials. However, therapy in children is based on experience. Future clinical trials must take into account the unique aspects of PAH in children, including pharmacokinetics, clinical endpoints, and long-term toxicity.
Acknowledgements / Conflicts of Interest
This work was supported in part by the National Institutes of Health (NIH) Specialized Centers of Clinically Oriented Research grant HL-HL684923, NIH/NCRR Colorado CTSI Grant Number UL1 RR025780, the Jayden DeLuca Foundation and the Leah Bult Pulmonary Hypertension Fund.
The University of Colorado Denver School of Medicine receives fees from Actelion, Gilead, Pfizer, and United Therapeutics for Dr Ivy to be a consultant. Dr Ivy serves on the Gilead Sciences Research Scholars Program in Pulmonary Hypertension review panel.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Full text links
Read article at publisher's site: https://doi.org/10.1097/hco.0b013e32835018cd
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3319159?pdf=render
Citations & impact
Impact metrics
Article citations
Right ventricular-vascular coupling ratio in pediatric pulmonary arterial hypertension: A comparison between cardiac magnetic resonance and right heart catheterization measurements.
Int J Cardiol, 293:211-217, 10 May 2019
Cited by: 13 articles | PMID: 31109778 | PMCID: PMC6710117
The Role of Biomarkers and Surrogate End Points in Drug Development for Neonatal Pulmonary Arterial Hypertension.
Neoreviews, 17(2):e87-e92, 01 Feb 2016
Cited by: 1 article | PMID: 28943808 | PMCID: PMC5609821
Pediatric Cardiac Intensive Care Society 2014 Consensus Statement: Pharmacotherapies in Cardiac Critical Care Pulmonary Hypertension.
Pediatr Crit Care Med, 17(3 suppl 1):S89-100, 01 Mar 2016
Cited by: 8 articles | PMID: 26945333 | PMCID: PMC4820013
Review Free full text in Europe PMC
Continuous inhaled iloprost in a neonate with d-transposition of the great arteries and severe pulmonary arterial hypertension.
Cardiol Young, 26(3):571-573, 29 Jul 2015
Cited by: 2 articles | PMID: 26220108
Pulmonary Hypertension in Children - a Practical Approach.
Maedica (Bucur), 10(3):237-242, 01 Sep 2015
Cited by: 0 articles | PMID: 28261360 | PMCID: PMC5327836
Go to all (15) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phosphodiesterase 5 inhibitors for pulmonary hypertension.
Cochrane Database Syst Rev, 1:CD012621, 31 Jan 2019
Cited by: 63 articles | PMID: 30701543 | PMCID: PMC6354064
Review Free full text in Europe PMC
Current challenges in pediatric pulmonary hypertension.
Semin Respir Crit Care Med, 34(5):627-644, 13 Sep 2013
Cited by: 14 articles | PMID: 24037630 | PMCID: PMC3909673
Review Free full text in Europe PMC
Pediatric pulmonary arterial hypertension.
Curr Hypertens Rep, 15(6):606-613, 01 Dec 2013
Cited by: 4 articles | PMID: 24163011
Review
Recent progress in understanding pediatric pulmonary hypertension.
Curr Opin Pediatr, 23(3):298-304, 01 Jun 2011
Cited by: 26 articles | PMID: 21572384 | PMCID: PMC3128451
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCRR NIH HHS (2)
Grant ID: UL1 RR025780
Grant ID: UL1 RR025780-04
NHLBI NIH HHS (3)
Grant ID: P50 HL084923-05S1
Grant ID: P50 HL084923
Grant ID: HL684923




