Abstract
Free full text

The vaginal microbiome: rethinking health and diseases
Abstract
Vaginal microbiota form a mutually beneficial relationship with their host and have major impact on health and disease. In recent years our understanding of vaginal bacterial community composition and structure has significantly broadened as a result of investigators using cultivation-independent methods based on the analysis of 16S ribosomal RNA (rRNA) gene sequences. In asymptomatic, otherwise healthy women, several kinds of vaginal microbiota exist, the majority often dominated by species of Lactobacillus, while others comprise a diverse array of anaerobic microorganisms. Bacterial vaginosis is the most common vaginal conditions and is vaguely characterized as the disruption of the equilibrium of the ‘normal’ vaginal microbiots. A better understanding of ‘normal’ and ‘healthy’ vaginal ecosystems that is based on its ‘true’ function and not simply on its composition would help better define health and further improve disease diagnostics as well as the development of more personalized regimens to promote health and treat diseases.
INTRODUCTION
The microbiota normally associated with the human body have an important influence on human development, physiology, immunity, and nutrition (18; 23; 65; 66; 70; 111). The vast majority of these indigenous microbiota exist in a mutualistic relationship with their human host, while few are opportunistic pathogens that can cause both chronic infections and life-threatening diseases. These microbial communities are believed to constitute the first line of defense against infection by competitively excluding invasive nonindigenous organisms that cause diseases. Despite their importance, surprisingly little is known about how these communities differ between individuals in composition and function, but more importantly, how their constituent members interact with each other and the host to form a dynamic ecosystem that responds to environmental disturbances. Major efforts are now underway to better understand the true role of these communities in health and diseases (84).
The human vagina and the bacterial communities that reside therein are an example of this finely balanced mutualistic association. In this relationship, the host provides benefit to the microbial communities in the form of the nutrients needed to support bacterial growth. This is of obvious importance since bacteria are continually shed from the body in vaginal secretions, and bacterial growth must occur to replenish their numbers. Some of the required nutrients are derived from sloughed cells, while others are from glandular secretions. The indigenous bacterial communities, on the other hand, play a protective role in preventing colonization of the host by potentially pathogenic organisms, including those responsible for symptomatic bacterial vaginosis, yeast infections, sexually transmitted infections (STI), and urinary tract infections (42; 47; 96; 98; 113; 118). Lactobacilli have long been thought to be the keystone species of vaginal communities in reproductive-age women. These microorganisms benefit the host by producing lactic acid as a fermentation product that lowers the vaginal pH to ~3.5–4.5 (12). While a wide range of other species are known to be members of vaginal bacterial communities, their ecological functions and influence on the overall community dynamics and function are largely undetermined. The vaginal ecosystem is thought to have been shaped by co-evolutionary processes between the human host and specific microbial partners, although the selective forces (traits) behind this mutualistic association are still not clear.
The development of culture-independent approaches has greatly facilitated comprehensive surveys of the composition of vaginal microbial communities. These studies have shown that several distinct kinds of vaginal communities with markedly different species composition occur and the frequency of these types of microbiota varies in different ethnic groups (86; 122–124). It is hypothesized that differences in species composition may correlate with how vaginal communities respond to disturbances (52; 104; 115; 123). Conceptually this is important since vaginal communities continually experience various kinds of chronic and acute disturbances caused by human behaviors such as the use of antibiotics, hormonal contraceptives and other methods of birth control, sexual activity, vaginal lubricants, douching, and so forth, in addition to many other intrinsic factors such as the innate and adaptive immune systems of hosts (64; 88; 110). Further, a disturbed state itself may constitute the clinical syndrome known as bacterial vaginosis (BV), which as a disruption of ecological equilibria is believed to increase the risk to invasion by infectious agents. While knowledge accumulated over the past few decades has provided some insights into the vaginal ecosystem, there remains a need to define and better understand factors that affect the composition and dynamics of vaginal microbiota, including the role of human genetic and physiology in both health and diseases. This knowledge will facilitate the development of new strategies for disease diagnosis and personalized treatments to promote health and improve the quality of women’s lives. This cannot be accomplished without addressing a fundamental issue as to what constitutes a ‘normal’ and ‘healthy’ vaginal microbiota and understanding its function in health and diseases.
THE COMPOSITION AND STRUCTURE OF THE VAGINAL MICROBIOTA
Comprehensive surveys of vaginal microbial community using culture-independent approaches have revealed that Lactobacillus species are the dominant vaginal bacterial species in a majority of women. However, an appreciable proportion of asymptomatic, otherwise healthy individuals have vaginal microbiota lacking significant numbers of Lactobacillus sp. and harbor a diverse array of facultative and strictly anaerobic microorganisms.
Culture-dependent and culture-independent approaches to survey microbial community composition and structure
Most of our knowledge of the composition, metabolic function, and ecology of indigenous microbial communities associated with humans has come from studies that depended on cultivating microbial populations. Hence our current understanding of microbe-host interactions is limited and skewed because the overwhelming majority of microbial species (>99%) resist cultivation in the laboratory (8). Our limited ability to culture may result from strict, yet unknown, growth requirements, such as the optimal combination of nutrients, growth temperatures, dissolved-oxygen levels, or potentially the need to co-cultivate with key microbial partners (3; 27). Recently our knowledge of microbial diversity has expanded enormously through the use of culture-independent approaches based on the analysis of 16S rRNA gene sequences (50; 107). These strategies circumvent the need to cultivate organisms by directly extracting genetic materials from environmental or biological samples. This is followed by amplification of the 16S rRNA genes using primers that anneal to highly conserved regions of the gene, followed by sequencing and classification of the phylotypes present. This constitutes an efficient way to comprehensively characterize microbial diversity. The development of next-generation sequencing technologies including the use of massively parallel DNA sequencing of short, hypervariable regions of the 16S rRNA gene now afford us the opportunity to obtain detailed surveys of microbial communities, including the identification of taxa present in low abundance that comprise the rare biospheres (27; 102). Other conserved genes such as cpn60, rpoC, uvrB or RecA have also been used for these purposes (92; 114).
Culture-independent methods have demonstrated that when surveyed cross-sectionally several kinds of vaginal communities (community state types) exist in normal and otherwise healthy women, each with a markedly different bacterial species composition. These communities are either dominated by one of four common Lactobacillus sp. (L. crispatus, L. iners, L. gasseri and L. jensenii) or do not contain significant numbers of lactobacilli, but instead have a diverse array of strict and facultative anaerobes (86).
Lactobacillus-dominated vaginal microbiota
Members of the genus Lactobacillus are commonly identified as the hallmark of normal or healthy vagina (25; 42; 69; 98). Since they were first identified by cultivation in vaginal secretion in late 19th century by Donderlein (24; 90; 106), Lactobacillus sp. are thought to play a major role in protecting the vaginal environment from non-indigenous and potentially harmful microorganisms. This is accomplished through the production of lactic acid, resulting in a low and protective pH (3.5–4.5) (1; 11; 12; 54; 87; 91). Interestingly, lactic acid has been shown to be more effective than acidity alone as a microbicide against HIV or against pathogens like Neisseria gonorrhea (38; 60). Exposure to Gram-negative bacteria, in the presence of lactic acid is believed to have stimulatory effects on the host innate immune defense system (120). A recent study using in vitro colonization of vaginal epithelial cell monolayers with common bacteria such as L. crispatus, Prevotella bivia and Atopobium vaginae species, demonstrated that these key vaginal bacteria appear to regulate the epithelial innate immunity in a species-specific manner (32).
L. crispatus was previously thought to be one of the most common species of lactobacilli in the vagina (5). However, the application of culture-independent method has identified L. iners, an organism that is difficult to cultivate and does not grow on traditional culture media, as the most prevalent vaginal bacterial species (30; 122). In these studies, vaginal microbiota of 42% (17) and 66% (123) of the reproductive age women sampled were dominated by L. iners. A recent large-scale cross-sectional study of 396 healthy asymptomatic women revealed that L. iners was detected in 83.5% of the subjects and dominated 34.1% of the communities analyzed (86), while L. crispatus, L. gasseri and L. jensenii were present in 64.5, 42.9 and 48.2% of the subjects and dominated in 26.2, 6.3 and 5.3% of the samples, respectively (86). This large study showed that vaginal bacterial communities that had similar species composition and abundance could be classified into five groups, which are referred to as community state types (Figure 1). The four community state types dominated by Lactobacillus sp. represented 73% of the samples, which supports the prevailing view that Lactobacillus sp. are important members of vaginal microbiota. The remaining 27% represented communities that lacked significant numbers of Lactobacillus sp. but instead were comprised of a diverse array of facultative or strictly anaerobic bacteria. Interestingly, the distribution of Lactobacillus sp. dominated community state types varies significantly among individuals with different ethnic background (86; 123; 124). White and Asian women are more likely than Hispanic and Black women to have vaginal community dominated by Lactobacilli (86). When Lactobacillus sp. is present, vaginal communities of Hispanic and Black women are more often dominated by L. iners (86). The study also noted a higher average pH in Black and Hispanic women, 4.7 and 5.0 respectively, compare to 4.4 and 4.2 for Asian and White women. This observation supports the hypothesis that host factors may play an important role in determining vaginal microbial community composition and structure.
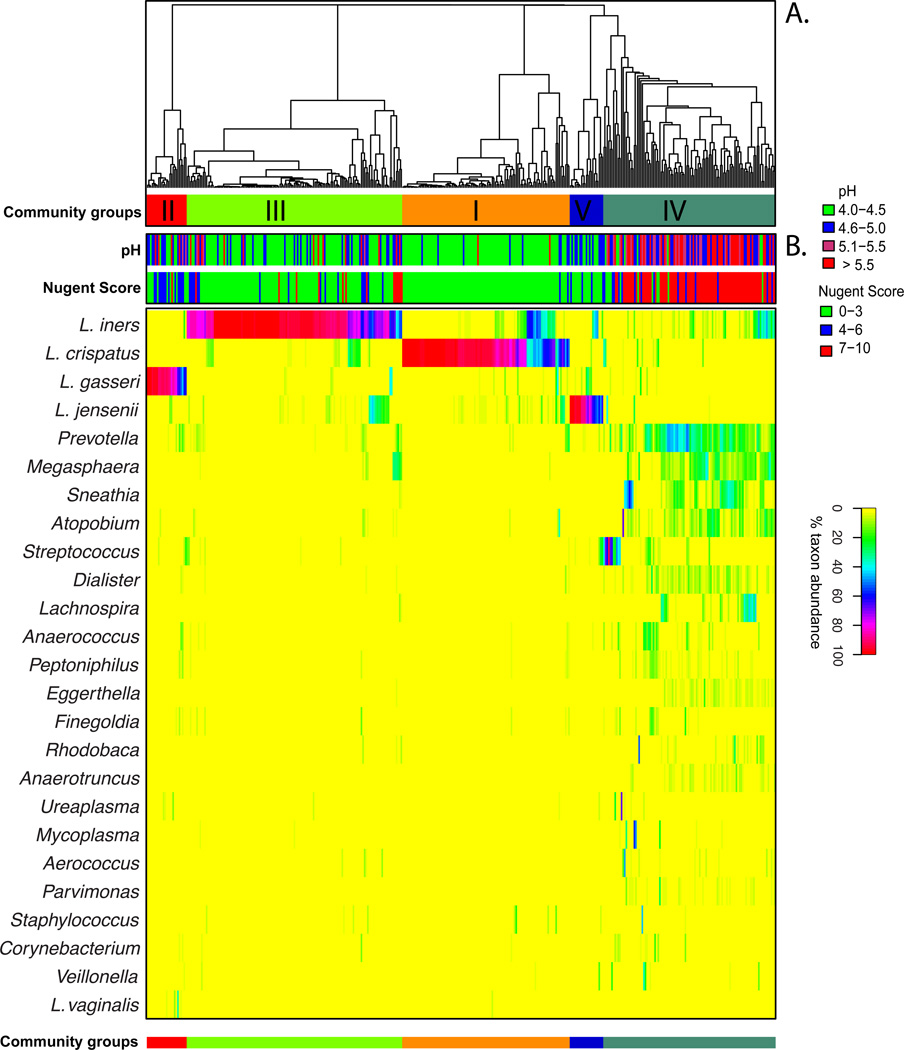
Heatmap of percentage abundance of microbial taxa found in the vaginal microbial communities of 394 reproductive-age women. (A) Complete linkage clustering of samples based on species composition and abundance in communities defining five community state types (CST I–V). (B) Nugent scores and pH measurements for each of the 394 samples. [Adapted from (86)]
These findings highlight potential differences in the protective capabilities of vaginal Lactobacillus species. Statistically significant differences have been observed in the ability of different Lactobacillus species to lower pH between different community state types, with L. crispatus dominated communities able to acidify the vaginal milieu to pH 4.0, while others achieved pH ranging from 4.4 to 5.0 (86). While Lactobacillus sp. drive these processes, other community members could contribute by either producing or utilizing lactic acid. Moreover, it is anticipated that strains of the same species will also demonstrates genomic differences that will results in specific physiological and biochemical traits. Previous comparative genomic analyses have identified high level of genetic diversity and varied metabolic potential of closely related bacterial species or strains of the same species. For example, Escherichia coli stains can vary by as much as 25% in their gene content (80), while strains of the same Serovars of Salmonella enterica can vary by more than 20% (61). Much of the variations occur in the form of large genomic islands or lineage-specific regions that may be involved with adaptation to host microenvironment (10; 82). While no comparative genomic studies of vaginal Lactobacillus sp. have been reported, we show on Figure 2, that strains of Gardnerella vaginalis, which is commonly found in vaginal microbiota and its overgrowth has been associated with the condition bacteria vaginosis (73; 116), can differ by 31% in gene content and gene order (synteny). Advanced knowledge of genetic variation among Lactobacillus species (or strains) may provide further insight into their functional potential that may have significant implications to health and diseases. Because of species or strain-level genomic heterogeneity, it is important to note that the analysis of 16S rRNA gene sequences, while taxonomically informative, is not able to identify functional differences without considerable speculation, and attempts to inferring function of any bacterial community only knowing ‘who is there’ should be made with caution.
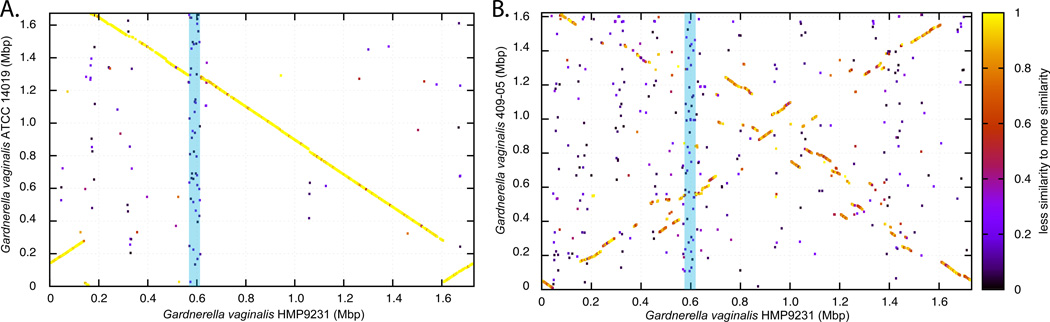
Whole genome comparative analysis of G. vaginalis using Blast Score Ratio Analysis. A protein match between two genomes is represented by a point plotted using the genomic coordinate of both matched proteins as X and Y coordinates. The level of protein sequence similarity is represented by the color of the points (see scale on right). (A) High degree of protein similarity and synteny is observed between G. vaginalis strains HMP9231 and ATCC 14019. (B) Lack of synteny and low degree of protein similarity is observed between G. vaginalis strains HMP9231 and 409-05. The blue bar highlights a set of syntenic genes that are unique to G. vaginalis HMP9231 and are not present in the other two genomes.
Lactobacillus species antimicrobial substances production
Vaginal Lactobacillus species are also known to produce other antimicrobial compounds besides lactic acid, including target-specific bacteriocins (2; 6) and broad-spectrum hydrogen peroxide (28; 43). Bacteriocins are proteinaceous, bactericidal substances synthesized by bacteria that have a narrow spectrum of activity (53). Their antimicrobial activity is usually based on permeabilization of the target cell membrane (81). In the vagina Bacteriocins could play a major role in fending off the growth of non-indigenous or pathogenic organisms (26). Many Lactobacillus sp. are also known to produce hydrogen peroxide in vitro under aerobic conditions. This is another antimicrobial compound that could inhibit colonization of potential pathogenic bacteria in vivo (43; 48; 112). However, the vagina is virtually an anaerobic environment wherein dissolved oxygen levels are low. Therefore it is unlikely that significant amounts of hydrogen peroxide are produced and accumulate to a toxic level. Further, a recent study showed that physiological concentrations of hydrogen peroxide had no detectable effect on 17 bacterial vaginosis-associated bacteria under anaerobic growth conditions and the presence of vaginal fluid could actually block its antimicrobial activity (78). In addition, it appears that high concentrations of hydrogen peroxide are even more toxic to vaginal Lactobacillus than to bacterial vaginosis-associated bacteria (78). Interestingly, some Lactobacillus species, such as L. iners, fail to produce hydrogen peroxide. This feature that has been used to differentiate beneficial versus non-beneficial vaginal Lactobacillus isolates (5), and it has been suggested that hydrogen peroxide producing vaginal Lactobacillus sp. are more likely to be protective against acquisition of bacterial vaginosis (43). Given the information summarized above, it is more likely that in vitro hydrogen peroxide production is not a significant factor in preventing the emergence of disease causing organisms, however it could be a surrogate marker for other yet unknown biochemical or physiological traits. Overall, this work suggests that lactic acid, not hydrogen peroxide, is more likely to contribute to the protective role of vaginal microbiota.
Other types of vaginal microbiota
Recent studies have found that 20–30% of asymptomatic, otherwise healthy women harbor vaginal communities that lack appreciable numbers of Lactobacillus but include a diverse array of facultative or strictly anaerobic bacteria that are associated with a somewhat higher pH (5.3–5.5) (86; 122–124). This proportion of communities can reach 40% among Black and Hispanic women (86). These microbiota include of members of the genera Atopobium, Corynebacterium, Anaerococcus, Peptoniphilus, Prevotella, Gardnerella, Sneathia, Eggerthella, Mobiluncus and Finegoldia among others (52; 86; 115; 122–124). These findings challenge the common wisdom that the occurrence of high numbers of lactobacilli and a vaginal pH <4.5 is synonymous with ‘normal’ and ‘healthy’. Previous studies have hypothesized non-Lactobacillus dominant vaginal microbiota may be nonetheless able to maintain functional vaginal ecosystems, by preserving lactic acid production and possibly other important functions (36; 86; 122). Actually many underappreciated microorganisms, such as members from Atopobium, Streptococcus, Straphylococcus, Megasphaera, and Leptotrichia, are capable of homolactic or heterolactic acid fermentations (89; 122). The highly diversified microbial community may have accommodated functional redundancy, allowing for the function of the ecosystem to persist in the face of perturbations (117). In the absence of symptomology, these types of vaginal bacterial communities might be considered ‘normal’ and ‘healthy’, even though the composition of these communities closely resemble those associated with symptomatic bacterial vaginosis.
BACTERIAL VAGINOSIS
Bacterial vaginosis (BV) is a highly prevalent vaginal disorder in reproductive age women, but its diagnostics and treatment are disappointingly ineffective. BV is often vaguely characterized as the disruption of the equilibrium of the ‘normal’ vaginal ecosystem.
The bacterial vaginosis enigma
Bacterial vaginosis (BV) is the most frequently cited cause of vaginal discharge and malodor, and the most common vaginal condition of reproductive-age women, resulting in millions of health care visits annually in the United States alone (99). In a cross-sectional study of reproductive age women in 2001 the National Health and Nutrition Examination Survey (NHANES) found that the prevalence of BV in the U.S. was 29.2% (59). BV has been shown to be an independent risk factor for the acquisition of sexually transmitted infections (19; 69; 83; 118), the acquisition and transmission of HIV (20–22; 69; 103; 105), the development of pelvic inflammatory disease (PID) (76), as well as reproductive tract and obstetric sequelae (37; 39; 49; 71; 72). Numerous investigations have been performed to identify factors that increase a woman’s risk to BV. Menstrual blood, a new sexual partner, vaginal douching, smoking and lack of condom use have shown to be among the strongest risk factors for BV (9; 15; 44; 45; 51; 57; 75; 95; 119). In general, these suspected factors often manifest themselves as relatively minor risks in clinical studies, and many women without the above risk factors have BV. In most women, the symptoms of BV resolve on their own without intervention (58). When necessary, the treatment of BV typically includes antibiotics such as metronidazole (oral or vaginal gel) or clindamycin vaginal cream (121). However, recurrence of BV after treatment is common: 15–30% of women have symptomatic BV 30–90 days following antibiotic therapy, while 70% of patients experience a recurrence within nine months (13; 62; 101). Strategies for the management of recurrent BV are not standardized (100), and since the etiology of BV remains unknown, the causes of relapse remain unclear (13; 97).
The confusion about BV stems in part from the subjective diagnostic criteria used. In clinical settings, BV is commonly diagnosed based on the clinical criteria described by Amsel et al. (121), wherein three of the following four symptoms must be evident: 1) a homogenous, white, non-inflammatory discharge that smoothly coats the vaginal walls; 2) the presence of “clue” cells on microscopic examination (squamous epithelial cells covered with adherent bacteria); 3) a vaginal fluid pH over 4.5; and 4) a fishy odor of vaginal discharge before or after addition of 10% KOH (121). The reliability of the Amsel criteria have been subject to debate, particularly in reference to pregnancy given the increased vaginal discharge that is often experienced by pregnant women, and the variation of pH depending on how and where samples are taken (41). However, not all symptoms are observed in every case (56), and because the diagnosis is subjective, controversy persists about the definition of BV. The sensitivity and specificity of the Amsel criteria is 70 and 94%, respectively (93) when compared to another diagnostic assay, the Nugent Gram stain score which is used in research and laboratories. In these settings, BV is traditionally diagnosed by scoring a Gram stained vaginal smear using the criteria defined by Nugent et al. (77). The Nugent score reflects the relative abundance of large Gram-positive rods (lactobacilli), Gram-negative and Gram-variable rods and cocci (i.e., G. vaginalis, Prevotella, Porphyromonas, and Peptostreptococcus), and curved Gram-negative rods (Mobiluncus). This technique allows assessment of relative numbers of bacteria, allowing for a rough evaluation of bacterial load, as well as the presence of polymorphonuclear leukocytes, candidal spores, fungal hyphae and sperm. It is based on a linear scale ranging from 0–10. A score of 0–3 is normal, 4–6 intermediate, and 7–10 is considered BV. While the Nugent criteria are commonly used to assess BV, the scoring of specimens can be subjective. Nonetheless with a sensitivity of 89% and specificity of 83% (93) compared to Amsel criteria, the Nugent Gram-stain test remains the preferred diagnostic tool (41; 59) and it can be performed on self-collected vaginal smears (74), thus facilitating longitudinal field-based studies (14; 94). Interestingly, as much as 50% of all women with BV (as defined by Nugent score) are asymptomatic (4), which led to the use of the term “Nugent-score BV” (85). It is unclear whether these women are truly without symptoms or whether the symptoms were poorly recognized or under reported. The meaning and implications of asymptomatic BV are not known. Even so, and because of growing concern for the complications linked to BV, there is a practice of treating asymptomatic disease under certain circumstances, such as prior to a hysterectomy procedure or in women at high risk for pre-term birth (121).
The complex etiology of BV
Despite decades of research attempts to find a single causative agent for BV failed. Consequently, Koch’s postulates are not fulfilled in which the etiologic agent is both necessary and sufficient to cause disease and should not be found in subjects without disease (29; 35). Indeed, there is growing evidence that BV is characterized, and perhaps caused by, disruption of the vaginal ecosystem that is reflected in alterations to the composition and structure of vaginal microbial communities, such that the numbers of lactic acid producing bacteria are decreased and the diversity and numbers of strictly anaerobic bacteria are increased, including species of Gardnerella, Atopobium, Mobiluncus, Prevotella, and other taxa of the order Clostridiales (34). The vaginal microbial community composition associated with BV is somewhat similar to the community state type described above that is found in asymptomatic healthy women that lack a significant number of Lactobacillus species. Culture-independent methods have identified potentially BV-associated bacteria that could not be identified by traditional culture-based methods (31; 34). These are now referred to as BV-associated bacteria (BVAB) and are distantly related to known species of the phyla Actinobacteria and Firmicutes. However, the significance of these findings remains unclear, as it is not known whether these microorganisms are pathogens that cause BV or if they simply are opportunistic organisms that take advantage of the temporary higher pH environment and thus increase in numerical dominance. Overall, these molecular studies have shown that the diversity, composition, and relative abundances of microbial species in the vagina vary dramatically in both normal healthy women and women presenting with BV. These diverse organisms accumulate to form different communities or profiles, which support the hypothesis that BV is not a single entity, but a syndrome linked to various community types that cause somewhat similar physiological symptoms. This suggests that an as yet unknown, common community functional output may account for BV and these respond differently to antibiotic therapies.
However, because these studies often rely on a single sample collected from women presenting to their physician with symptomatic BV, it is not possible to elucidate the causes of BV (microbiological, biochemical, molecular or behavioral) without access to samples collected prior to the diagnosis and during the events leading to BV. Prospective longitudinal studies, where samples are collected frequently along with detailed behavioral metadata, are necessary to understand the causes of BV. Such information is expected to suggest strategies to identify women who are at risk of acquiring symptomatic BV, new targets for intervention and prevention strategies, and enable development of more accurate diagnostic criteria.
RETHINKING NORMAL AND HEALTHY
The paradigm that healthy women are always colonized with high numbers of Lactobacillus species (46; 48) has previously been challenged as discussed above. Although numerous studies have shown that women with abundant Lactobacillus species do not have BV, the corollary that women whose vaginal communities have few or no Lactobacillus species also have BV is faulty logic. Unfortunately, the commonly used diagnostic criteria (both Amsel and Nugent) wherein the degree of ‘healthiness’ is in part assessed by scoring the abundance of Lactobacillus morphotypes, will tend to over-diagnose BV. This could at least partly account for the reported high incidence (as high as 42% (56)) of so-called asymptomatic BV in reproductive age women as defined by a positive Nugent score and no reported vaginal symptoms. It could also explain a portion of BV treatment failures and apparent recurrences of BV (33). To better understand symptomatic BV, its causes and the factors predisposing or triggering the condition, it is essential to apply molecular analyses of vaginal communities on a statistically significant number of women sampled longitudinally and prospectively, in order to define the diversity and dynamics of the vaginal ecosystem in the general populace. These studies would help us better understand what constitutes ‘normal’ and ‘healthy’ vaginal microbiota and the fluctuations that commonly occur in ‘normal’ and ‘healthy’ communities. As suggested by Marrazzo et al. (67), it is only by using such approaches will we be able to change and refine the definition, etiology, and epidemiology of BV.
The fine line that separates a ‘normal’ and ‘healthy’ from an ‘abnormal’ and ‘unhealthy’ vaginal microbiota is further complicated by potential confusion between health and the predisposition to diseases such as sexually transmitted infections, and by the lack of a complete understanding of the functional intricacies of the host and its vaginal microbiota. It is difficult to envision the evolutionary processes that led to a vaginal microbiota with sole function of protecting the host from STI, mainly because only a small proportion of women have been or are exposed to STI pathogens. Interestingly, human are among very few mammals with a vaginal microbiota often dominated with Lactobacillus sp, and with such a low pH. Hence, It appears that other yet unknown functions must have driven the composition of the human vaginal microbiota. For example, one potential function could relate to immune stimulation or microbial protection of the newborn in the first days of life. Without more knowledge of these functions, one might consider separating the concept of health and that of resistance to STI. That said, it is essential to understand the factors that increase the risks to acquiring STI and the community types that might be more susceptible to infection. It is conceivable that from time to time a dynamic system such as the vaginal microbiota might enter states (define by their composition or their function) that would increase the risks to infections. The frequency and duration of these states might represent better predictors of risk to infections than the abundance of a single community members or a given microbial community profile. With knowledge of the factors driving these dynamics and a better understanding of the function of the vaginal microbiota, novel prevention strategies could be developed to lower the risks. These might include driving the maintenance of more protective and highly functional vaginal microbiota, or possibly using more personalized probiotics or prebiotic mixtures. In addition, it is important to note that these risks are only at play when a woman has the potential to be exposed to the pathogens. If exposure is not likely (perhaps celibate or a monogamous couple), it might not be appropriate to define the healthiness of a woman’s vaginal microbiota by factors associated with its predisposition to infections. Hence, a new thinking would involve dissociating the concept of ‘normal’ and ‘healthy’ vaginal microbiota from that of predisposition to STI. A healthy vaginal microbiota could then be defined as a microbial community with a functional output that is adequately beneficial to the host and not solely defined by its composition, and this function could be provided by several kinds of vaginal communities. In this context, different types of vaginal microbiota could be considered ‘healthy’ in the absence of symptoms, with or without lactobacilli, while having differing degrees of predisposition to infections by sexually transmitted pathogens.
Temporal dynamics of vaginal communities
To date most studies of vaginal microbiology have employed cross-sectional designs in which samples are obtained from individuals at a single time point or with long intervals between sampling times (weeks or months). While these studies have provided important information on the species composition of vaginal communities they yield little insight to the normal temporal dynamics of these bacterial communities within individuals and do not allow for estimation of community stability. Daily fluctuations in the composition of the vaginal microbiota have been previously documented by microscopy (16; 44; 55; 95). Even more recently a longitudinal study conducted by our group described the temporal dynamics of vaginal community composition in 32 healthy reproductive aged women sampled twice-weekly over a 16-week period. The study showed that communities sometimes changed markedly over short period of time, while others were relatively stable, including communities lacking significant number of Lactobacillus sp. (Figure 3). In an effort to model the dependence of stability of vaginal bacterial communities on the time in the menstrual cycle and other time-varying factors, menses was identified as having the most negative effect on stability, along with the type of communities and sexual activity to a lesser extend. On the other hand, periods of the menstrual cycle corresponding with high level of estrogen or estrogen and progesterone were associated with higher stability. This study highlights the great potential of prospective longitudinal studies to elucidate the cause and etiology of multifactorial diseases such as BV over studies that often rely on a single sample collected from women presenting to their physician with symptomatic BV. Longitudinal study designs, where samples are collected frequently along with detailed behavioral metadata would afford access to samples collected prior to the diagnosis and during the events leading to BV. The knowledge gained from such studies is expected to factors that govern this dynamic ecosystem, to forecast symptomatic BV susceptibility, and enable the development of innovative diagnostic, intervention and prevention strategies.
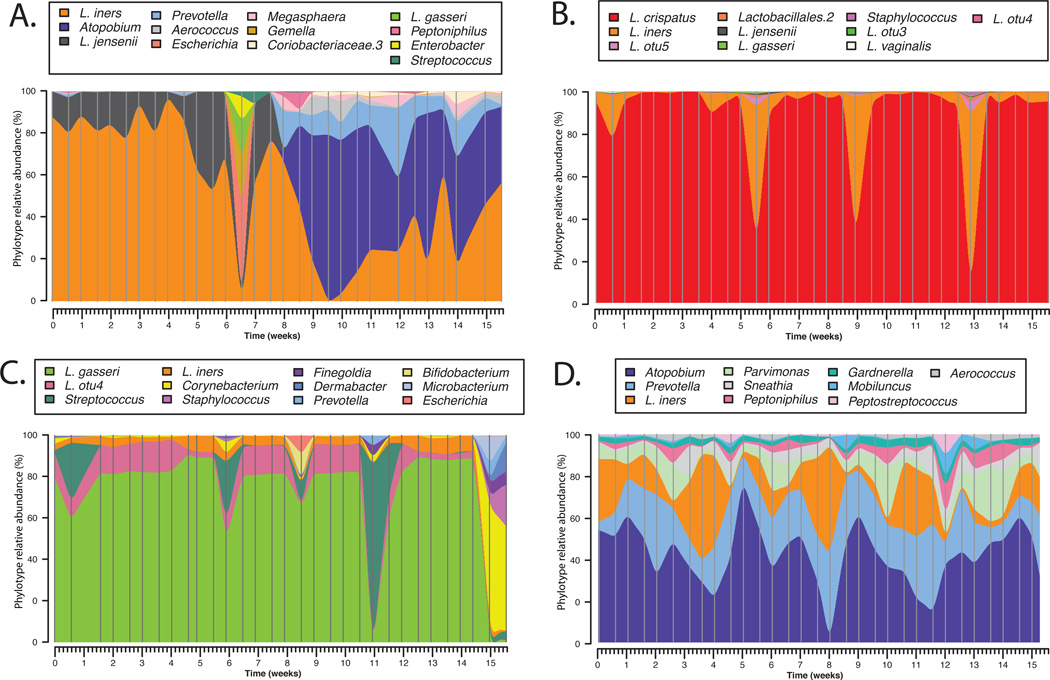
Temporal dynamics of vaginal bacterial communities in women sampled twice-weekly over 16 weeks. Interpolated bar plot of phylotypes relative abundance for four subjects (A–D) with different community dynamics profiles. Color key for each phylotype is shown on top each graph. Vertical lines represents sampling time points.
Toward a system-level understanding of the vaginal ecosystem
While comprehensive molecular community surveys have provided a great deal of information on the composition of the vaginal microbiota, our understanding of it role and the intrinsic dynamics that drive its interaction with its host are still unknown. In order to have translational impact on women’s health, it is essential to develop an understanding of healthy and disease states that include knowledge the functional characteristics of vaginal microbiota and the types of vaginal microbiota that can provide the needed functions. For example, the notion of an “enterotype” in the gut microbiome is defined not by the presence of a core set of organisms, but a core set of available conserved genes that are involved in critical metabolic pathways (7; 86; 108; 109). This notion is informative for subject stratification, but still limited to an understanding of the functional potential of a microbial community, and not of its true function and benefit to the host. State-of-the-art ‘omics’ technologies combined with statistical modeling framework offer an opportunity to develop systems-level understanding of the vaginal ecosystem by measuring biological components of a system to derive functional modules that reflect specific phenotypic traits. This could be accomplished using a multi-level approach based on the following approaches: metagenomics to catalogue (1) the relative abundance of all microbial and to some extent human genes and their polymorphisms; (2) the functional potentials and their degree of redundancy; and (3) metatranscriptomics and metaproteomics to assess levels of differential expression of microbial and host genes in health and disease states or in response to various perturbations. This would also provide insight to the functional interaction between the vaginal microbiota and the host using metabolomics to characterize the products of ecosystemlevel physiological processes and metabolic output. Predictive statistical models used in a systems biology framework could be used to integrate these various datasets and to quantitatively assess critical biological processes, environmental conditions, or behaviors associated with healthy states, as well as disease initiation, progression and symptomatology (40; 63; 68; 79). The goal of these efforts would be to develop a system-level understanding of the molecular events that promote health or lead to disease, and spur the development of novel diagnostic screens and enable more holistic prevention and treatment regimens.
CONCLUDING REMARKS
The dynamics of vaginal bacterial communities over the menstrual cycle, and the dramatic changes associated with transitions between the physiological stages of a women’s life span, from the first week of life to puberty, the reproductive years, and menopause is a reflection of the interplay of the mutualistic relationship between the vaginal microbiota and its human host. The ever-changing yet finely-tuned vagina ecosystem is result of adaptive co-evolutionary processes that integrates many different aspects such as sexual hormone levels, features of host physiology, as well as composition and functional output of the vaginal microbiota. Understanding the system-level temporal dynamics of the vaginal ecosystem and its functional output would contribute to understanding of what truly constitutes ‘normal’ and ‘healthy’. It is expected that the application of the modern –omics technologies to the study of the vaginal ecosystem will translate into better diagnostics and improved personalized treatments.
ACKNOWLEDGMENTS
This work was supported by the National Institute of Allergies and Infectious Diseases, National Institutes of Health (grant number U19 AI084044, UO1 AI070921 and UH2AI083264).
Glossary
| Microbiome | microbial community gene content |
| Microbiota | microbial community composition and structure |
| Phylotype | Community members represented by a set of 16S rRNA gene sequences phylogenetically related |
| Community states | Phylotypes composition and relative abundance of a single sample or a sample in a time series |
| Community state types | Clusters of community states that have similar phylotypes composition and relative abundance |
| Community class | Clusters of community state types profiles that have similar temporal pattern of bacterial community dynamics (applies to time series) |
| Resilience | Capability of an ecosystem to regain its previous state after disturbance |
| Stability | Capability of an ecosystem to resist change in the face of disturbance |
| Nugent-score BV | BV that is diagnosed based on Nugent Gram-stain test, symptomology is not taken into account |
LITERATURE CITED
Full text links
Read article at publisher's site: https://doi.org/10.1146/annurev-micro-092611-150157
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3780402?pdf=render
Subscription required at AnnualReviews
http://arjournals.annualreviews.org/doi/pdf/10.1146/annurev-micro-092611-150157
Subscription required at AnnualReviews
http://arjournals.annualreviews.org/doi/full/10.1146/annurev-micro-092611-150157
Free to read at AnnualReviews
http://arjournals.annualreviews.org/doi/abs/10.1146/annurev-micro-092611-150157
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1146/annurev-micro-092611-150157
Article citations
Urogenital colonization and pathogenicity of E. Coli in the vaginal microbiota during pregnancy.
Sci Rep, 14(1):25523, 26 Oct 2024
Cited by: 0 articles | PMID: 39462143 | PMCID: PMC11513020
Association between gut microbiota, plasma metabolites, and ovarian cancer: A Mendelian randomization study.
Medicine (Baltimore), 103(45):e40479, 01 Nov 2024
Cited by: 0 articles | PMID: 39533575 | PMCID: PMC11556969
The cAMP receptor protein from Gardnerella vaginalis is not regulated by ligands.
Commun Biol, 7(1):1233, 01 Oct 2024
Cited by: 0 articles | PMID: 39354127 | PMCID: PMC11445507
The In Vivo Effect of Water-Based Lubricants on the Vaginal Microbiome of Women from Varying Age Groups: Exploratory Analysis of a Randomized Controlled Trial.
Microorganisms, 12(9):1917, 20 Sep 2024
Cited by: 0 articles | PMID: 39338590 | PMCID: PMC11434374
Strong evidence supports the use of estradiol therapy for the treatment of vaginal inflammation: a two-way Mendelian randomization study.
Eur J Med Res, 29(1):339, 18 Jun 2024
Cited by: 0 articles | PMID: 38890725 | PMCID: PMC11186076
Go to all (358) article citations
Other citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Vaginal microbiome.
Ceska Gynekol, 83(5):371-379, 01 Jan 2018
Cited by: 31 articles | PMID: 30848142
Review
Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria.
PLoS One, 7(6):e37818, 18 Jun 2012
Cited by: 353 articles | PMID: 22719852 | PMCID: PMC3377712
Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women.
PLoS One, 8(1):e54379, 23 Jan 2013
Cited by: 137 articles | PMID: 23372716 | PMCID: PMC3553157
Molecular methods to describe the spectrum and dynamics of the vaginal microbiota.
Anaerobe, 17(4):191-195, 03 Mar 2011
Cited by: 45 articles | PMID: 21376827 | PMCID: PMC3126881
Funding
Funders who supported this work.
NIAID NIH HHS (4)
Grant ID: U01 AI070921
Grant ID: UO1 AI070921
Grant ID: U19 AI084044
Grant ID: UH2 AI083264





