Abstract
Free full text

Wnt Signaling in Vertebrate Axis Specification
Abstract
The Wnt pathway is a major embryonic signaling pathway that controls cell proliferation, cell fate, and body-axis determination in vertebrate embryos. Soon after egg fertilization, Wnt pathway components play a role in microtubule-dependent dorsoventral axis specification. Later in embryogenesis, another conserved function of the pathway is to specify the anteroposterior axis. The dual role of Wnt signaling in Xenopus and zebrafish embryos is regulated at different developmental stages by distinct sets of Wnt target genes. This review highlights recent progress in the discrimination of different signaling branches and the identification of specific pathway targets during vertebrate axial development.
Wnt pathways play major roles in cell-fate specification, proliferation and differentiation, cell polarity, and morphogenesis (Clevers 2006; van Amerongen and Nusse 2009). Signaling is initiated in the responding cell by the interaction of Wnt ligands with different receptors and coreceptors, including Frizzled, LRP5/6, ROR1/2, RYK, PTK7, and proteoglycans (Angers and Moon 2009; Kikuchi et al. 2009; MacDonald et al. 2009). Receptor activation is accompanied by the phosphorylation of Dishev-elled (Yanagawa et al. 1995), which appears to transduce the signal to both the cell membrane and the nucleus (Cliffe et al. 2003; Itoh et al. 2005; Bilic et al. 2007). Another common pathway component is β-catenin, an abundant component of adherens junctions (Nelson and Nusse 2004; Grigoryan et al. 2008). In response to signaling, β-catenin associates with T-cell factors (TCFs) and translocates to the nucleus to stimulate Wnt target gene expression (Behrens et al. 1996; Huber et al. 1996; Molenaar et al. 1996).
This β-catenin-dependent activation of specific genes is often referred to as the “canonical” pathway. In the absence of Wnt signaling, β-catenin is destroyed by the protein complex that includes Axin, GSK3, and the tumor suppressor APC (Clevers 2006; MacDonald et al. 2009). Wnt proteins, such as Wnt1, Wnt3, and Wnt8, stimulate Frizzled and LRP5/6 receptors to inactivate this β-catenin destruction complex, and, at the same time, trigger the phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2 (HIPK2) (Hikasa et al. 2010; Hikasa and Sokol 2011). Both β-catenin stabilization and the regulation of TCF protein function by phosphorylation appear to represent general strategies that are conserved in multiple systems (Sokol 2011). Thus, the signaling pathway consists of two branches that together regulate target gene expression (Fig. 1).
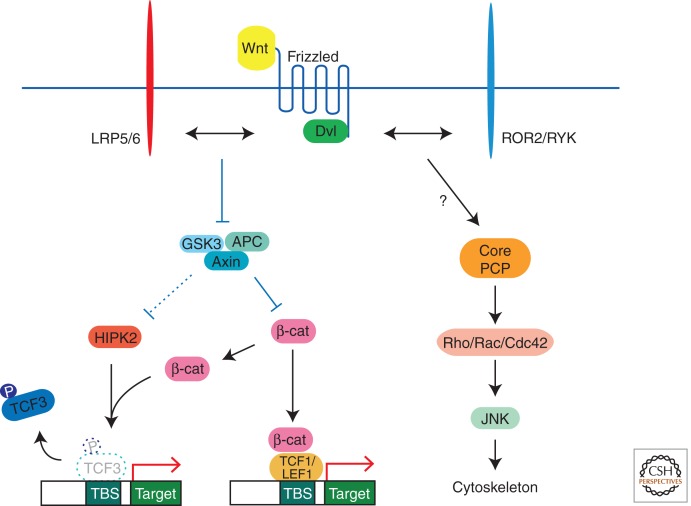
Conserved Wnt pathway branches and components. In the absence of Wnt signals, glycogen synthase kinase 3 (GSK3) binds Axin and APC to form the β-catenin destruction complex. Some Wnt proteins, such as Wnt8 and Wnt3a, stimulate Frizzled and LRP5/6 receptors to inhibit GSK3 activity and stabilize β-catenin (β-cat). Stabilized β-cat forms a complex with T-cell factors (e.g., TCF1/LEF1) to activate target genes. Moreover, GSK3 inhibition leads to target gene derepression by promoting TCF3 phosphorylation by homeodomain-interacting protein kinase 2 (HIPK2) through an unknown mechanism, for which β-catenin is required as a scaffold. This phosphorylation results in TCF3 removal from target promoters and gene activation. Other Wnt proteins, such as Wnt5a and Wnt11, use distinct receptors such as ROR2 and RYK, in addition to Frizzled, to control the the cytoskeletal organization through core planar cell polarity (PCP) proteins, small GTPases (Rho/Rac/Cdc42), and c-Jun amino-terminal kinase (JNK).
Other Wnt proteins, such as Wnt5a or Wnt11, strongly affect the cytoskeletal organization and morphogenesis without stabilizing β-catenin (Torres et al. 1996; Angers and Moon 2009; Wu and Mlodzik 2009). These “noncanonical” ligands do not influence TCF3 phosphorylation (Hikasa and Sokol 2011), but may use distinct receptors such as ROR1/2 and RYK instead of or in addition to Frizzled (Hikasa et al. 2002; Lu et al. 2004; Mikels and Nusse 2006; Nishita et al. 2006, 2010; Schambony and Wedlich 2007; Grumolato et al. 2010; Lin et al. 2010; Gao et al. 2011). In such cases, signaling mechanisms are likely to include planar cell polarity (PCP) components, such as Vangl2, Flamingo, Prickle, Diversin, Rho GTPases, and c-Jun amino-terminal kinases (JNKs), which do not directly affect β-catenin stability (Fig. 1) (Sokol 2000; Schwarz-Romond et al. 2002; Schambony and Wedlich 2007; Komiya and Habas 2008; Axelrod 2009; Itoh et al. 2009; Tada and Kai 2009; Sato et al. 2010; Gao et al. 2011). This simplistic dichotomy of the Wnt pathway does not preclude some Wnt ligands from using both β-catenin-dependent and -independent routes in a context-specific manner.
Despite the existence of many pathway branches, only the β-catenin-dependent branch has been implicated in body-axis specification. Recent experiments in lower vertebrates have identified additional pathway components and targets and provided new insights into the underlying mechanisms.
ROLE OF CYTOPLASMIC DETERMINANTS AND WNT SIGNALING IN DORSOVENTRAL AXIS SPECIFICATION
The specification of the dorsoventral axis coincides with the formation of an essential embryonic signaling center known as the Spemann organizer in amphibians, the shield in zebrafish, and the node in the mouse (Harland and Gerhart 1997; Schier and Talbot 2005; Marlow 2010). During egg fertilization, sperm entry triggers microtubule-dependent rearrangement of the cortical cytoplasm from the vegetal pole toward the future dorsal side, a process known as the cortical rotation (Houliston and Elinson 1992). Experiments with cytoplasm microinjections or removal revealed vegetally localized dorsalizing activity that moves to the dorsal region before the first cleavage in Xenopus or zebrafish eggs (Fujisue et al. 1993; Holowacz and Elinson 1993; Mizuno et al. 1999; Ober and Schulte-Merker 1999). Treatment of fertilized Xenopus or zebrafish eggs with microtubule-depolymerizing agents, such as UV irradiation or nocodazole, causes embryo ventralization (Chung and Malacinski 1980; Scharf and Gerhart 1980; Jesuthasan and Stahle 1997). These observations indicated that the movement of the dorsalizing activity is essential for the establishment of the primary dorsoventral axis in both Xenopus and zebrafish.
One of the important outcomes of the cortical rotation is the accumulation of β-catenin in dorsal nuclei of early blastulae, whereas β-catenin remains largely cytoplasmic and cortical in ventral cells (Schneider et al. 1996; Schohl and Fagotto 2002). This difference in β-catenin localization can be detected as early as the 2- to 4-cell stage (Larabell et al. 1997). Supporting the critical role of this localization for dorsal development, several maternal effect mutants in zebrafish such as hecate, ichabod, and tokkaebi result in the reduction of β-catenin dorsal accumulation and defects in dorsal development (Kelly et al. 2000; Nojima et al. 2004; Lyman Gingerich et al. 2005; Bellipanni et al. 2006). The ichabod mutation has been mapped to the proximity of the gene encoding β-catenin-2, a second zebrafish β-catenin that is essential for maternal Wnt signaling (Bellipanni et al. 2006). Consistent with the ventralized phenotype of the ichabod embryos, maternal β-catenin-2 RNA is down-regulated. Hecate is another zebrafish mutation that is manifested by deficient dorsal development and enhanced intracellular calcium release; however, the mutated gene has not yet been identified (Lyman Gingerich et al. 2005). Positional cloning revealed that the tokkaebi gene encodes syntabulin, a microtubule-binding protein connecting kinesin I to syntaxin-containing cargo vesicles (Nojima et al. 2010). This finding further implicates microtubule-dependent trafficking in dorsoventral axis specification. Reminiscent of the key function of β-catenin in Xenopus and zebrafish, mouse embryos lacking β-catenin reveal abnormal visceral endoderm patterning before gastrulation and do not form the primitive streak (Haegel et al. 1995; Huelsken et al. 2000).
An essential role for Wnt/β-catenin signaling in dorsoventral axis determination has been initially suggested in gain-of-function experiments, in which Wnt and Dishevelled messenger RNAs (mRNAs) triggered ectopic organizer formation (McMahon and Moon 1989; Smith and Harland 1991; Sokol et al. 1991, 1995; Rothbacher et al. 1995). The requirement for β-catenin has been established by antisense oligonucleotide-mediated depletion of β-catenin in Xenopus and zebrafish embryos (Heasman et al. 1994; Bellipanni et al. 2006). β-catenin activates several major targets in Xenopus embryos that are responsible for Spemann organizer formation. Activation of many organizer-specific genes depends on functional TCF sites in their promoters (Brannon et al. 1997; Laurent et al. 1997; McKendry et al. 1997; Fan et al. 1998; Ryu et al. 2001; Leung et al. 2003). These early β-catenin targets include Nodal group genes that are essential for dorsal mesoderm formation in frogs, zebrafish, and mammals (Conlon et al. 1994; Feldman et al. 1998; Osada and Wright 1999; Takahashi et al. 2000; Yang et al. 2002; Hilton et al. 2003; Zhang et al. 2003). Besides Nodals, other direct targets of β-catenin are Siamois and Twin in Xenopus (Lemaire et al. 1995; Brannon et al. 1997; Laurent et al. 1997; Fan et al. 1998), and Bozozok/Dharma/Nieuwkoid in zebrafish (Ryu et al. 2001), which are required for organizer formation (Fig. 2; Table 1) (Fan and Sokol 1997; Kessler 1997; Koos and Ho 1998; Yamanaka et al. 1998; Fekany et al. 1999). Taken together, available data establish a key role for Wnt signaling and its early targets in early dorsoventral axis specification (Moon and Kimelman 1998; Schroeder et al. 1999; Sokol 1999; Tao et al. 2005).
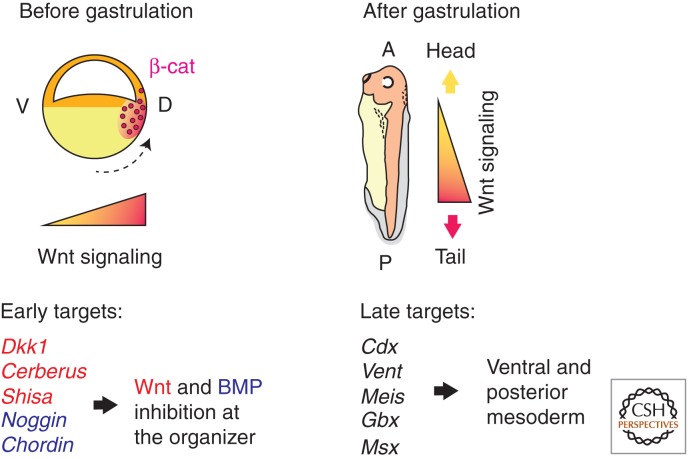
Axis specification by early and late Wnt signaling involves distinct targets. After cortical rotation (black dotted arrow), early β-catenin accumulation in the dorsal equatorial region activates gene targets to generate the Spemann organizer. β-Catenin up-regulates several Wnt and bone morphogenetic protein (BMP) antagonists, including Dkk1, Cerberus, Shisa, Noggin, and Chordin. The pathway is inhibited by Wnt antagonists in the anterior tissues, but the zygotic activation of Wnt8 causes ventral and posterior accumulation of β-catenin during gastrulation. The target genes of this late Wnt signaling, including Cdx, Vent, Meis, Gbx, and Msx, are critical for the specification of ventroposterior mesodermal fates and lead to tail formation.
Table 1.
Putative Wnt targets during anteroposterior axis specification
| Genes activated by Wnt3a | Genes inhibited by Dickkopf-1 | References |
|---|---|---|
| Axin1/2 | Axin1/2 | Zeng et al. 1997; Jho et al. 2002 |
| Kremen2 | Kremen2 | Mao et al. 2002 |
| Cdx2, 4 | Cdx2, 4 | Isaacs et al. 1998 |
| Gbx2 | Gbx2 | von Bubnoff et al. 1996 |
| HoxA1/D1 | HoxA1/D1 | Kolm and Sive 1995 |
| Esr9/10 | Esr9/10 | Li et al. 2003 |
| Fz10 | Fz10 | Wheeler and Hoppler 1999; Garcia-Morales et al. 2009 |
| Foxi1 | Suri et al. 2005; Mir et al. 2007 | |
| Meis3 | Meis-like | Elkouby et al. 2010 |
| Msx1/2 | Msx1/2 | Maeda et al. 1997; Marazzi et al. 1997; Mathers et al. 1997; Suzuki et al. 1997; Willert et al. 2002 |
| Nzl1 | Nzl1 | Andreazzoli et al. 2001; Nakamura et al. 2008 |
| Nrh1 | Bromley et al. 2004; Sasai et al. 2004 | |
| Riddle2 | Shibata et al. 2008 | |
| Spr2 | Weidinger et al. 2005 | |
| Vent/PV1 | Vent/PV1 | Onichtchouk et al. 1996 |
| XARP | XARP | Itoh et al. 2000 |
| Xmc | Frazzetto et al. 2002 | |
| Xpo | Xpo | Sato and Sargent 1991; Amaya et al. 1993; Hoppler et al. 1996 |
Only some of these genes have been shown to contain TCF/LEF sites in their promoters.
Although lack of effect of a dominant–negative form of Dishevelled (Dvl) on axis specification does not eliminate Dvl participation; it suggests that β-catenin is stabilized by a downstream Wnt pathway component that is translocated dorsally during the cortical rotation (Sokol 1996; Harland and Gerhart 1997). One of the key factors is GSK3-binding protein or Frat, which inhibits GSK3 and is essential for dorsal development in Xenopus embryos (Yost et al. 1998). However, the triple knockout of the existing Frat homologs in the mouse does not produce a morphological phenotype, suggesting that this mechanism is not conserved in mammals (van Amerongen et al. 2005). More recently, antisense oligonucleotide-mediated depletion of maternal Wnt5a, Wnt11, and Fz7 indicated their roles in dorsoventral axis specification (Sumanas et al. 2000; Tao et al. 2005; Cha et al. 2008). These observations suggest a different model, in which Wnt5a and Wnt11 influence β-catenin levels in the oocyte, raising the question how β-catenin becomes localized to the dorsal side of the embryo. Because maternal Wnt11 RNA is vegetally localized (Ku and Melton 1993), one possibility is that Wnt11 is delivered to its future site of action by the cortical rotation (Tao et al. 2005). So far, dorsoventral patterning defects have not been described in embryos with genetically reduced Wnt5a or Wnt11 function (Rauch et al. 1997; Yamaguchi et al. 1999; Heisenberg et al. 2000; Kilian et al. 2003; Majumdar et al. 2003; Ulrich et al. 2003; Fossat et al. 2011). Potentially, these observations could be explained by functional redundancy or by the presence of maternally encoded proteins. On the other hand, overexpression of Wnt5a or Wnt11 does not lead to β-catenin stabilization (Torres et al. 1996; Mikels and Nusse 2006). Owing to these contradictory observations, the final conclusion regarding the involvement of these Wnt proteins in β-catenin stabilization and body-axis formation awaits additional experimental insights. Despite the variability in the existing models of vertebrate axis specification, there is a common outcome: the establishment of a signaling center that dorsalizes all three germ layers and mediates anteroposterior patterning.
ZYGOTIC WNT SIGNALING DURING ANTEROPOSTERIOR AXIS SPECIFICATION
Early studies revealed that the effect of Wnt proteins on embryogenesis is stage specific. When injected as mRNA, Wnt8 triggers secondary axis formation (Smith and Harland 1991; Sokol et al. 1991). However, when supplied as a plasmid, Wnt8 expression is initiated only at midblastula transition and the delayed stimulation leads to posteriorization, characterized by lack of head structures (Christian and Moon 1993). These observations correlated well with the time-sensitive effect of lithium chloride, a GSK3 inhibitor (Klein and Melton 1996), on anteroposterior patterning (Yamaguchi and Shinagawa 1989). Of note, Dkk1 RNA does not interfere with the early dorsal signaling, although being fully capable of inhibiting ectopic Wnt expression (Glinka et al. 1998; Brott and Sokol 2002), consistent with the idea that Wnt ligands are not accessible to Dkk-dependent inhibition at this early stage. These observations indicate that Wnt signaling plays different roles in axis specification at different developmental stages.
Several Wnt ligands, including Wnt3a, Wnt5a, Wnt8, and Wnt11, are expressed in the ventral or posterior region of the embryo (Christian et al. 1991a; Krauss et al. 1992; Moon et al. 1993; Kelly et al. 1995; Hong et al. 2008). On the other hand, multiple Wnt antagonists, such as Frzb/Sfrp3, Crescent, Shisa, and Dkk1, are expressed in the head region (Leyns et al. 1997; Wang et al. 1997; Glinka et al. 1998; Shibata et al. 2005; Yamamoto et al. 2005). This complementary expression pattern indicates a function for the Wnt pathway in anteroposterior axis specification. The involvement of Wnt signaling in anteroposterior patterning was first revealed in studies with overexpressed Wnt pathway modulators. Twofold titration of Xenopus Dishevelled RNA caused graded appearance of region-specific positional markers, indicating a morphogenlike dose response with the highest point at the posterior of the embryo (Itoh and Sokol 1997). Wnt pathway antagonists, such as GSK3, Dkk1, and Shisa, cause anteriorization, in agreement with the proposed posteriorizing activity of Wnt ligands (Itoh et al. 1995; Glinka et al. 1998; Yamamoto et al. 2005). Further supporting this hypothesis, TCF-dependent reporter activity and the nuclear localization of endogenous β-catenin are elevated in the anterior-to-posterior gradient (Kiecker and Niehrs 2001; Dorsky et al. 2002).
The critical role of Wnt signaling in anteroposterior axis specification has been corroborated by depletion experiments for pathway components (Heasman et al. 2000; Erter et al. 2001; Lekven et al. 2001; Shimizu et al. 2005; Bellipanni et al. 2006; Hikasa et al. 2010) and the analysis of the corresponding zebrafish mutations. Headless (hdl) was identified as a point mutation in a tcf3 gene homolog, which represses Wnt target genes in zebrafish embryos (Kim et al. 2000). Loss of forebrain and eyes and the modest expansion of ventral mesoderm in hdl embryos are consistent with up-regulated Wnt signaling. When both Headless and a closely related protein Tcf3b are down-regulated, this posteriorization is more severe (Dorsky et al. 2003). Similarly, mastermind zebrafish embryos with a mutation in Axin1 are posteriorized with telencephalon and eyes transformed to diencephalon (Heisenberg et al. 2001). Thus, interference with Wnt pathway antagonists mimics Wnt protein overexpression and results in posteriorization. Phenotypes of mice lacking the genes for Dkk1, APC, TCF3, Wnt3, ICAT, and β-catenin also support the role of the Wnt pathway in posterior development (Takada et al. 1994; Liu et al. 1999b; Mukhopadhyay et al. 2001; Ishikawa et al. 2003; Merrill et al. 2004; Satoh et al. 2004). Combined with more recent studies of Cnidaria and Planaria (Holstein 2008; Martin and Kimelman 2009; Petersen and Reddien 2009), these findings establish a conserved key role for Wnt signaling in anteroposterior axis specification (Fig. 2).
PUTATIVE TRANSCRIPTIONAL TARGETS OF THE WNT PATHWAY IN AXIS SPECIFICATION
Molecular screens have identified transcriptional Wnt targets in early embryos and embryonic stem cells (Willert et al. 2002; Li et al. 2004; Hufton et al. 2006). A major obstacle to identifying Wnt gene targets is the stage and context dependence of cellular responses to Wnt ligands. Therefore, a special effort is needed to elucidate the Wnt targets that are relevant to anteroposterior axis specification.
Hybridization of RNA probes prepared from the control and Wnt3a-stimulated ectoderm cells with Xenopus copy DNA (cDNA) microarrays (Affymetrix) has identified a number of candidate Wnt targets (Table 1) (H Hikasa and SY Sokol, unpubl.). Among these are Wnt pathway components and modulators, such as Fz9, Fz10, Kremen 2, and the Axin family, highlighting the need for feedback regulation at this developmental stage. A second class contains many of the previously identified Wnt targets that are expressed ventrally or posteriorly. These genes are Gbx2 (von Bubnoff et al. 1996), the Cdx group (Epstein et al. 1997; Haremaki et al. 2003; Shimizu et al. 2005; Keenan et al. 2006; Pilon et al. 2006), the Vent/Vox/Ved/Xom group (Gawantka et al. 1995; Ladher et al. 1996; Onichtchouk et al. 1996; Schmidt et al. 1996; Imai et al. 2001; Ramel and Lekven 2004; Thorpe and Moon 2004), and the Meis group (Meis3) (Choe et al. 2009; Elkouby et al. 2010). The remaining genes include some known bone morphogenetic protein (BMP)- and fibroblast growth factor (FGF)-inducible genes, reiterating the importance of the cross talk between these pathways, and novel putative Wnt targets (Table 1). Whereas some of these genes may be activated indirectly, Meis3, Gbx2, Cdx4, and Vent1/2 are known to contain functional TCF-binding sites in their promoters that are required for Wnt responsiveness (Haremaki et al. 2003; Li et al. 2009; Elkouby et al. 2010; Hikasa et al. 2010). Supporting these findings, a complementary analysis revealed a similar list of genes that are inhibited in ventral mesoderm by Dkk1 (Table 1) (Hufton et al. 2006).
The Cdx genes, a group of four conserved vertebrate homologes of Drosophila caudal, have been widely implicated in posterior development (Northrop and Kimelman 1994; Isaacs et al. 1998; Pillemer et al. 1998; van den Akker et al. 2002; Shimizu et al. 2005, 2006; Faas and Isaacs 2009; Young and Deschamps 2009; Young et al. 2009; Beck and Stringer 2010). Loss of the Cdx4 gene function in the zebrafish mutant kugelig shows its essential role in posterior development (Davidson et al. 2003). The promoter of the Xenopus and mammalian Cdx4 gene is Wnt inducible and contains multiple TCF/LEF binding sites (Haremaki et al. 2003; Shimizu et al. 2005; Pilon et al. 2006). Up-regulation of Cdx genes causes the anterior shift of posterior markers in Xenopus and zebrafish (Isaacs et al. 1998; Shimizu et al. 2005; Faas and Isaacs 2009). Similar to Cdx genes, Vent family proteins (Vent/Vox/Ved/Xom) in Xenopus and zebrafish are homeodomain-containing transcriptional repressors that promote ventroposterior fates by suppressing organizer genes such as bozozok (in zebrafish), chordin, and goosecoid (Schmidt et al. 1996; Melby et al. 1999, 2000; Imai et al. 2001; Ramel and Lekven 2004; Sander et al. 2007). Inactivation of Vent genes results in a severe loss of ventroposterior structures and enhanced dorsoanterior fate, similar to the Wnt8 knockdown (Ramel and Lekven 2004; Sander et al. 2007). Together, these findings show that the Wnt pathway controls posterior development through multiple gene targets.
Putative Wnt target genes that are involved in anteroposterior patterning differ from the early β-catenin targets that mark the Spemann organizer region. These organizer-specific genes include Xenopus Siamois (Lemaire et al. 1995; Brannon et al. 1997; Fan et al. 1998) and Twin (Laurent et al. 1997), zebrafish Bozozok/dharma/nieuwkoid (Koos and Ho 1998; Yamanaka et al. 1998; Fekany et al. 1999), Xnr3 (Smith et al. 1995), Xnr5/6 (Takahashi et al. 2000; Yang et al. 2002) and several Wnt antagonists: Frzb/Sfrp3 (Leyns et al. 1997), Dkk1 (Glinka et al. 1998), Crescent (Shibata et al. 2000), Cerberus (Bouwmeester et al. 1996), Shisa (Yamamoto et al. 2005), as well as other genes listed in Table 2. The difference between the early and late targets for the Wnt pathway corresponds to the switch between stage-specific Wnt signaling mechanisms that are critical for vertebrate axis specification. For additional information about Wnt targets in different experimental systems, the reader is referred to Roel Nusse’s Wnt Window (http://www.stanford.edu/group/nusselab/cgi-bin/wnt/target_genes).
Table 2.
Putative Wnt targets during dorsoventral axis specification
| Genes activated by maternal β-catenin | References |
|---|---|
| Siamois | Lemaire et al. 1995; Brannon et al. 1997; Fan et al. 1998 |
| Twin | Laurent et al. 1997 |
| Xnr3 | Smith et al. 1995 |
| Goosecoid | Blumberg et al. 1991; Cho et al. 1991 |
| Cerberus | Bouwmeester et al. 1996 |
| Chordin | Sasai et al. 1994 |
| Crescent | Shibata et al. 2000; Shibata et al. 2001 |
| Noggin | Smith and Harland 1992 |
| Xlim-1 | Taira et al. 1994 |
| Xnr5/6 | Takahashi et al. 2000; Yang et al. 2002 |
| Xnot1 | von Dassow et al. 1993 |
| Shisa | Yamamoto et al. 2005 |
| Dkk1 | Glinka et al. 1998 |
| Frzb | Leyns et al. 1997 |
| Frizzled 8 | Deardorff et al. 1998; Itoh et al. 1998a |
| Bozozok | Koos and Ho 1998; Yamanaka et al. 1998; Fekany et al. 1999 (zebrafish-specific, also known as Dharma and Nieuwkoid) |
Known antagonists of Wnt and BMP signaling are shown in bold.
REGULATION OF TCF PROTEIN FUNCTION DURING AXIS SPECIFICATION
How does Wnt signaling lead to target gene activation during anteroposterior patterning? A critical event seems to be TCF3 phosphorylation by HIPK2 allowing one to derepress the target genes (Hikasa et al. 2010; Sokol 2011), although in some cases TCF3 is likely to function in a Wnt-independent manner (Cole et al. 2008; Gribble et al. 2009). Homeodomain-interacting protein kinases (HIPKs) have been shown to function in a context-dependent manner in response to Wnt signaling (Wei et al. 2007; Lee et al. 2009; Louie et al. 2009; Kim et al. 2010). Loss-of-function studies reveal that HIPK2 is required in vivo for Wnt8-dependent ventroposterior development and antagonizes the function of TCF3 in axis specification (Hikasa et al. 2010). Besides HIPK2, TCF3 phosphorylation requires β-catenin. TCF3 with a mutation that disrupts β-catenin binding is not phosphorylated (Hikasa et al. 2010). Thus, β-catenin appears to act by providing a scaffold for TCF3 phosphorylation by HIPK2. This is a novel function of β-catenin that differs from its commonly proposed role as transcriptional coactivator (Behrens et al. 1996; van de Wetering et al. 1997; Cavallo et al. 1998; Daniels and Weis 2005). Although TCF3 phosphorylation has been shown to require LRP6 activity and GSK3 inhibition (Hikasa and Sokol 2011), the mechanistic role of GSK3 in HIPK2 activation remains a mystery.
Nemolike kinase (Nlk) has been previously reported to phosphorylate TCF proteins (Ishitani et al. 1999; Meneghini et al. 1999) and is a positive regulator of Wnt8 signaling in anteroposterior patterning in zebrafish embryos (Thorpe and Moon 2004). In mammalian cells, Nlk and HIPK2 have been shown to regulate the stability of Myb oncoprotein in response to Wnt1 (Kanei-Ishii et al. 2004), indicating that these kinases might also function together during axis specification. The pathway leading to TCF regulation in vertebrates is reminiscent of Wnt signaling in C. elegans, because LIT-1, a homolog of Nlk, and WRM-1, a β-catenin paralog, phosphorylates POP-1/TCF to enhance its nuclear export, leading to transcriptional derepression (Rocheleau et al. 1997, 1999; Meneghini et al. 1999; Lo et al. 2004). Because POP-1 plays a dual role in gene target regulation, the reader is referred to other reviews for additional information (Phillips and Kimble 2009; Sokol 2011).
Chromatin precipitation analysis has shown that phosphorylated TCF3 is removed from target promoters, resulting in gene activation (Hikasa et al. 2010). Subsequent experiments showed that Vent2 gene activation is accompanied by the removal of TCF3 from the promoter and its replacement by TCF1, an activator form of TCF that is resistant to HIPK2-mediated phosphorylation (Hikasa and Sokol 2011). This finding strongly supports the view that the four vertebrate homologs of TCF, expressed in a tissue-specific manner (Molenaar et al. 1998; Dorsky et al. 1999, 2003; Konig et al. 2000; Roel et al. 2003; Veien et al. 2005), play diverse roles in Wnt signaling and axis specification (Hamilton et al. 2001; Houston et al. 2002; Roel et al. 2002; Liu et al. 2005; Standley et al. 2006). Although both TCF3 and TCF1 can bind β-catenin and Groucho corepressors (Roose et al. 1998; Brantjes et al. 2001), the two proteins appear to function very differently. TCF3 serves exclusively as a transcriptional repressor, whereas TCF1 is a transcriptional activator (Kim et al. 2000; Gradl et al. 2002; Houston et al. 2002; Merrill et al. 2004; Liu et al. 2005; Nguyen et al. 2006; Gribble et al. 2009; Hikasa and Sokol 2011). Additional studies are needed to explain the difference in the activity of TCF1 and TCF3, which is likely caused by a distinct set of interacting partners.
PATHWAY SWITCH MECHANISMS
Whereas the early dorsal accumulation of β-catenin is essential for the activation of organizer genes, it is later followed by the stabilization of β-catenin ventrally (Kiecker and Niehrs 2001; Schohl and Fagotto 2002; Hikasa et al. 2010). This reversed distribution of β-catenin correlates with the ventroposterior expression of Wnt8 (Christian et al. 1991a,b; Kelly et al. 1995; Lekven et al. 2001) and dorsoanterior expression of several Wnt antagonists in both Xenopus and zebrafish (Fig. 2). Thus, a likely explanation for this change is that dorsal β-catenin stimulates the transcription of pathway antagonists, such as Dkk1, Shisa, FrzB, and, likely, Axin family proteins (Leyns et al. 1997; Glinka et al. 1998; Yamamoto et al. 2005). The negative feedback from Wnt antagonists decreases β-catenin in the dorsoanterior region, whereas ventroposterior activity of Wnt8 up-regulates β-catenin ventrally. In agreement with this model, the increased level of β-catenin in ventroposterior tissues relies mostly on Wnt8 activity, rather than dorsally derived signals (Hikasa et al. 2010).
Although the molecular cause for the switch from early dorsal Wnt signaling to late ventroposterior Wnt signaling has not yet been identified, the gene targets for each round of signaling appear to be very different. A number of questions remain to be answered. (1) What prevents late Wnt targets from being activated early at the dorsal side? (2) Why are the early targets silent ventrally at gastrulation when β-catenin is stabilized in response to Wnt8 signals at later stages? A possible explanation of this context dependence is related to epigenetic changes in chromatin that underlie the developmental control of transcription (Li 2002; Kouzarides 2007; Ng and Gurdon 2008; Akkers et al. 2009; Xu et al. 2010). A plasmid reporter for the Siamois gene failed to respond to a constitutive TCF activator after gastrulation (Darken and Wilson 2001), suggesting that the stage-dependent regulation of target genes depends on some downstream components of the pathway. Moreover, β-catenin can modulate histone methylation of poised genes, indicating local epigenetic changes in chromatin (Blythe et al. 2010). Thus, ventroposterior Wnt targets, such as Vent or Cdx, may be poised for activation at a later time, predicting the existence of epigenetic differences in the chromatin state that are critical for axis specification.
Another explanation is that functionally diverse vertebrate TCF proteins are responsible for the early to late transition in Wnt target specificity. Besides the distinct roles of TCF proteins as discussed above, the switch from early to late Wnt signaling can be linked to the phosphorylation of TCF by Wnt8/HIPK2, attenuating target DNA binding. This takes place only after the beginning of gastrulation, likely depending on a factor that becomes available after the onset of zygotic transcription. Notably, Wnt8/HIPK2 signal can trigger the phosphorylation of TCF3, TCF4, and Lef1 (but not TCF1), owing to the conservation of the HIPK2 phosphorylation sites. Thus, context-dependent activation of early and late Wnt target genes may be explained by the opposing action of tissue-specific TCF proteins and their distinct response to Wnt8-mediated phosphorylation (Sokol 2011). Whether this explanation is correct remains to be experimentally tested.
CROSS TALK WITH OTHER PATHWAYS
Organizer-derived posteriorizing signals reach neuroectoderm during gastrulation to specify the anteroposterior axis (Nieuwkoop 1952; Doniach et al. 1992; Holowacz and Sokol 1999). Besides Wnt signaling, this process requires additional pathways, such as BMP and FGF signaling. The region- and stage-specific integration of distinct signals contributes to the differential selection of pathway targets that is crucial for the establishment of embryonic axes. The best known examples are the cross talk between the Wnt pathway and the BMP pathway in the ventroposterior region (Itasaki and Hoppler 2010) or FGF/IGF (insulinlike growth factor) receptor tyrosine kinase signaling dorsally and posteriorly (Schohl and Fagotto 2002; Pera et al. 2003; Kudoh et al. 2004; Marchal et al. 2009).
Both BMP and Wnt signaling are involved in setting up ventroposterior gene expression in vertebrate embryos (Hoppler and Moon 1998; De Robertis and Kuroda 2004; Schier and Talbot 2005). The synergistic function of Wnt8 and BMP4 in ventral mesoderm patterning has been first shown in Xenopus embryos (Hoppler and Moon 1998) and confirmed by genetic studies in zebrafish. Reduced Vent expression in zebrafish embryos depleted of β-catenin or containing a mutation in the Wnt8 gene (Ramel and Lekven 2004; Bellipanni et al. 2006) indicates a requirement for the Wnt pathway in Vent activation. The Wnt8 and Swr (Bmp2) double mutants display a progressive reduction of Vent gene expression and a concomitant expansion of the dorsal domain (Ramel and Lekven 2004; Ramel et al. 2005). The synergy between BMP and Wnt signaling has been documented in many other developmental processes, including bone, kidney, limb, and tooth development (Perantoni 2003; Tucker and Sharpe 2004; Hartmann 2006; Yokoyama 2008; Itasaki and Hoppler 2010).
In contrast to the BMP pathway that is active in the ventral region of the blastula embryo, FGF signaling seems to be essential for dorsal posterior patterning (Amaya et al. 1991; Cox and Hemmati-Brivanlou 1995; Lamb and Harland 1995; Holowacz and Sokol 1999; Schohl and Fagotto 2002; Kudoh et al. 2004; Marchal et al. 2009; Martin and Kimelman 2009). Many Wnt-inducible genes, including Cdx4, Xpo, Meis, and Marginal coil (Xmc), have been previously identified as FGF-responsive genes (Table 1) (Amaya et al. 1993; Frazzetto et al. 2002; Aamar and Frank 2004; Chung et al. 2004; Keenan et al. 2006). In studies using dominant–negative FGF receptor constructs and specific morpholino oligonucleotides, FGF signaling has been reported to be essential for the Wnt pathway to activate its targets during anteroposterior axis development (McGrew et al. 1997; Maegawa et al. 2006; Burks et al. 2009). The cross talk between the Wnt and the FGF pathway has been preserved in many other models, including brain patterning, limb formation, tail regeneration, and lung and kidney morphogenesis (Martinez et al. 1999; Schedl and Hastie 2000; Kawakami et al. 2001; Lupo et al. 2002; Villanueva et al. 2002; Lin and Slack 2008).
The mechanisms underlying these examples of pathway cross talk are being revealed. In some cases two pathways operate sequentially, with one pathway inducing the other. For example, FGF8a signaling transcriptionally activates Wnt8 to promote neural crest development (Fig. 3A) (Hong et al. 2008). Alternatively, two pathways may function in parallel, when a target promoter contains transcription factor binding sites that are specific for each pathway (Fig. 3B). Both Wnt and BMP signals promote ventral fate and directly activate the expression of Vent genes. The Vent2 promoter contains a unique Wnt-responsive TCF-binding site and the Smad and OAZ sites needed for the BMP response (Candia et al. 1997; Hata et al. 2000; Karaulanov et al. 2004; Hikasa et al. 2010). Mutagenesis of individual promoter elements indicates that the Wnt and the BMP pathways separately control the Vent2 gene (Hikasa et al. 2010).
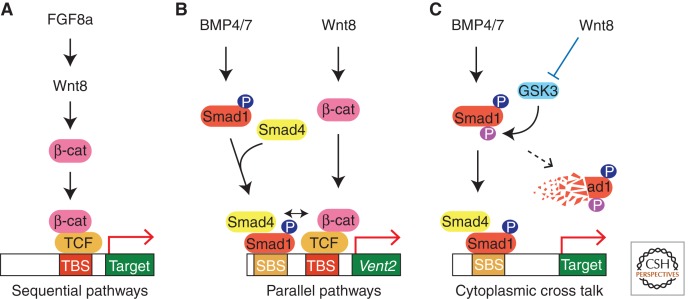
Cross talk between Wnt, BMP, and FGF pathways during axis specification. (A) Sequential interaction of two pathways. FGF8a induces the expression of Wnt8 transcripts to promote neural crest formation in Xenopus neurulae. (B) Parallel interaction of two pathways at a target promoter (Vent2). Vent2 is up-regulated by BMP proteins acting through Smad1 interacting with the Smad binding site (SBS), whereas Wnt proteins act through TCF and the TCF-binding site (TBS). Both signals are responsible for the maximal activation of Vent2. (C) The cytoplasmic integration of two pathways. GSK3 phosphorylates the activated form of Smad1, marking it for degradation. Wnt proteins signal to inhibit or sequester GSK3, thereby promoting BMP target gene activation.
Similarly, a combination of Wnt and FGF signals leads to a robust expression of Cdx4 in Xenopus and zebrafish (Shimizu et al. 2005; Keenan et al. 2006). The regulatory region of the Cdx4 gene contains multiple TCF and Ets DNA-binding elements that are located in close proximity (Haremaki et al. 2003). This structure of the Cdx4 regulatory region explains the observation that Cdx4 is a direct target of both Wnt and FGF pathways, because Ets factors are known to regulate gene expression downstream from FGF (Nentwich et al. 2009; Znosko et al. 2010). This transcriptional cross talk does not preclude a more close interaction, in which two transcription factors interact physically, resulting in a synergistic response (Fig. 3B). The physical interaction of LEF1 with Smad3 and Smad4 may help explain the cross talk between Wnt and TGFβ signaling, relevant to the specification of the primary dorsoventral axis by early Wnt signaling (Sokol and Melton 1992; Sokol 1993; Labbe et al. 2000; Nishita et al. 2000).
A direct cytoplasmic mechanism has been proposed for Wnt-BMP and FGF-BMP pathway cross talk. Smad1 proteins are phosphorylated by MAPK in response to FGF and IGF, targeting Smad1 for degradation by the proteasome (Massague 2003; Pera et al. 2003; Sapkota et al. 2007). Similarly, GSK3 phosphorylates Smad1 and this phosphorylation can be inhibited by Wnt signaling in tissue culture cells (Fig. 3C) (Fuentealba et al. 2007; Sapkota et al. 2007). These phosphorylation events may provide specific interfaces for pathway cross talk. Nevertheless, this mode of regulation may not be the main mechanism for Wnt8-mediated anteroposterior axis determination, because no significant change in the amount of active Smad1 (phosphorylated at the carboxyl terminus) is observed in Xenopus embryos with altered Wnt8 activity (Hikasa et al. 2010). On the contrary, the requirement for the TCF-binding site in Vent2 reporter activation and the regulation of TCF by Wnt signaling reiterates the importance of TCF regulation for Wnt signaling (Hikasa et al. 2010).
Besides FGF and BMP signaling, there are examples of Wnt pathway cross talk with other signaling pathways, especially those linked to proteasome-mediated protein degradation. The β-transducin repeat-containing protein (βTrCP) is an E3 ubiquitin ligase that represents a nodal point for down-regulation of β-catenin, Gli, and NF-κB levels (Liu et al. 1999a; Maniatis 1999). As Wnt signaling has been shown to up-regulate βTrCP activity in a negative-feedback loop (Spiegelman et al. 2000), the effect on the Hh and NF-κB signaling may be expected. Also, the Wnt pathway negatively regulates the activity of GSK3 (Cook et al. 1996; Itoh et al. 1998b; Taelman et al. 2010), an enzyme that has been implicated in the regulation (e.g., degradation) of a large number of proteins, in addition to β-catenin (Zhou et al. 2004; Xu et al. 2009). These findings suggest the interaction of the Wnt pathway with many signaling pathways, which is likely to be better understood in the course of future studies.
CONCLUDING REMARKS
Recent studies support the view that Wnt signaling plays a dual role in vertebrate axis specification. Initially, the maternally encoded components of the pathway help to establish the dorsoventral axis, whereas at a later stage the zygotic Wnt pathway is involved in anteroposterior axis specification. These two roles are mediated by two distinct sets of specific targets, yet the underlying mechanisms for target selection are unclear. Further work is needed to understand the role of maternally produced Wnt ligands in early axis determination in the context of the abundant evidence for the involvement of microtubule-dependent trafficking in the dorsal accumulation of β-catenin. Although HIPK2-mediated phosphorylation of TCF3 is a major route for anteroposterior target activation, this phosphorylation is undetectable before gastrulation, suggesting an alternative mechanism for early target derepression (Hikasa et al. 2010). Other possibilities should also be explored, given that β-catenin can use other cofactors, besides TCFs, for transcriptional control (Olson et al. 2006; Takao et al. 2007; Abu-Remaileh et al. 2010). Future research will be driven by the need to better understand pathway target selection and the contribution of Wnt5 and Wnt11 signaling to body-axis specification.
ACKNOWLEDGMENTS
We thank Stefan Hoppler, Keiji Itoh, and Jenya Grinblat for comments on the manuscript. We apologize to those investigators, whose work has not been cited here because of limited space. The work in the Sokol laboratory is supported by National Institutes of Health grants.
Footnotes
Editors: Roel Nusse, Xi He, and Renee van Amerongen
Additional Perspectives on Wnt Signaling available at www.cshperspectives.org
REFERENCES
- Aamar E, Frank D 2004. Xenopus Meis3 protein forms a hindbrain-inducing center by activating FGF/MAP kinase and PCP pathways. Development 131: 153–163 [Abstract] [Google Scholar]
- Abu-Remaileh M, Gerson A, Farago M, Nathan G, Alkalay I, Zins Rousso S, Gur M, Fainsod A, Bergman Y 2010. Oct-3/4 regulates stem cell identity and cell fate decisions by modulating Wnt/β-catenin signalling. EMBO J 29: 3236–3248 [Europe PMC free article] [Abstract] [Google Scholar]
- Akkers RC, van Heeringen SJ, Jacobi UG, Janssen-Megens EM, Francoijs KJ, Stunnenberg HG, Veenstra GJ 2009. A hierarchy of H3K4me3 and H3K27me3 acquisition in spatial gene regulation in Xenopus embryos. Dev Cell 17: 425–434 [Europe PMC free article] [Abstract] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW 1991. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell 66: 257–270 [Abstract] [Google Scholar]
- Amaya E, Stein PA, Musci TJ, Kirschner MW 1993. FGF signalling in the early specification of mesoderm in Xenopus. Development 118: 477–487 [Abstract] [Google Scholar]
- Andreazzoli M, Broccoli V, Dawid IB 2001. Cloning and expression of noz1, a zebrafish zinc finger gene related to Drosophila nocA. Mech Dev 104: 117–120 [Abstract] [Google Scholar]
- Angers S, Moon RT 2009. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol 10: 468–477 [Abstract] [Google Scholar]
- Axelrod JD 2009. Progress and challenges in understanding planar cell polarity signaling. Semin Cell Dev Biol 20: 964–971 [Abstract] [Google Scholar]
- Beck F, Stringer EJ 2010. The role of Cdx genes in the gut and in axial development. Biochem Soc Trans 38: 353–357 [Abstract] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382: 638–642 [Abstract] [Google Scholar]
- Bellipanni G, Varga M, Maegawa S, Imai Y, Kelly C, Myers AP, Chu F, Talbot WS, Weinberg ES 2006. Essential and opposing roles of zebrafish β-catenins in the formation of dorsal axial structures and neurectoderm. Development 133: 1299–1309 [Abstract] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C 2007. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316: 1619–1622 [Abstract] [Google Scholar]
- Blumberg B, Wright CV, De Robertis EM, Cho KW 1991. Organizer-specific homeobox genes in Xenopus laevis embryos. Science 253: 194–196 [Abstract] [Google Scholar]
- Blythe SA, Cha SW, Tadjuidje E, Heasman J, Klein PS 2010. β-Catenin primes organizer gene expression by recruiting a histone H3 arginine 8 methyltransferase, Prmt2. Dev Cell 19: 220–231 [Europe PMC free article] [Abstract] [Google Scholar]
- Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis EM 1996. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann’s organizer. Nature 382: 595–601 [Abstract] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D 1997. A β-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 11: 2359–2370 [Europe PMC free article] [Abstract] [Google Scholar]
- Brantjes H, Roose J, van De Wetering M, Clevers H 2001. All Tcf HMG box transcription factors interact with Groucho-related co-repressors. Nucleic Acids Res 29: 1410–1419 [Europe PMC free article] [Abstract] [Google Scholar]
- Bromley E, Knapp D, Wardle FC, Sun BI, Collins-Racie L, LaVallie E, Smith JC, Sive HL 2004. Identification and characterisation of the posteriorly-expressed Xenopus neurotrophin receptor homolog genes fullback and fullback-like. Gene Expr Patterns 5: 135–140 [Abstract] [Google Scholar]
- Brott BK, Sokol SY 2002. Regulation of Wnt/LRP signaling by distinct domains of Dickkopf proteins. Mol Cell Biol 22: 6100–6110 [Europe PMC free article] [Abstract] [Google Scholar]
- Burks PJ, Isaacs HV, Pownall ME 2009. FGF signalling modulates transcriptional repression by Xenopus groucho-related-4. Biol Cell 101: 301–308 [Abstract] [Google Scholar]
- Candia AF, Watabe T, Hawley SH, Onichtchouk D, Zhang Y, Derynck R, Niehrs C, Cho KW 1997. Cellular interpretation of multiple TGF-β signals: Intracellular antagonism between activin/BVg1 and BMP-2/4 signaling mediated by Smads. Development 124: 4467–4480 [Abstract] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A 1998. Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 395: 604–608 [Abstract] [Google Scholar]
- Cha SW, Tadjuidje E, Tao Q, Wylie C, Heasman J 2008. Wnt5a and Wnt11 interact in a maternal Dkk1-regulated fashion to activate both canonical and non-canonical signaling in Xenopus axis formation. Development 135: 3719–3729 [Abstract] [Google Scholar]
- Cho KW, Blumberg B, Steinbeisser H, De Robertis EM 1991. Molecular nature of Spemann’s organizer: The role of the Xenopus homeobox gene goosecoid. Cell 67: 1111–1120 [Europe PMC free article] [Abstract] [Google Scholar]
- Choe SK, Lu P, Nakamura M, Lee J, Sagerstrom CG 2009. Meis cofactors control HDAC and CBP accessibility at Hox-regulated promoters during zebrafish embryogenesis. Dev Cell 17: 561–567 [Europe PMC free article] [Abstract] [Google Scholar]
- Christian JL, Moon RT 1993. Interactions between Xwnt-8 and Spemann organizer signaling pathways generate dorsoventral pattern in the embryonic mesoderm of Xenopus. Genes Dev 7: 13–28 [Abstract] [Google Scholar]
- Christian JL, Gavin BJ, McMahon AP, Moon RT 1991a. Isolation of cDNAs partially encoding four Xenopus Wnt-1/int-1-related proteins and characterization of their transient expression during embryonic development. Dev Biol 143: 230–234 [Abstract] [Google Scholar]
- Christian JL, McMahon JA, McMahon AP, Moon RT 1991b. Xwnt-8, a Xenopus Wnt-1/int-1-related gene responsive to mesoderm-inducing growth factors, may play a role in ventral mesodermal patterning during embryogenesis. Development 111: 1045–1055 [Abstract] [Google Scholar]
- Chung HM, Malacinski GM 1980. Establishment of the dorsal/ventral polarity of the amphibian embryo: Use of ultraviolet irradiation and egg rotation as probes. Dev Biol 80: 120–133 [Abstract] [Google Scholar]
- Chung HA, Hyodo-Miura J, Kitayama A, Terasaka C, Nagamune T, Ueno N 2004. Screening of FGF target genes in Xenopus by microarray: Temporal dissection of the signalling pathway using a chemical inhibitor. Genes Cells 9: 749–761 [Abstract] [Google Scholar]
- Clevers H 2006. Wnt/β-catenin signaling in development and disease. Cell 127: 469–480 [Abstract] [Google Scholar]
- Cliffe A, Hamada F, Bienz M 2003. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol 13: 960–966 [Abstract] [Google Scholar]
- Cole MF, Johnstone SE, Newman JJ, Kagey MH, Young RA 2008. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev 22: 746–755 [Europe PMC free article] [Abstract] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ 1994. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development 120: 1919–1928 [Abstract] [Google Scholar]
- Cook D, Fry MJ, Hughes K, Sumathipala R, Woodgett JR, Dale TC 1996. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J 15: 4526–4536 [Europe PMC free article] [Abstract] [Google Scholar]
- Cox WG, Hemmati-Brivanlou A 1995. Caudalization of neural fate by tissue recombination and bFGF. Development 121: 4349–4358 [Abstract] [Google Scholar]
- Daniels DL, Weis WI 2005. β-Catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol 12: 364–371 [Abstract] [Google Scholar]
- Darken RS, Wilson PA 2001. Axis induction by wnt signaling: Target promoter responsiveness regulates competence. Dev Biol 234: 42–54 [Abstract] [Google Scholar]
- Davidson AJ, Ernst P, Wang Y, Dekens MP, Kingsley PD, Palis J, Korsmeyer SJ, Daley GQ, Zon LI 2003. cdx4 mutants fail to specify blood progenitors and can be rescued by multiple hox genes. Nature 425: 300–306 [Abstract] [Google Scholar]
- Deardorff MA, Tan C, Conrad LJ, Klein PS 1998. Frizzled-8 is expressed in the Spemann organizer and plays a role in early morphogenesis. Development 125: 2687–2700 [Abstract] [Google Scholar]
- De Robertis EM, Kuroda H 2004. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20: 285–308 [Europe PMC free article] [Abstract] [Google Scholar]
- Doniach T, Phillips CR, Gerhart JC 1992. Planar induction of anteroposterior pattern in the developing central nervous system of Xenopus laevis. Science 257: 542–545 [Abstract] [Google Scholar]
- Dorsky RI, Snyder A, Cretekos CJ, Grunwald DJ, Geisler R, Haffter P, Moon RT, Raible DW 1999. Maternal and embryonic expression of zebrafish lef1. Mech Dev 86: 147–150 [Abstract] [Google Scholar]
- Dorsky RI, Sheldahl LC, Moon RT 2002. A transgenic Lef1/β-catenin-dependent reporter is expressed in spatially restricted domains throughout zebrafish development. Dev Biol 241: 229–237 [Abstract] [Google Scholar]
- Dorsky RI, Itoh M, Moon RT, Chitnis A 2003. Two tcf3 genes cooperate to pattern the zebrafish brain. Development 130: 1937–1947 [Abstract] [Google Scholar]
- Elkouby YM, Elias S, Casey ES, Blythe SA, Tsabar N, Klein PS, Root H, Liu KJ, Frank D 2010. Mesodermal Wnt signaling organizes the neural plate via Meis3. Development 137: 1531–1541 [Europe PMC free article] [Abstract] [Google Scholar]
- Epstein M, Pillemer G, Yelin R, Yisraeli JK, Fainsod A 1997. Patterning of the embryo along the anterior-posterior axis: The role of the caudal genes. Development 124: 3805–3814 [Abstract] [Google Scholar]
- Erter CE, Wilm TP, Basler N, Wright CV, Solnica-Krezel L 2001. Wnt8 is required in lateral mesendodermal precursors for neural posteriorization in vivo. Development 128: 3571–3583 [Abstract] [Google Scholar]
- Faas L, Isaacs HV 2009. Overlapping functions of Cdx1, Cdx2, and Cdx4 in the development of the amphibian Xenopus tropicalis. Dev Dyn 238: 835–852 [Europe PMC free article] [Abstract] [Google Scholar]
- Fan MJ, Sokol SY 1997. A role for Siamois in Spemann organizer formation. Development 124: 2581–2589 [Abstract] [Google Scholar]
- Fan MJ, Gruning W, Walz G, Sokol SY 1998. Wnt signaling and transcriptional control of Siamois in Xenopus embryos. Proc Natl Acad Sci 95: 5626–5631 [Europe PMC free article] [Abstract] [Google Scholar]
- Fekany K, Yamanaka Y, Leung T, Sirotkin HI, Topczewski J, Gates MA, Hibi M, Renucci A, Stemple D, Radbill A, et al. 1999. The zebrafish bozozok locus encodes Dharma, a homeodomain protein essential for induction of gastrula organizer and dorsoanterior embryonic structures. Development 126: 1427–1438 [Abstract] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS 1998. Zebrafish organizer development and germ-layer formation require nodal-related signals. Nature 395: 181–185 [Abstract] [Google Scholar]
- Fossat N, Jones V, Khoo PL, Bogani D, Hardy A, Steiner K, Mukhopadhyay M, Westphal H, Nolan PM, Arkell R, et al. 2011. Stringent requirement of a proper level of canonical WNT signalling activity for head formation in mouse embryo. Development 138: 667–676 [Abstract] [Google Scholar]
- Frazzetto G, Klingbeil P, Bouwmeester T 2002. Xenopus marginal coil (Xmc), a novel FGF inducible cytosolic coiled-coil protein regulating gastrulation movements. Mech Dev 113: 3–14 [Abstract] [Google Scholar]
- Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM, De Robertis EM 2007. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131: 980–993 [Europe PMC free article] [Abstract] [Google Scholar]
- Fujisue M, Kobayakawa Y, Yamana K 1993. Occurrence of dorsal axis-inducing activity around the vegetal pole of an uncleaved Xenopus egg and displacement to the equatorial region by cortical rotation. Development 118: 163–170 [Abstract] [Google Scholar]
- Gao B, Song H, Bishop K, Elliot G, Garrett L, English MA, Andre P, Robinson J, Sood R, Minami Y, et al. 2011. Wnt signaling gradients establish planar cell polarity by inducing Vangl2 phosphorylation through Ror2. Dev Cell 20: 163–176 [Europe PMC free article] [Abstract] [Google Scholar]
- Garcia-Morales C, Liu CH, Abu-Elmagd M, Hajihosseini MK, Wheeler GN 2009. Frizzled-10 promotes sensory neuron development in Xenopus embryos. Dev Biol 335: 143–155 [Abstract] [Google Scholar]
- Gawantka V, Delius H, Hirschfeld K, Blumenstock C, Niehrs C 1995. Antagonizing the Spemann organizer: Role of the homeobox gene Xvent-1. EMBO J 14: 6268–6279 [Europe PMC free article] [Abstract] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C 1998. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature 391: 357–362 [Abstract] [Google Scholar]
- Gradl D, Konig A, Wedlich D 2002. Functional diversity of Xenopus lymphoid enhancer factor/T-cell factor transcription factors relies on combinations of activating and repressing elements. J Biol Chem 277: 14159–14171 [Abstract] [Google Scholar]
- Gribble SL, Kim HS, Bonner J, Wang X, Dorsky RI 2009. Tcf3 inhibits spinal cord neurogenesis by regulating sox4a expression. Development 136: 781–789 [Europe PMC free article] [Abstract] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W 2008. Deciphering the function of canonical Wnt signals in development and disease: Conditional loss- and gain-of-function mutations of β-catenin in mice. Genes Dev 22: 2308–2341 [Europe PMC free article] [Abstract] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA 2010. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev 24: 2517–2530 [Europe PMC free article] [Abstract] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R 1995. Lack of β-catenin affects mouse development at gastrulation. Development 121: 3529–3537 [Abstract] [Google Scholar]
- Hamilton FS, Wheeler GN, Hoppler S 2001. Difference in XTcf-3 dependency accounts for change in response to β-catenin-mediated Wnt signalling in Xenopus blastula. Development 128: 2063–2073 [Abstract] [Google Scholar]
- Haremaki T, Tanaka Y, Hongo I, Yuge M, Okamoto H 2003. Integration of multiple signal transducing pathways on Fgf response elements of the Xenopus caudal homologue Xcad3. Development 130: 4907–4917 [Abstract] [Google Scholar]
- Harland R, Gerhart J 1997. Formation and function of Spemann’s organizer. Annu Rev Cell Dev Biol 13: 611–667 [Abstract] [Google Scholar]
- Hartmann C 2006. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol 16: 151–158 [Abstract] [Google Scholar]
- Hata A, Seoane J, Lagna G, Montalvo E, Hemmati-Brivanlou A, Massague J 2000. OAZ uses distinct DNA- and protein-binding zinc fingers in separate BMP-Smad and Olf signaling pathways. Cell 100: 229–240 [Abstract] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C 1994. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopus embryos. Cell 79: 791–803 [Abstract] [Google Scholar]
- Heasman J, Kofron M, Wylie C 2000. β-Catenin signaling activity dissected in the early Xenopus embryo: A novel antisense approach. Dev Biol 222: 124–134 [Abstract] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW 2000. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature 405: 76–81 [Abstract] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, et al. 2001. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev 15: 1427–1434 [Europe PMC free article] [Abstract] [Google Scholar]
- Hikasa H, Sokol SY 2011. Phosphorylation of TCF proteins by homeodomain-interacting protein kinase 2. J Biol Chem 286: 12093–12100 [Europe PMC free article] [Abstract] [Google Scholar]
- Hikasa H, Shibata M, Hiratani I, Taira M 2002. The Xenopus receptor tyrosine kinase Xror2 modulates morphogenetic movements of the axial mesoderm and neuroectoderm via Wnt signaling. Development 129: 5227–5239 [Abstract] [Google Scholar]
- Hikasa H, Ezan J, Itoh K, Li X, Klymkowsky MW, Sokol SY 2010. Regulation of TCF3 by Wnt-dependent phosphorylation during vertebrate axis specification. Dev Cell 19: 521–532 [Europe PMC free article] [Abstract] [Google Scholar]
- Hilton E, Rex M, Old R 2003. VegT activation of the early zygotic gene Xnr5 requires lifting of Tcf-mediated repression in the Xenopus blastula. Mech Dev 120: 1127–1138 [Abstract] [Google Scholar]
- Holowacz T, Elinson RP 1993. Cortical cytoplasm, which induces dorsal axis formation in Xenopus, is inactivated by UV irradiation of the oocyte. Development 119: 277–285 [Abstract] [Google Scholar]
- Holowacz T, Sokol S 1999. FGF is required for posterior neural patterning but not for neural induction. Dev Biol 205: 296–308 [Abstract] [Google Scholar]
- Holstein TW 2008. Wnt signaling in cnidarians. Methods Mol Biol 469: 47–54 [Abstract] [Google Scholar]
- Hong CS, Park BY, Saint-Jeannet JP 2008. Fgf8a induces neural crest indirectly through the activation of Wnt8 in the paraxial mesoderm. Development 135: 3903–3910 [Europe PMC free article] [Abstract] [Google Scholar]
- Hoppler S, Moon RT 1998. BMP-2/-4 and Wnt-8 cooperatively pattern the Xenopus mesoderm. Mech Dev 71: 119–129 [Abstract] [Google Scholar]
- Hoppler S, Brown JD, Moon RT 1996. Expression of a dominant-negative Wnt blocks induction of MyoD in Xenopus embryos. Genes Dev 10: 2805–2817 [Abstract] [Google Scholar]
- Houliston E, Elinson RP 1992. Microtubules and cytoplasmic reorganization in the frog egg. Curr Top Dev Biol 26: 53–70 [Abstract] [Google Scholar]
- Houston DW, Kofron M, Resnik E, Langland R, Destree O, Wylie C, Heasman J 2002. Repression of organizer genes in dorsal and ventral Xenopus cells mediated by maternal XTcf3. Development 129: 4015–4025 [Abstract] [Google Scholar]
- Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann BG, Kemler R 1996. Nuclear localization of β-catenin by interaction with transcription factor LEF-1. Mech Dev 59: 3–10 [Abstract] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W 2000. Requirement for β-catenin in anterior-posterior axis formation in mice. J Cell Biol 148: 567–578 [Europe PMC free article] [Abstract] [Google Scholar]
- Hufton AL, Vinayagam A, Suhai S, Baker JC 2006. Genomic analysis of Xenopus organizer function. BMC Dev Biol 6: 27. [Europe PMC free article] [Abstract] [Google Scholar]
- Imai Y, Gates MA, Melby AE, Kimelman D, Schier AF, Talbot WS 2001. The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development 128: 2407–2420 [Abstract] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM 1998. Regulation of Hox gene expression and posterior development by the Xenopus caudal homologue Xcad3. EMBO J 17: 3413–3427 [Europe PMC free article] [Abstract] [Google Scholar]
- Ishikawa TO, Tamai Y, Li Q, Oshima M, Taketo MM 2003. Requirement for tumor suppressor Apc in the morphogenesis of anterior and ventral mouse embryo. Dev Biol 253: 230–246 [Abstract] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H, et al. 1999. The TAK1-NLK-MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature 399: 798–802 [Abstract] [Google Scholar]
- Itasaki N, Hoppler S 2010. Crosstalk between Wnt and bone morphogenic protein signaling: A turbulent relationship. Dev Dyn 239: 16–33 [Abstract] [Google Scholar]
- Itoh K, Sokol SY 1997. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mech Dev 61: 113–125 [Abstract] [Google Scholar]
- Itoh K, Tang TL, Neel BG, Sokol SY 1995. Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development 121: 3979–3988 [Abstract] [Google Scholar]
- Itoh K, Jacob J, Sokol YS 1998a. A role for Xenopus Frizzled 8 in dorsal development. Mech Dev 74: 145–157 [Abstract] [Google Scholar]
- Itoh K, Krupnik VE, Sokol SY 1998b. Axis determination in Xenopus involves biochemical interactions of axin, glycogen synthase kinase 3 and β-catenin. Curr Biol 8: 591–594 [Abstract] [Google Scholar]
- Itoh K, Antipova A, Ratcliffe MJ, Sokol S 2000. Interaction of dishevelled and Xenopus axin-related protein is required for wnt signal transduction. Mol Cell Biol 20: 2228–2238 [Europe PMC free article] [Abstract] [Google Scholar]
- Itoh K, Brott BK, Bae GU, Ratcliffe MJ, Sokol SY 2005. Nuclear localization is required for Dishevelled function in Wnt/β-catenin signaling. J Biol 4: 3. [Europe PMC free article] [Abstract] [Google Scholar]
- Itoh K, Jenny A, Mlodzik M, Sokol SY 2009. Centrosomal localization of Diversin and its relevance to Wnt signaling. J Cell Sci 122: 3791–3798 [Europe PMC free article] [Abstract] [Google Scholar]
- Jesuthasan S, Stahle U 1997. Dynamic microtubules and specification of the zebrafish embryonic axis. Curr Biol 7: 31–42 [Abstract] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F 2002. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183 [Europe PMC free article] [Abstract] [Google Scholar]
- Kanei-Ishii C, Ninomiya-Tsuji J, Tanikawa J, Nomura T, Ishitani T, Kishida S, Kokura K, Kurahashi T, Ichikawa-Iwata E, Kim Y, et al. 2004. Wnt-1 signal induces phosphorylation and degradation of c-Myb protein via TAK1, HIPK2, and NLK. Genes Dev 18: 816–829 [Europe PMC free article] [Abstract] [Google Scholar]
- Karaulanov E, Knochel W, Niehrs C 2004. Transcriptional regulation of BMP4 synexpression in transgenic Xenopus. EMBO J 23: 844–856 [Europe PMC free article] [Abstract] [Google Scholar]
- Kawakami Y, Capdevila J, Buscher D, Itoh T, Rodriguez Esteban C, Izpisua Belmonte JC 2001. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell 104: 891–900 [Abstract] [Google Scholar]
- Keenan ID, Sharrard RM, Isaacs HV 2006. FGF signal transduction and the regulation of Cdx gene expression. Dev Biol 299: 478–488 [Abstract] [Google Scholar]
- Kelly GM, Greenstein P, Erezyilmaz DF, Moon RT 1995. Zebrafish wnt8 and wnt8b share a common activity but are involved in distinct developmental pathways. Development 121: 1787–1799 [Abstract] [Google Scholar]
- Kelly C, Chin AJ, Leatherman JL, Kozlowski DJ, Weinberg ES 2000. Maternally controlled β-catenin-mediated signaling is required for organizer formation in the zebrafish. Development 127: 3899–3911 [Abstract] [Google Scholar]
- Kessler DS 1997. Siamois is required for formation of Spemann’s organizer. Proc Natl Acad Sci 94: 13017–13022 [Europe PMC free article] [Abstract] [Google Scholar]
- Kiecker C, Niehrs C 2001. A morphogen gradient of Wnt/β-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128: 4189–4201 [Abstract] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A 2009. Selective activation mechanisms of Wnt signaling pathways. Trends Cell Biol 19: 119–129 [Abstract] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP 2003. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev 120: 467–476 [Abstract] [Google Scholar]
- Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, Driever W, Chitnis AB 2000. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407: 913–916 [Europe PMC free article] [Abstract] [Google Scholar]
- Kim EA, Kim JE, Sung KS, Choi DW, Lee BJ, Choi CY 2010. Homeodomain-interacting protein kinase 2 (HIPK2) targets β-catenin for phosphorylation and proteasomal degradation. Biochem Biophys Res Commun 394: 966–971 [Abstract] [Google Scholar]
- Klein PS, Melton DA 1996. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci 93: 8455–8459 [Europe PMC free article] [Abstract] [Google Scholar]
- Kolm PJ, Sive HL 1995. Regulation of the Xenopus labial homeodomain genes, HoxA1 and HoxD1: Activation by retinoids and peptide growth factors. Dev Biol 167: 34–49 [Abstract] [Google Scholar]
- Komiya Y, Habas R 2008. Wnt signal transduction pathways. Organogenesis 4: 68–75 [Europe PMC free article] [Abstract] [Google Scholar]
- Konig A, Gradl D, Kuhl M, Wedlich D 2000. The HMG-box transcription factor XTcf-4 demarcates the forebrain-midbrain boundary. Mech Dev 93: 211–214 [Abstract] [Google Scholar]
- Koos DS, Ho RK 1998. The nieuwkoid gene characterizes and mediates a Nieuwkoop-center-like activity in the zebrafish. Curr Biol 8: 1199–1206 [Abstract] [Google Scholar]
- Kouzarides T 2007. Chromatin modifications and their function. Cell 128: 693–705 [Abstract] [Google Scholar]
- Krauss S, Korzh V, Fjose A, Johansen T 1992. Expression of four zebrafish wnt-related genes during embryogenesis. Development 116: 249–259 [Abstract] [Google Scholar]
- Ku M, Melton DA 1993. Xwnt-11: A maternally expressed Xenopus wnt gene. Development 119: 1161–1173 [Abstract] [Google Scholar]
- Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW 2004. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development 131: 3581–3592 [Europe PMC free article] [Abstract] [Google Scholar]
- Labbe E, Letamendia A, Attisano L 2000. Association of Smads with lymphoid enhancer binding factor 1/T cell-specific factor mediates cooperative signaling by the transforming growth factor-β and wnt pathways. Proc Natl Acad Sci 97: 8358–8363 [Europe PMC free article] [Abstract] [Google Scholar]
- Ladher R, Mohun TJ, Smith JC, Snape AM 1996. Xom: A Xenopus homeobox gene that mediates the early effects of BMP-4. Development 122: 2385–2394 [Abstract] [Google Scholar]
- Lamb TM, Harland RM 1995. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development 121: 3627–3636 [Abstract] [Google Scholar]
- Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, Kimelman D, Moon RT 1997. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in β-catenin that are modulated by the Wnt signaling pathway. J Cell Biol 136: 1123–1136 [Europe PMC free article] [Abstract] [Google Scholar]
- Laurent MN, Blitz IL, Hashimoto C, Rothbacher U, Cho KW 1997. The Xenopus homeobox gene twin mediates Wnt induction of goosecoid in establishment of Spemann’s organizer. Development 124: 4905–4916 [Abstract] [Google Scholar]
- Lee W, Swarup S, Chen J, Ishitani T, Verheyen EM 2009. Homeodomain-interacting protein kinases (Hipks) promote Wnt/Wg signaling through stabilization of β-catenin/Arm and stimulation of target gene expression. Development 136: 241–251 [Abstract] [Google Scholar]
- Lekven AC, Thorpe CJ, Waxman JS, Moon RT 2001. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell 1: 103–114 [Abstract] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB 1995. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell 81: 85–94 [Abstract] [Google Scholar]
- Leung T, Soll I, Arnold SJ, Kemler R, Driever W 2003. Direct binding of Lef1 to sites in the boz promoter may mediate pre-midblastula-transition activation of boz expression. Dev Dyn 228: 424–432 [Abstract] [Google Scholar]
- Leyns L, Bouwmeester T, Kim SH, Piccolo S, De Robertis EM 1997. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88: 747–756 [Europe PMC free article] [Abstract] [Google Scholar]
- Li E 2002. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 3: 662–673 [Abstract] [Google Scholar]
- Li Y, Fenger U, Niehrs C, Pollet N 2003. Cyclic expression of esr9 gene in Xenopus presomitic mesoderm. Differentiation 71: 83–89 [Abstract] [Google Scholar]
- Li H, Malbon CC, Wang HY 2004. Gene profiling of Frizzled-1 and Frizzled-2 signaling: Expression of G-protein-coupled receptor chimeras in mouse F9 teratocarcinoma embryonal cells. Mol Pharmacol 65: 45–55 [Abstract] [Google Scholar]
- Li B, Kuriyama S, Moreno M, Mayor R 2009. The posteriorizing gene Gbx2 is a direct target of Wnt signalling and the earliest factor in neural crest induction. Development 136: 3267–3278 [Europe PMC free article] [Abstract] [Google Scholar]
- Lin G, Slack JM 2008. Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Dev Biol 316: 323–335 [Abstract] [Google Scholar]
- Lin S, Baye LM, Westfall TA, Slusarski DC 2010. Wnt5b-Ryk pathway provides directional signals to regulate gastrulation movement. J Cell Biol 190: 263–278 [Europe PMC free article] [Abstract] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X 1999a. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci 96: 6273–6278 [Europe PMC free article] [Abstract] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A 1999b. Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22: 361–365 [Abstract] [Google Scholar]
- Liu F, van den Broek O, Destree O, Hoppler S 2005. Distinct roles for Xenopus Tcf/Lef genes in mediating specific responses to Wnt/β-catenin signalling in mesoderm development. Development 132: 5375–5385 [Abstract] [Google Scholar]
- Lo MC, Gay F, Odom R, Shi Y, Lin R 2004. Phosphorylation by the β-catenin/MAPK complex promotes 14–3-3-mediated nuclear export of TCF/POP-1 in signal-responsive cells in C. elegans. Cell 117: 95–106 [Abstract] [Google Scholar]
- Louie SH, Yang XY, Conrad WH, Muster J, Angers S, Moon RT, Cheyette BN 2009. Modulation of the β-catenin signaling pathway by the dishevelled-associated protein Hipk1. PLoS ONE 4: e4310. [Europe PMC free article] [Abstract] [Google Scholar]
- Lu W, Yamamoto V, Ortega B, Baltimore D 2004. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119: 97–108 [Abstract] [Google Scholar]
- Lupo G, Harris WA, Barsacchi G, Vignali R 2002. Induction and patterning of the telencephalon in Xenopus laevis. Development 129: 5421–5436 [Abstract] [Google Scholar]
- Lyman Gingerich J, Westfall TA, Slusarski DC, Pelegri F 2005. Hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev Biol 286: 427–439 [Abstract] [Google Scholar]
- MacDonald BT, Tamai K, He X 2009. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev Cell 17: 9–26 [Europe PMC free article] [Abstract] [Google Scholar]
- Maeda R, Kobayashi A, Sekine R, Lin JJ, Kung H, Maeno M 1997. Xmsx-1 modifies mesodermal tissue pattern along dorsoventral axis in Xenopus laevis embryo. Development 124: 2553–2560 [Abstract] [Google Scholar]
- Maegawa S, Varga M, Weinberg ES 2006. FGF signaling is required for β-catenin-mediated induction of the zebrafish organizer. Development 133: 3265–3276 [Abstract] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP 2003. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130: 3175–3185 [Abstract] [Google Scholar]
- Maniatis T 1999. A ubiquitin ligase complex essential for the NF-κB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev 13: 505–510 [Abstract] [Google Scholar]
- Mao B, Wu W, Davidson G, Marhold J, Li M, Mechler BM, Delius H, Hoppe D, Stannek P, Walter C, et al. 2002. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nature 417: 664–667 [Abstract] [Google Scholar]
- Marazzi G, Wang Y, Sassoon D 1997. Msx2 is a transcriptional regulator in the BMP4-mediated programmed cell death pathway. Dev Biol 186: 127–138 [Abstract] [Google Scholar]
- Marchal L, Luxardi G, Thome V, Kodjabachian L 2009. BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc Natl Acad Sci 106: 17437–17442 [Europe PMC free article] [Abstract] [Google Scholar]
- Marlow FL 2010. Maternal control of development in vertebrates: My mother made me do it! Morgan & Claypool Life Sciences, San Rafel, CA [Abstract] [Google Scholar]
- Martin BL, Kimelman D 2009. Wnt signaling and the evolution of embryonic posterior development. Curr Biol 19: R215–R219 [Europe PMC free article] [Abstract] [Google Scholar]
- Martinez S, Crossley PH, Cobos I, Rubenstein JL, Martin GR 1999. FGF8 induces formation of an ectopic isthmic organizer and isthmocerebellar development via a repressive effect on Otx2 expression. Development 126: 1189–1200 [Abstract] [Google Scholar]
- Massague J 2003. Integration of Smad and MAPK pathways: A link and a linker revisited. Genes Dev 17: 2993–2997 [Abstract] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M 1997. The Rx homeobox gene is essential for vertebrate eye development. Nature 387: 603–607 [Abstract] [Google Scholar]
- McGrew LL, Hoppler S, Moon RT 1997. Wnt and FGF pathways cooperatively pattern anteroposterior neural ectoderm in Xenopus. Mech Dev 69: 105–114 [Abstract] [Google Scholar]
- McKendry R, Hsu SC, Harland RM, Grosschedl R 1997. LEF-1/TCF proteins mediate wnt-inducible transcription from the Xenopus nodal-related 3 promoter. Dev Biol 192: 420–431 [Abstract] [Google Scholar]
- McMahon AP, Moon RT 1989. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell 58: 1075–1084 [Abstract] [Google Scholar]
- Melby AE, Clements WK, Kimelman D 1999. Regulation of dorsal gene expression in Xenopus by the ventralizing homeodomain gene Vox. Dev Biol 211: 293–305 [Abstract] [Google Scholar]
- Melby AE, Beach C, Mullins M, Kimelman D 2000. Patterning the early zebrafish by the opposing actions of bozozok and vox/vent. Dev Biol 224: 275–285 [Abstract] [Google Scholar]
- Meneghini MD, Ishitani T, Carter JC, Hisamoto N, Ninomiya-Tsuji J, Thorpe CJ, Hamill DR, Matsumoto K, Bowerman B 1999. MAP kinase and Wnt pathways converge to downregulate an HMG-domain repressor in Caenorhabditis elegans. Nature 399: 793–797 [Abstract] [Google Scholar]
- Merrill BJ, Pasolli HA, Polak L, Rendl M, Garcia-Garcia MJ, Anderson KV, Fuchs E 2004. Tcf3: A transcriptional regulator of axis induction in the early embryo. Development 131: 263–274 [Abstract] [Google Scholar]
- Mikels AJ, Nusse R 2006. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol 4: e115. [Europe PMC free article] [Abstract] [Google Scholar]
- Mir A, Kofron M, Zorn AM, Bajzer M, Haque M, Heasman J, Wylie CC 2007. FoxI1e activates ectoderm formation and controls cell position in the Xenopus blastula. Development 134: 779–788 [Abstract] [Google Scholar]
- Mizuno T, Yamaha E, Kuroiwa A, Takeda H 1999. Removal of vegetal yolk causes dorsal deficencies and impairs dorsal-inducing ability of the yolk cell in zebrafish. Mech Dev 81: 51–63 [Abstract] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H 1996. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [Abstract] [Google Scholar]
- Molenaar M, Roose J, Peterson J, Venanzi S, Clevers H, Destree O 1998. Differential expression of the HMG box transcription factors XTcf-3 and XLef-1 during early Xenopus development. Mech Dev 75: 151–154 [Abstract] [Google Scholar]
- Moon RT, Kimelman D 1998. From cortical rotation to organizer gene expression: Toward a molecular explanation of axis specification in Xenopus. Bioessays 20: 536–545 [Abstract] [Google Scholar]
- Moon RT, Campbell RM, Christian JL, McGrew LL, Shih J, Fraser S 1993. Xwnt-5A: A maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development 119: 97–111 [Abstract] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, et al. 2001. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell 1: 423–434 [Abstract] [Google Scholar]
- Nakamura M, Choe SK, Runko AP, Gardner PD, Sagerstrom CG 2008. Nlz1/Znf703 acts as a repressor of transcription. BMC Dev Biol 8: 108. [Europe PMC free article] [Abstract] [Google Scholar]
- Nelson WJ, Nusse R 2004. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303: 1483–1487 [Europe PMC free article] [Abstract] [Google Scholar]
- Nentwich O, Dingwell KS, Nordheim A, Smith JC 2009. Downstream of FGF during mesoderm formation in Xenopus: The roles of Elk-1 and Egr-1. Dev Biol 336: 313–326 [Abstract] [Google Scholar]
- Ng RK, Gurdon JB 2008. Epigenetic memory of an active gene state depends on histone H3.3 incorporation into chromatin in the absence of transcription. Nat Cell Biol 10: 102–109 [Abstract] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E 2006. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 127: 171–183 [Abstract] [Google Scholar]
- Nieuwkoop PD 1952. Activation and organization of the central nervous system in amphibians. J Exp Zool 120: 83–108 [Google Scholar]
- Nishita M, Hashimoto MK, Ogata S, Laurent MN, Ueno N, Shibuya H, Cho KW 2000. Interaction between Wnt and TGF-β signalling pathways during formation of Spemann’s organizer. Nature 403: 781–785 [Abstract] [Google Scholar]
- Nishita M, Yoo SK, Nomachi A, Kani S, Sougawa N, Ohta Y, Takada S, Kikuchi A, Minami Y 2006. Filopodia formation mediated by receptor tyrosine kinase Ror2 is required for Wnt5a-induced cell migration. J Cell Biol 175: 555–562 [Europe PMC free article] [Abstract] [Google Scholar]
- Nishita M, Itsukushima S, Nomachi A, Endo M, Wang Z, Inaba D, Qiao S, Takada S, Kikuchi A, Minami Y 2010. Ror2/Frizzled complex mediates Wnt5a-induced AP-1 activation by regulating Dishevelled polymerization. Mol Cell Biol 30: 3610–3619 [Europe PMC free article] [Abstract] [Google Scholar]
- Nojima H, Shimizu T, Kim CH, Yabe T, Bae YK, Muraoka O, Hirata T, Chitnis A, Hirano T, Hibi M 2004. Genetic evidence for involvement of maternally derived Wnt canonical signaling in dorsal determination in zebrafish. Mech Dev 121: 371–386 [Abstract] [Google Scholar]
- Nojima H, Rothhamel S, Shimizu T, Kim CH, Yonemura S, Marlow FL, Hibi M 2010. Syntabulin, a motor protein linker, controls dorsal determination. Development 137: 923–933 [Abstract] [Google Scholar]
- Northrop JL, Kimelman D 1994. Dorsal-ventral differences in Xcad-3 expression in response to FGF-mediated induction in Xenopus. Dev Biol 161: 490–503 [Abstract] [Google Scholar]
- Ober EA, Schulte-Merker S 1999. Signals from the yolk cell induce mesoderm, neuroectoderm, the trunk organizer, and the notochord in zebrafish. Dev Biol 215: 167–181 [Abstract] [Google Scholar]
- Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, et al. 2006. Homeodomain-mediated β-catenin-dependent switching events dictate cell-lineage determination. Cell 125: 593–605 [Abstract] [Google Scholar]
- Onichtchouk D, Gawantka V, Dosch R, Delius H, Hirschfeld K, Blumenstock C, Niehrs C 1996. The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling dorsoventral patterning of Xenopus mesoderm. Development 122: 3045–3053 [Abstract] [Google Scholar]
- Osada SI, Wright CV 1999. Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development 126: 3229–3240 [Abstract] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM 2003. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 17: 3023–3028 [Europe PMC free article] [Abstract] [Google Scholar]
- Perantoni AO 2003. Renal development: Perspectives on a Wnt-dependent process. Semin Cell Dev Biol 14: 201–208 [Abstract] [Google Scholar]
- Petersen CP, Reddien PW 2009. Wnt signaling and the polarity of the primary body axis. Cell 139: 1056–1068 [Abstract] [Google Scholar]
- Phillips BT, Kimble J 2009. A new look at TCF and β-catenin through the lens of a divergent C. elegans Wnt pathway. Dev Cell 17: 27–34 [Europe PMC free article] [Abstract] [Google Scholar]
- Pillemer G, Yelin R, Epstein M, Gont L, Frumkin Y, Yisraeli JK, Steinbeisser H, Fainsod A 1998. The Xcad-2 gene can provide a ventral signal independent of BMP-4. Mech Dev 74: 133–143 [Abstract] [Google Scholar]
- Pilon N, Oh K, Sylvestre JR, Bouchard N, Savory J, Lohnes D 2006. Cdx4 is a direct target of the canonical Wnt pathway. Dev Biol 289: 55–63 [Abstract] [Google Scholar]
- Ramel MC, Lekven AC 2004. Repression of the vertebrate organizer by Wnt8 is mediated by Vent and Vox. Development 131: 3991–4000 [Abstract] [Google Scholar]
- Ramel MC, Buckles GR, Baker KD, Lekven AC 2005. WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation. Dev Biol 287: 237–248 [Abstract] [Google Scholar]
- Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strahle U, Ingham PW, McMahon AP, Haffter P 1997. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb Symp Quant Biol 62: 227–234 [Abstract] [Google Scholar]
- Rocheleau CE, Downs WD, Lin R, Wittmann C, Bei Y, Cha YH, Ali M, Priess JR, Mello CC 1997. Wnt signaling and an APC-related gene specify endoderm in early C. elegans embryos. Cell 90: 707–716 [Abstract] [Google Scholar]
- Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC 1999. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell 97: 717–726 [Abstract] [Google Scholar]
- Roel G, Hamilton FS, Gent Y, Bain AA, Destree O, Hoppler S 2002. Lef-1 and Tcf-3 transcription factors mediate tissue-specific Wnt signaling during Xenopus development. Curr Biol 12: 1941–1945 [Abstract] [Google Scholar]
- Roel G, van den Broek O, Spieker N, Peterson-Maduro J, Destree O 2003. Tcf-1 expression during Xenopus development. Gene Expr Patterns 3: 123–126 [Abstract] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clevers H 1998. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395: 608–612 [Abstract] [Google Scholar]
- Rothbacher U, Laurent MN, Blitz IL, Watabe T, Marsh JL, Cho KWY 1995. Functional conservation of the Wnt signaling pathway revealed by ectopic expression of Drosophila dishevelled in Xenopus. Dev Biol 170: 717–721 [Abstract] [Google Scholar]
- Ryu SL, Fujii R, Yamanaka Y, Shimizu T, Yabe T, Hirata T, Hibi M, Hirano T 2001. Regulation of dharma/bozozok by the Wnt pathway. Dev Biol 231: 397–409 [Abstract] [Google Scholar]
- Sander V, Reversade B, De Robertis EM 2007. The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. EMBO J 26: 2955–2965 [Europe PMC free article] [Abstract] [Google Scholar]
- Sapkota G, Alarcon C, Spagnoli FM, Brivanlou AH, Massague J 2007. Balancing BMP signaling through integrated inputs into the Smad1 linker. Mol Cell 25: 441–454 [Abstract] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM 1994. Xenopus chordin: A novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79: 779–790 [Europe PMC free article] [Abstract] [Google Scholar]
- Sasai N, Nakazawa Y, Haraguchi T, Sasai Y 2004. The neurotrophin-receptor-related protein NRH1 is essential for convergent extension movements. Nat Cell Biol 6: 741–748 [Abstract] [Google Scholar]
- Sato SM, Sargent TD 1991. Localized and inducible expression of Xenopus-posterior (Xpo), a novel gene active in early frog embryos, encoding a protein with a “CCHC” finger domain. Development 112: 747–754 [Abstract] [Google Scholar]
- Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A 2010. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J 29: 41–54 [Europe PMC free article] [Abstract] [Google Scholar]
- Satoh K, Kasai M, Ishidao T, Tago K, Ohwada S, Hasegawa Y, Senda T, Takada S, Nada S, Nakamura T, et al. 2004. Anteriorization of neural fate by inhibitor of β-catenin and T cell factor (ICAT), a negative regulator of Wnt signaling. Proc Natl Acad Sci 101: 8017–8021 [Europe PMC free article] [Abstract] [Google Scholar]
- Schambony A, Wedlich D 2007. Wnt-5A/Ror2 regulate expression of XPAPC through an alternative noncanonical signaling pathway. Dev Cell 12: 779–792 [Abstract] [Google Scholar]
- Scharf SR, Gerhart JC 1980. Determination of the dorsal-ventral axis in eggs of Xenopus laevis: Complete rescue of uv-impaired eggs by oblique orientation before first cleavage. Dev Biol 79: 181–198 [Abstract] [Google Scholar]
- Schedl A, Hastie ND 2000. Cross-talk in kidney development. Curr Opin Genet Dev 10: 543–549 [Abstract] [Google Scholar]
- Schier AF, Talbot WS 2005. Molecular genetics of axis formation in zebrafish. Annu Rev Genet 39: 561–613 [Abstract] [Google Scholar]
- Schmidt JE, von Dassow G, Kimelman D 1996. Regulation of dorsal-ventral patterning: The ventralizing effects of the novel Xenopus homeobox gene Vox. Development 122: 1711–1721 [Abstract] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P 1996. β-Catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57: 191–198 [Abstract] [Google Scholar]
- Schohl A, Fagotto F 2002. β-Catenin, MAPK and Smad signaling during early Xenopus development. Development 129: 37–52 [Abstract] [Google Scholar]
- Schroeder KE, Condic ML, Eisenberg LM, Yost HJ 1999. Spatially regulated translation in embryos: Asymmetric expression of maternal Wnt-11 along the dorsal-ventral axis in Xenopus. Dev Biol 214: 288–297 [Abstract] [Google Scholar]
- Schwarz-Romond T, Asbrand C, Bakkers J, Kuhl M, Schaeffer HJ, Huelsken J, Behrens J, Hammerschmidt M, Birchmeier W 2002. The ankyrin repeat protein Diversin recruits Casein kinase Iε to the ε-catenin degradation complex and acts in both canonical Wnt and Wnt/JNK signaling. Genes Dev 16: 2073–2084 [Europe PMC free article] [Abstract] [Google Scholar]
- Shibata M, Ono H, Hikasa H, Shinga J, Taira M 2000. Xenopus crescent encoding a Frizzled-like domain is expressed in the Spemann organizer and pronephros. Mech Dev 96: 243–246 [Abstract] [Google Scholar]
- Shibata M, Itoh M, Ohmori SY, Shinga J, Taira M 2001. Systematic screening and expression analysis of the head organizer genes in Xenopus embryos. Dev Biol 239: 241–256 [Abstract] [Google Scholar]
- Shibata M, Itoh M, Hikasa H, Taira S, Taira M 2005. Role of crescent in convergent extension movements by modulating Wnt signaling in early Xenopus embryogenesis. Mech Dev 122: 1322–1339 [Abstract] [Google Scholar]
- Shibata T, Takahashi Y, Tasaki J, Saito Y, Izutsu Y, Maeno M 2008. A role of D domain-related proteins in differentiation and migration of embryonic cells in Xenopus laevis. Mech Dev 125: 284–298 [Abstract] [Google Scholar]
- Shimizu T, Bae YK, Muraoka O, Hibi M 2005. Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev Biol 279: 125–141 [Abstract] [Google Scholar]
- Shimizu T, Bae YK, Hibi M 2006. Cdx-Hox code controls competence for responding to Fgfs and retinoic acid in zebrafish neural tissue. Development 133: 4709–4719 [Abstract] [Google Scholar]
- Smith WC, Harland RM 1991. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell 67: 753–765 [Abstract] [Google Scholar]
- Smith WC, Harland RM 1992. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell 70: 829–840 [Abstract] [Google Scholar]
- Smith WC, McKendry R, Ribisi S Jr, Harland RM 1995. A nodal-related gene defines a physical and functional domain within the Spemann organizer. Cell 82: 37–46 [Abstract] [Google Scholar]
- Sokol SY 1993. Mesoderm formation in Xenopus ectodermal explants overexpressing Xwnt8: Evidence for a cooperating signal reaching the animal pole by gastrulation. Development 118: 1335–1342 [Abstract] [Google Scholar]
- Sokol SY 1996. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol 6: 1456–1467 [Abstract] [Google Scholar]
- Sokol SY 1999. Wnt signaling and dorso-ventral axis specification in vertebrates. Curr Opin Genet Dev 9: 405–410 [Abstract] [Google Scholar]
- Sokol SY 2000. A role for Wnts in morpho-genesis and tissue polarity. Nat Cell Biol 2: E124–E125 [Abstract] [Google Scholar]
- Sokol SY 2011. Wnt signaling through T-cell factor phosphorylation. Cell Res 21: 1002–1012 [Europe PMC free article] [Abstract] [Google Scholar]
- Sokol SY, Melton DA 1992. Interaction of Wnt and activin in dorsal mesoderm induction in Xenopus. Dev Biol 154: 348–355 [Abstract] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA 1991. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67: 741–752 [Abstract] [Google Scholar]
- Sokol SY, Klingensmith J, Perrimon N, Itoh K 1995. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development 121: 3487. [Abstract] [Google Scholar]
- Spiegelman VS, Slaga TJ, Pagano M, Minamoto T, Ronai Z, Fuchs SY 2000. Wnt/β-catenin signaling induces the expression and activity of βTrCP ubiquitin ligase receptor. Mol Cell 5: 877–882 [Abstract] [Google Scholar]
- Standley HJ, Destree O, Kofron M, Wylie C, Heasman J 2006. Maternal XTcf1 and XTcf4 have distinct roles in regulating Wnt target genes. Dev Biol 289: 318–328 [Abstract] [Google Scholar]
- Sumanas S, Strege P, Heasman J, Ekker SC 2000. The putative wnt receptor Xenopus frizzled-7 functions upstream of β-catenin in vertebrate dorsoventral mesoderm patterning. Development 127: 1981–1990 [Abstract] [Google Scholar]
- Suri C, Haremaki T, Weinstein DC 2005. Xema, a foxi-class gene expressed in the gastrula stage Xenopus ectoderm, is required for the suppression of mesendoderm. Development 132: 2733–2742 [Europe PMC free article] [Abstract] [Google Scholar]
- Suzuki A, Ueno N, Hemmati-Brivanlou A 1997. Xenopus msx1 mediates epidermal induction and neural inhibition by BMP4. Development 124: 3037–3044 [Abstract] [Google Scholar]
- Tada M, Kai M 2009. Noncanonical Wnt/PCP signaling during vertebrate gastrulation. Zebrafish 6: 29–40 [Abstract] [Google Scholar]
- Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM 2010. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell 143: 1136–1148 [Europe PMC free article] [Abstract] [Google Scholar]
- Taira M, Otani H, Saint-Jeannet JP, Dawid IB 1994. Role of the LIM class homeodomain protein Xlim-1 in neural and muscle induction by the Spemann organizer in Xenopus. Nature 372: 677–679 [Abstract] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP 1994. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev 8: 174–189 [Abstract] [Google Scholar]
- Takahashi S, Yokota C, Takano K, Tanegashima K, Onuma Y, Goto J, Asashima M 2000. Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development 127: 5319–5329 [Abstract] [Google Scholar]
- Takao Y, Yokota T, Koide H 2007. β-Catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem Biophys Res Commun 353: 699–705 [Abstract] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J 2005. Maternal wnt11 activates the canonical wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120: 857–871 [Abstract] [Google Scholar]
- Thorpe CJ, Moon RT 2004. Nemo-like kinase is an essential co-activator of Wnt signaling during early zebrafish development. Development 131: 2899–2909 [Abstract] [Google Scholar]
- Torres MA, Yang-Snyder JA, Purcell SM, DeMarais AA, McGrew LL, Moon RT 1996. Activities of the Wnt-1 class of secreted signaling factors are antagonized by the Wnt-5A class and by a dominant negative cadherin in early Xenopus development. J Cell Biol 133: 1123–1137 [Europe PMC free article] [Abstract] [Google Scholar]
- Tucker A, Sharpe P 2004. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet 5: 499–508 [Abstract] [Google Scholar]
- Ulrich F, Concha ML, Heid PJ, Voss E, Witzel S, Roehl H, Tada M, Wilson SW, Adams RJ, Soll DR, et al. 2003. Slb/Wnt11 controls hypoblast cell migration and morphogenesis at the onset of zebrafish gastrulation. Development 130: 5375–5384 [Europe PMC free article] [Abstract] [Google Scholar]
- van Amerongen R, Nusse R 2009. Towards an integrated view of Wnt signaling in development. Development 136: 3205–3214 [Abstract] [Google Scholar]
- van Amerongen R, Nawijn M, Franca-Koh J, Zevenhoven J, van der Gulden H, Jonkers J, Berns A 2005. Frat is dispensable for canonical Wnt signaling in mammals. Genes Dev 19: 425–430 [Europe PMC free article] [Abstract] [Google Scholar]
- van den Akker E, Forlani S, Chawengsaksophak K, de Graaff W, Beck F, Meyer BI, Deschamps J 2002. Cdx1 and Cdx2 have overlapping functions in anteroposterior patterning and posterior axis elongation. Development 129: 2181–2193 [Abstract] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, et al. 1997. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799 [Abstract] [Google Scholar]
- Veien ES, Grierson MJ, Saund RS, Dorsky RI 2005. Expression pattern of zebrafish tcf7 suggests unexplored domains of Wnt/β-catenin activity. Dev Dyn 233: 233–239 [Abstract] [Google Scholar]
- Villanueva S, Glavic A, Ruiz P, Mayor R 2002. Posteriorization by FGF, Wnt, and retinoic acid is required for neural crest induction. Dev Biol 241: 289–301 [Abstract] [Google Scholar]
- von Bubnoff A, Schmidt JE, Kimelman D 1996. The Xenopus laevis homeobox gene Xgbx-2 is an early marker of anteroposterior patterning in the ectoderm. Mech Dev 54: 149–160 [Abstract] [Google Scholar]
- von Dassow G, Schmidt JE, Kimelman D 1993. Induction of the Xenopus organizer: Expression and regulation of Xnot, a novel FGF and activin-regulated homeo box gene. Genes Dev 7: 355–366 [Abstract] [Google Scholar]
- Wang S, Krinks M, Lin K, Luyten FP, Moos M Jr 1997. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88: 757–766 [Abstract] [Google Scholar]
- Wei G, Ku S, Ma GK, Saito S, Tang AA, Zhang J, Mao JH, Appella E, Balmain A, Huang EJ 2007. HIPK2 represses β-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc Natl Acad Sci 104: 13040–13045 [Europe PMC free article] [Abstract] [Google Scholar]
- Weidinger G, Thorpe CJ, Wuennenberg-Stapleton K, Ngai J, Moon RT 2005. The Sp1-related transcription factors sp5 and sp5-like act downstream of Wnt/β-catenin signaling in mesoderm and neuroectoderm patterning. Curr Biol 15: 489–500 [Abstract] [Google Scholar]
- Wheeler GN, Hoppler S 1999. Two novel Xenopus frizzled genes expressed in developing heart and brain. Mech Dev 86: 203–207 [Abstract] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R 2002. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol 2: 8. [Europe PMC free article] [Abstract] [Google Scholar]
- Wu J, Mlodzik M 2009. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol 19: 295–305 [Europe PMC free article] [Abstract] [Google Scholar]
- Xu C, Kim NG, Gumbiner BM 2009. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle 8: 4032–4039 [Europe PMC free article] [Abstract] [Google Scholar]
- Xu PF, Zhu KY, Jin Y, Chen Y, Sun XJ, Deng M, Chen SJ, Chen Z, Liu TX 2010. Setdb2 restricts dorsal organizer territory and regulates left-right asymmetry through suppressing fgf8 activity. Proc Natl Acad Sci 107: 2521–2526 [Europe PMC free article] [Abstract] [Google Scholar]
- Yamaguchi Y, Shinagawa A 1989. Marked alteration at midblastula transition in the effect of lithium on formation of the larval body pattern of Xenopus laevis. Dev Growth Differ 31: 531–541 [Abstract] [Google Scholar]
- Yamaguchi TP, Bradley A, McMahon AP, Jones S 1999. A Wnt5a pathway underlies outgrowth of multiple structures in the vertebrate embryo. Development 126: 1211–1223 [Abstract] [Google Scholar]
- Yamamoto A, Nagano T, Takehara S, Hibi M, Aizawa S 2005. Shisa promotes head formation through the inhibition of receptor protein maturation for the caudalizing factors, Wnt and FGF. Cell 120: 223–235 [Abstract] [Google Scholar]
- Yamanaka Y, Mizuno T, Sasai Y, Kishi M, Takeda H, Kim CH, Hibi M, Hirano T 1998. A novel homeobox gene, dharma, can induce the organizer in a non-cell-autonomous manner. Genes Dev 12: 2345–2353 [Europe PMC free article] [Abstract] [Google Scholar]
- Yanagawa S, van Leeuwen F, Wodarz A, Klingensmith J, Nusse R 1995. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev 9: 1087–1097 [Abstract] [Google Scholar]
- Yang J, Tan C, Darken RS, Wilson PA, Klein PS 2002. β-Catenin/Tcf-regulated transcription prior to the midblastula transition. Development 129: 5743–5752 [Abstract] [Google Scholar]
- Yokoyama H 2008. Initiation of limb regeneration: The critical steps for regenerative capacity. Dev Growth Differ 50: 13–22 [Abstract] [Google Scholar]
- Yost C, Farr GH III, Pierce SB, Ferkey DM, Chen MM, Kimelman D 1998. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell 93: 1031–1041 [Abstract] [Google Scholar]
- Young T, Deschamps J 2009. Hox, Cdx, and anteroposterior patterning in the mouse embryo. Curr Top Dev Biol 88: 235–255 [Abstract] [Google Scholar]
- Young T, Rowland JE, van de Ven C, Bialecka M, Novoa A, Carapuco M, van Nes J, de Graaff W, Duluc I, Freund JN, et al. 2009. Cdx and Hox genes differentially regulate posterior axial growth in mammalian embryos. Dev Cell 17: 516–526 [Abstract] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL III, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90: 181–192 [Abstract] [Google Scholar]
- Zhang C, Basta T, Jensen ED, Klymkowsky MW 2003. The β-catenin/VegT-regulated early zygotic gene Xnr5 is a direct target of SOX3 regulation. Development 130: 5609–5624 [Abstract] [Google Scholar]
- Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC 2004. Dual regulation of Snail by GSK-3β-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6: 931–940 [Abstract] [Google Scholar]
- Znosko WA, Yu S, Thomas K, Molina GA, Li C, Tsang W, Dawid IB, Moon AM, Tsang M 2010. Overlapping functions of Pea3 ETS transcription factors in FGF signaling during zebrafish development. Dev Biol 342: 11–25 [Europe PMC free article] [Abstract] [Google Scholar]
Articles from Cold Spring Harbor Perspectives in Biology are provided here courtesy of Cold Spring Harbor Laboratory Press
Full text links
Read article at publisher's site: https://doi.org/10.1101/cshperspect.a007955
Read article for free, from open access legal sources, via Unpaywall:
http://cshperspectives.cshlp.org/content/5/1/a007955.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1101/cshperspect.a007955
Article citations
Carboxy-terminal polyglutamylation regulates signaling and phase separation of the Dishevelled protein.
EMBO J, 43(22):5635-5666, 30 Sep 2024
Cited by: 0 articles | PMID: 39349846
Canonical and Non-Canonical Wnt Signaling Generates Molecular and Cellular Asymmetries to Establish Embryonic Axes.
J Dev Biol, 12(3):20, 02 Aug 2024
Cited by: 0 articles | PMID: 39189260 | PMCID: PMC11348223
Review Free full text in Europe PMC
Cord Blood Proteomic Profiles, Birth Weight, and Early Life Growth Trajectories.
JAMA Netw Open, 7(5):e2411246, 01 May 2024
Cited by: 0 articles | PMID: 38743419 | PMCID: PMC11094560
Tail and Spinal Cord Regeneration in Urodelean Amphibians.
Life (Basel), 14(5):594, 07 May 2024
Cited by: 0 articles | PMID: 38792615 | PMCID: PMC11122520
Review Free full text in Europe PMC
R-Spondin 2 governs Xenopus left-right body axis formation by establishing an FGF signaling gradient.
Nat Commun, 15(1):1003, 02 Feb 2024
Cited by: 0 articles | PMID: 38307837 | PMCID: PMC10837206
Go to all (99) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Spatial and temporal aspects of Wnt signaling and planar cell polarity during vertebrate embryonic development.
Semin Cell Dev Biol, 42:78-85, 15 May 2015
Cited by: 51 articles | PMID: 25986055 | PMCID: PMC4562884
Review Free full text in Europe PMC
Anteroposterior axis patterning by early canonical Wnt signaling during hemichordate development.
PLoS Biol, 16(1):e2003698, 16 Jan 2018
Cited by: 40 articles | PMID: 29337984 | PMCID: PMC5786327
Custos controls β-catenin to regulate head development during vertebrate embryogenesis.
Proc Natl Acad Sci U S A, 111(36):13099-13104, 25 Aug 2014
Cited by: 6 articles | PMID: 25157132 | PMCID: PMC4246939
Heads or tails? Amphioxus and the evolution of anterior-posterior patterning in deuterostomes.
Dev Biol, 241(2):209-228, 01 Jan 2002
Cited by: 47 articles | PMID: 11784106
Review




