Abstract
Free full text

Regulation of RhoA Signaling by the cAMP-dependent Phosphorylation of RhoGDIα*
Abstract
RhoA plays a pivotal role in regulating cell shape and movement. Protein kinase A (PKA) inhibits RhoA signaling and thereby induces a characteristic morphological change, cell rounding. This has been considered to result from cAMP-induced phosphorylation of RhoA at Ser-188, which induces a stable RhoA-GTP-RhoGDIα complex and sequesters RhoA to the cytosol. However, few groups have shown RhoA phosphorylation in intact cells. Here we show that phosphorylation of RhoGDIα but not RhoA plays an essential role in the PKA-induced inhibition of RhoA signaling and in the morphological changes using cardiac fibroblasts. The knockdown of RhoGDIα by siRNA blocks cAMP-induced cell rounding, which is recovered by RhoGDIα-WT expression but not when a RhoGDIα-S174A mutant is expressed. PKA phosphorylates RhoGDIα at Ser-174 and the phosphorylation of RhoGDIα is likely to induce the formation of a active RhoA-RhoGDIα complex. Our present results thus reveal a principal molecular mechanism underlying Gs/cAMP-induced cross-talk with Gq/G13/RhoA signaling.
Introduction
The RhoGTPases are small GTP-binding proteins of which 20 family members are found in mammals, including the well characterized RhoA, Rac1, and Cdc42 proteins, and play important roles in cellular functions such as cytoskeletal organization, morphology, proliferation, cell adherence, cell migration, cell contraction, and phagocytosis (1–6). Dysfunctional RhoGTPases have been found to be associated with many kinds of diseases such as inflammation, cancer, hypertension, stroke, heart failure, diabetes, and so on (7–11).
RhoA has been found to regulate cellular morphology, based on a number of observations. RhoA is a substrate of C3-toxin and Clostridium difficile toxin B by which it is inactivated through ADP-ribosylation at Asn-41 (12), and glycosylation at Thr-37 (13), respectively, resulting in morphological changes in which the cells become small and rounded (cell rounding). In 1996, Lang et al. (14) reported that PKA phosphorylates RhoA at Ser-188 in vitro and proposed that this plays a key role in the inactivation of this RhoGTPase. Their proposed mechanism is that RhoA-GTP is phosphorylated by PKA and extracted from the membrane in its GTP-bound form by RhoGDIα, thus terminating RhoA-GTP signaling prematurely (14). They further postulated that this RhoA inactivation underlies the morphological changes of cytotoxic lymphocytes such as those induced by C3 toxin. It is noteworthy that even in its GTP-bound active form, phosphorylated RhoA may bind to RhoGDIα. More recently many groups have reported similar findings for PKA or PKG in different types of cells, such as neuronal cells, renal fibroblasts, NIH3T3 cells, and so on, and speculated that a defective RhoA inactivation system might be related to diseases such as hypertension (15, 16).
Some previous studies have demonstrated the phosphorylation of geranylgeranylated RhoA at Ser-188 in intact cells (17, 18) and in vivo (19). Others have reported, however, that they could not detect the phosphorylation of RhoA in intact cells although many other studies have shown the in vitro phosphorylation of RhoA at Ser-188 by PKA or PKG (14, 20, 21). In addition, Bolz et al. (22) have reported that they failed to observe the PKG phospho-resistant effects of the RhoA-S188A mutant that were expected. By overexpression of the RhoA-S188A mutant in the resistant artery, they tried unsuccessfully to inhibit the effects of RhoA phosphorylation through the SNP-PKG pathway. These discrepancies might be due to the cells used, but another mechanism of inactivation of RhoA by PKA/PKG has also been postulated (23).
An alternative candidate for the PKA substrate may be RhoGDIα. RhoGDIα plays important roles in RhoGTPase regulation systems as a chaperon (6, 24, 25) and is well known as a partner of phosphorylated RhoA. The PKA-induced phosphorylation of RhoGDIα at Ser-174 was reported previously by two groups. DerMardlrosslan et al. (26) suggested in their study that PKA phosphorylates RhoGDIα only at Ser-174 in vitro, whereas Pak1 phosphorylates RhoGDIα at both Ser-101 and Ser-174. Qiao et al. (27, 28) demonstrated that PKA phosphorylates RhoGDIα at Ser-174 in vitro and when transfected into intact cells and speculated that phosphorylated RhoGDIα might inhibit RhoA. It must be noted, however, that these intact cell studies were conducted in the presence of endogenous RhoGDIα, which should exist at a 3-fold higher level than RhoA, and the analyses failed to show any interaction between RhoA and phosphorylated RhoGDIα.
In our current study, we observed that cardiac fibroblasts expressing a constitutively active and phosphorylation-resistant mutant RhoA, RhoA-G14V/S188A, undergo PKA-induced morphological changes, when RhoGDIα is co-expressed. This suggests that these morphological changes cannot be explained by the phosphorylation of RhoA alone. By performing a series of experiments involving: 1) a specific knockdown of endogenous RhoGDIα using siRNA; 2) re-expression of RhoGDIα-WT or -S174A; 3) phosphorylation analysis in immunoprecipitated RhoGDIα; and 4) coimmunoprecipitation studies between active RhoA and RhoGDIα, we have elucidated in our present analyses that the cAMP-induced phosphorylation of RhoGDIα at Ser-174 likely plays a key role in the cAMP-induced sequestration of RhoA in the cytosol and in the morphological changes in cardiac fibroblasts.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
The rat cardiac fibroblasts (29), HEK293 cells (30), and COS7 cells (31, 32) were maintained in DMEM containing 10% (v/v) fetal bovine serum. Transfections of rat cardiac fibroblasts and HEK293 cells were performed using Lipofectamine2000 (Invitrogen) via standard procedures and COS7 cells were transfected using adenovirus infection as described previously (31, 32). To obtain HEK293 cells stably expressing the human Myc-RhoA-G14V or G14V/S188A, clones were selected in medium containing 0.4 mg/ml of hygromycin, as described (31, 33).
Immunocytochemistry and Immunofluorescence Staining
Actin staining by Texas Red X-phalloidin (Invitrogen) was performed using standard procedures. Briefly, cells were placed onto the glass coverslips, washed twice with ice-cold PBS, and fixed with 4% fresh paraformaldehyde (Wako)/PBS for 10 min. After two further washes in PBS, the cells were permeabilized in 0.1% Triton X-100 for 5 min. After two further washes in PBS, the cells were blocked using 1% BSA/PBS at room temperature for 30 min and stained using Texas Red X/phalloidin (1:60) diluted in 1% BSA/PBS at room temperature for 30 min for actin.
Double staining with phalloidin and each antibody was performed as described previously (31, 34, 35). Briefly, rat cardiac fibroblasts with or without exogenously expressed Myc-RhoA mutant or HA-RhoGDIα were fixed in fresh 4% paraformaldehyde/PBS for 20 min, then permeabilized in 0.2% Triton X-100 for 10 min, washed twice with PBS, and blocked using 5% BSA/PBS for 30 min. Then cells were then incubated with anti-RhoGDIα (Santa Cruz, A20, 1:500 dilution) or anti-Myc (MBL, PL14, 1:2000 dilution) or anti-HA (Roche Applied Science, 12CA5, 1:500 dilution) antibodies diluted in 5% BSA/PBS at room temperature for 1 h. After three further washes, the cells were incubated with goat anti-mouse IgG tagged with Alexa 488 (Invitrogen, 1:400) or anti-rabbit IgG tagged with Alexa 488 (Invitrogen, 1:400) and Texas Red X-phalloidin (1:60) in 5% BSA/TBS for 1 h at room temperature. After another three washes in 0.1% Triton X-100/TBS and twice with TBS alone, the cells were mounted on glass slides for analysis. Fluorescence images were collected using an ECLIPSE E600 microscope (Nikon).
cAMP Assay
cAMP accumulation in cardiac fibroblasts was assayed as described previously (32, 33, 36). Briefly, cardiac fibroblasts were seeded into 24-well plates at 0.2 × 105 cells/well. Twenty-four hours later, the medium was replaced with serum-free medium to which [3H]adenine (2 μCi/ml, Amersham Biosciences) had been added. After a further 24 h, the cells were washed and incubated for 30 min with or without the indicated concentrations of agonists in the presence of 1 mm 3-isobutyl-1-methylxanthine. The reaction was terminated by removing the medium and lysing the cells in 5% trichloroacetic acid (500 μl/well) containing ATP and cAMP (each at 1 mm). [3H]cAMP and [3H]ATP fractions were separated on AG 50W-X4 and alumina columns, and cAMP accumulation was estimated by determining the ratio of cAMP radioactivity to the sum of the radioactivity of cAMP and ATP. The bars indicate cAMP accumulated for 30 min in cells cultured at 37 °C. Values shown represent mean ± S.D. of triplicate determinations.
Membrane Localization Assay
Cardiac fibroblasts (6 × 105) were seeded into 100-mm dishes or HEK293 cells (1.5 × 106) were seeded into 60-mm dishes without or with siRNA and DNA transfection. Twenty-four hours later (cardiac fibroblasts) or 36 h later (HEK293 cells), when the cells were subconfluent, the medium was replaced with serum-free medium. At 48 h, the cells were harvested in ice-cold PBS after incubation with isoproterenol for 1 h and in the case of cholera toxin (CTX)3 for 3 h. After centrifugation (400 × g for 5 min at 4 °C), the cells were lysed in hypotonic buffer (20 mm Tris-HCl, pH 7.5, 20 mm NaCl, 2 mm MgCl2, 1 mm EGTA, proteinase inhibitors) for 10 min, and then sheared using Dounce homogenization (15 times). The nuclear fractions and unbroken cells were removed by centrifugation (800 × g for 5 min at 4 °C). The supernatants were then ultracentrifuged (160,000 × g for 30 min at 4 °C) and the membrane and soluble cytosol fractions were separated. The pellet was lysed as a membrane fraction in lysis buffer (40 mm Tris-HCl, pH 7.5, 100 mm NaCl, 2 mm MgCl2, 1 mm EGTA, 1% Triton X-100, and proteinase inhibitors) for 1 h at 4 °C, and centrifuged (200 × g for 5 min at 4 °C). The supernatant was next mixed with the same volume of Laemmli sample buffer, and boiled for 5 min. Membrane protein extracts from the cardiac fibroblasts or HEK293 cells were then separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with monoclonal anti-RhoA antibody (1:500 dilution, Santa Cruz, 26C4) or anti-Myc antibody (1:2,000 dilution, MBL PL14).
RhoGDIα Knockdown by siRNA and Rescue
The sequences of siRNA molecules targeting rat RhoGDIα (#1 and #2; Invitrogen) are as follows: #1(RSS337674), GCUCGCUCAGAUAGCUGCAGAGAAU and #2(RSS337675), ACAAGGAUGAUGAAAGCCUUCGAAA and the homologous HA-Bos taurus RhoGDIα sequences are: for #1, GTTGGCACAGATTGCGGCAGAGAAC, and for #2, ACAAGGACGACGAGAGTCTGCGCAA. The underlined nucleotides are not complementary to the corresponding siRNA.
The sequences of siRNA molecules targeting human RhoGDIα (Ser-698; Ambion) are, CCAGCATACGTACAGGAAATT; and the homologous HA-B. taurus RhoGDIα sequences are, forward CCAGCACACGTACAGGAAAGG, and the added silent mutation to avoid siRNA interfering are: forward, CCAGCACACGTACCGCAAAGG. The underlined nucleotides are not complementary to the corresponding siRNA.
Transfection of siRNA alone or siRNA with DNA were performed using a standard protocol for Lipofectamine2000. First, rat cardiac fibroblasts were plated in 12-well dishes (0.2 × 105 cells/well) and after 24 h, when confluence of the cells was about 50%, siRNA (each at 20 nm) and EGFP-C3 as a control or HA-RhoGDIα-WT or -S174A in the pcDNA3.1 hygro+ vector (0.4 μg/well) were transfected to cells with Lipofectamine2000. Two days later, the cells were starved, and after an additional 24 h, the cells were collected using Laemmli sample buffer for Western blotting or fixed for immunocytochemistry. The bars indicate the ratio of the cells that responded to isoproterenol at 500 nm for 30 min. For each well, at least 100 cells were counted and the values shown represent mean ± S.D. of triplicate determinations.
HEK293 cells stably expressing Myc-RhoA-G14V or Myc-RhoA-G14V/S188A were plated in 24-well dishes (1.5 × 105 cells/24 well) or 60-mm dishes (1.5 × 106 cells/well), and after 24 h, when the confluence of the cells was about 50–60%, siRNA (5 nm) and HA-RhoGDIα-WT or -S174A in the pcDNA3.1 hygro+ vector (0.02 μg/24-well or 0.2 μg/60-mm dish) were transfected to the cells with Lipofectamine2000. 24 h later, the cells were re-seeded into collagen-coated glass or two 60-mm dishes, and after an additional 24 h, the cells were serum starved for 10 h, and collected using Laemmli sample buffer for Western blotting or fractionated for membrane analysis or fixed for immunocytochemistry.
Phosphorylation of RhoA and RhoGDIα
Cardiac fibroblasts (2 × 105) were seeded into 60-mm dishes. After 24 h, when the cells were subconfluent, the medium was replaced with serum-free medium. After a further 20 h, the cells were loaded with 100 μCi/ml of [32P]orthophospholic acid for 4 h in phosphate-free medium and then stimulated by 100 ng/ml of CTX for 3 h. The cells were then washed with ice-cold PBS and lysed with 200 μl of 1% SDS buffer (50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 10 mm EDTA, 10 mm EGTA, 1 mm DTT, 1% SDS). The lysates were then immediately boiled for 10 min, and diluted using Nonidet P-40 buffer to a final concentration of 0.5% SDS, 1% Nonidet P-40 buffer (0.5% SDS, 1% Nonidet P-40, 50 mm Tris-HCl, pH 7.5, 100 mm NaCl, 10 mm EDTA, 10 mm EGTA, 1 mm DTT). Lysates were incubated with anti-RhoA(26C4) antibody (1 μg/sample) or anti-RhoGDIα(A-20) antibody (1 μg/sample) (as described for the immunoprecipitation method below) at 4 °C for 1 h, followed by the addition of 20 μl of Protein G+, and incubated for an additional hour at 4 °C. After several washes with 0.5% SDS, 1% Nonidet P-40 buffer, the resins containing immunocomplexes were vortexed and boiled in a mixture of 30 μl of 0.5% SDS, 1% Nonidet P-40 buffer and 30 μl of Laemmli sample buffer. The proteins were then separated by SDS-PAGE, and immunoblotted against a rabbit polyclonal anti-phospho-RhoGDIα-S174 antibody (Abcam, 1:500) or anti-phospho-RhoA-S188 antibody (Abcam, 1:500). In the Western blotting procedure, 1% BSA/PBS was used as a blocking buffer and a dilution buffer for the anti-phosphoserine antibodies. Western blotting analysis was also performed with the anti-RhoGDIα antibody (A-20, 1:1000) or anti-RhoA antibody (26C4, 1:1000) to normalize the protein loading.
For COS7 cells, HA-RhoGDIα (WT) (0.06 μg of DNA per 0.8 × 106 cells/35-mm dish) or myc-RhoA-G14V (0.06 μg of DNA per 0.8 × 106 cells/35-mm dish) were transfected into COS7 cells 48 h before the assay. Phosphate-binding Tag (Phos-tag) Western blotting was performed using the running gel of SDS-PAGE added with 25 μm Phos-tag and 50 μm MnCl2 as described (37).
Phosphorylation of Myosin Phosphatase Target Subunit 1 (MYPT1) (Thr-696)
The whole cell lysate of cardiac fibroblasts or HEK293 cells were separated on SDS-PAGE (8% polyacrylamide gel), transferred to PVDF membranes (100 V, 3 h), and immunoblotted with rabbit polyclonal anti-phospho-MYPT1 (Thr-696) (Millipore, 1:5000 dilution) (38).
Co-immunoprecipitation Assay
Both HA-RhoGDIα (WT or S174A) (0.48 μg of DNA per 0.8 × 106 cells/35-mm dish) and GFP-RhoA (G14V, G14V/S188A, or WT) (0.12 μg of DNA per 0.8 × 106 cells/35-mm dish) or constitutively active myc-RhoGTPases (RhoA-G14V, Rac1-G12V, or Cdc42-G12V) (0.12 μg of DNA per 0.8 × 106 cells/35-mm dish) were transfected into COS7 cells, and after 24 h, the medium was replaced with serum-free medium. After a further 48 h, the cells were stimulated with or without 200 μm forskolin for 30 min. The reactions were terminated by washing with ice-cold PBS and the cells were lysed in immunoprecipitation buffer (50 mm Tris-HCl, pH 7.5, 40 mm NaCl, 10 mm EGTA, 10 mm sodium PPi, 100 mm NaF, 1 mm DTT, 1% Triton-X, and proteinase inhibitors) for 1 h at 4 °C. The lysates were incubated with 1 μg/sample of mouse monoclonal anti-GFP antibody (MBL, 1E4) or anti-Myc antibody (MBL, PL14) for 1 h at 4 °C with rocking. This was followed by the addition of 20 μl of Protein G+ solution (50% in resin bed) and tumbled for another 1 h at 4 °C. After several further washes with immunoprecipitation buffer, the resins were vortexed and boiled after adding equal volume of 2× Laemmli sample buffer. The proteins were separated by SDS-PAGE and Western blot analysis was performed using rabbit polyclonal anti-GFP (MBL, 1:4000) or anti-Myc (MBL, 1:2000) antibodies to analyze the immunoprecipitated GFP/Myc each or using anti-HA antibody (Roche Applied Science, 12CA5, 1:2000) to analyze co-immunoprecipitated HA-RhoGDIα.
Statistical Method and Analysis
The statistical method used was the Dunnett's multiple comparison procedures. Averaged data of three independent experiments are shown, and error bars represent the S.D. These analyses were performed using Microsoft Excel software, *, p < 0.02.
RESULTS
Morphological Changes in the Cardiac Fibroblasts Are Induced by cAMP Elevation and ROCK Inhibition
Cell rounding of cardiac fibroblasts, a characteristic morphological change, is induced by isoproterenol (Fig. 1A) at a 100–500 nm dosage range with almost complete rounding induced at 1 μm (data not shown). CTX (Fig. 1A) and forskolin (data not shown) also produce similar changes in cellular morphology, indicating that it is cAMP that primarily induces cell rounding. These changes are reversed in the absence of further stimulation for 24 h. In our current experiments, we inhibited the effector of RhoA, ROCK, using Y27632 (Fig. 1A, d), which induced a similar morphological change, as previously reported (39, 40). This suggested that this morphological change is due to inhibition of the RhoA-ROCK signaling pathway. We also obtained a similar result in HEK293 cells (Fig. 1A, e and f).
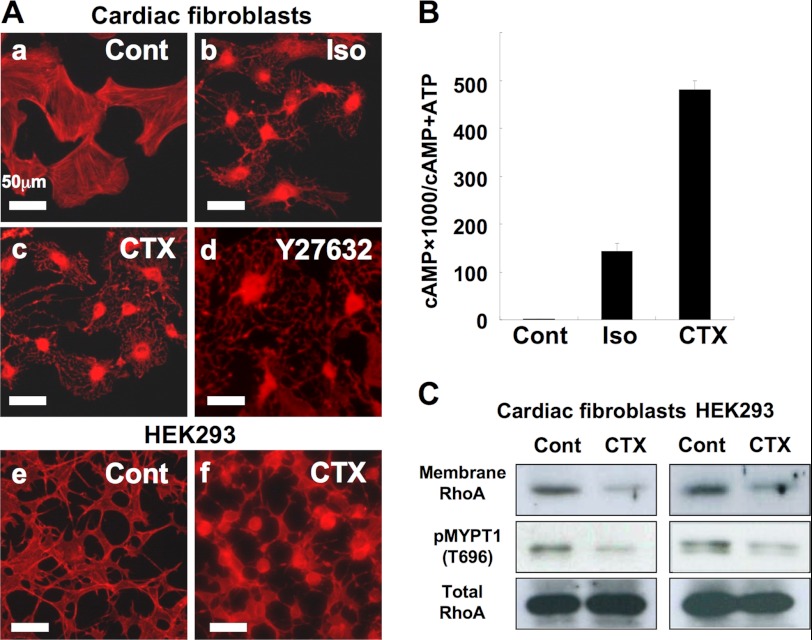
cAMP-induced regulation of cell morphology and RhoA localization. A, cAMP-induced morphological changes in cardiac fibroblasts and HEK293 cells. Cardiac fibroblasts were incubated with (a) serum-free medium for 1 h; (b) 10 μm isoproterenol for 1 h; (c) cholera toxin (100 ng/ml) for 3 h; (d) 10 μm Y27632 for 1 h. HEK293 cells were incubated with (e) serum-free medium for 1 h or (f) cholera toxin (1 μg/ml) for 3 h. B, cAMP accumulation by isoproterenol or cholera toxin in cardiac fibroblasts. Cardiac fibroblasts were seeded into 24-well plates at 0.2 × 105 cells/well. Twenty-four hours later, the medium was replaced with serum-free medium to which [3H]adenine (2 μCi/ml, Amersham Biosciences) was added. After a further 24 h, the cells were washed and incubated for 30 min at 37 °C in serum-free medium with or without 10 μm isoproterenol in the presence of 1 mm isobutylmethylxanthine. Cholera toxin (100 ng/ml) was added to the medium 3 h before adding isobutylmethylxanthine. cAMP accumulation was measured as described under “Experimental Procedures.” Data are the mean ± S.D. of three independent experiments. C, membrane localization of RhoA and phosphorylation of MYPT1 (Thr-696). The membrane localization of RhoA and phosphorylation of MYPT1 (Thr-696) in cardiac fibroblasts and HEK-293 was quantified using Western blotting. After a 24-h (cardiac fibroblasts) or 12-h (HEK293 cells) incubation in serum-free DMEM, the cells were incubated in serum-free medium without or with 1 μg/ml of cholera toxin for 3 h and harvested for whole cell lysate for phosphorylated MYPT1 and total RhoA or fractionated for membrane RhoA. RhoA (in 5 μg of membrane protein) and phosphorylation of MYPT1 (Thr-696) was detected by immunoblotting against a specific antibody as described under “Experimental Procedures.” Each set of results is representative of at least two additional experiments.
We next confirmed that cAMP accumulation is induced by isoproterenol or CTX in cardiac fibroblasts (Fig. 1B). As previously indicated, we suspected that the morphological change accompanying cAMP accumulation in these may be the result of RhoA inhibition, and investigated whether RhoA is blocked by treatment with isoproterenol or CTX. It has been shown that activated RhoA is localized in the membrane fraction. As previously reported (14, 21), RhoA in the membrane fraction of cardiac fibroblasts is decreased by treatments with cholera toxin (Fig. 1C) or isoproterenol (data not shown) and a similar result is observed in HEK293 cells (Fig. 1C). In parallel, phosphorylation of MYPT1 (Thr-696), which reflects Rho-associated kinase (ROCK activity), is decreased (Fig. 1C). These data suggest that cAMP inhibits RhoA activity.
A Phosphoresistant Mutant of RhoA Does Not Prevent Morphological Changes Induced by PKA Activation
Our results were consistent with previous reports that morphological changes in cardiac and other cell types are due to RhoA inactivation through PKA activation. We thus predicted that cells expressing RhoA-G14V/S188A would be resistant to CTX treatment, and that cells expressing RhoA-G14V would remain sensitive to this agent. Surprisingly, however, both RhoA-G14V and Rho-G14V/S188A were equally resistant to CTX treatment (Fig. 2). We speculated from this that the quantity of RhoA at the plasma membrane might exceed the capacity of endogenous RhoGDIα to sequester GTP-RhoA in the cytosol, and that remnant GTP-RhoA proteins might maintain cell morphology without being sequestered despite their PKA-dependent phosphorylation.
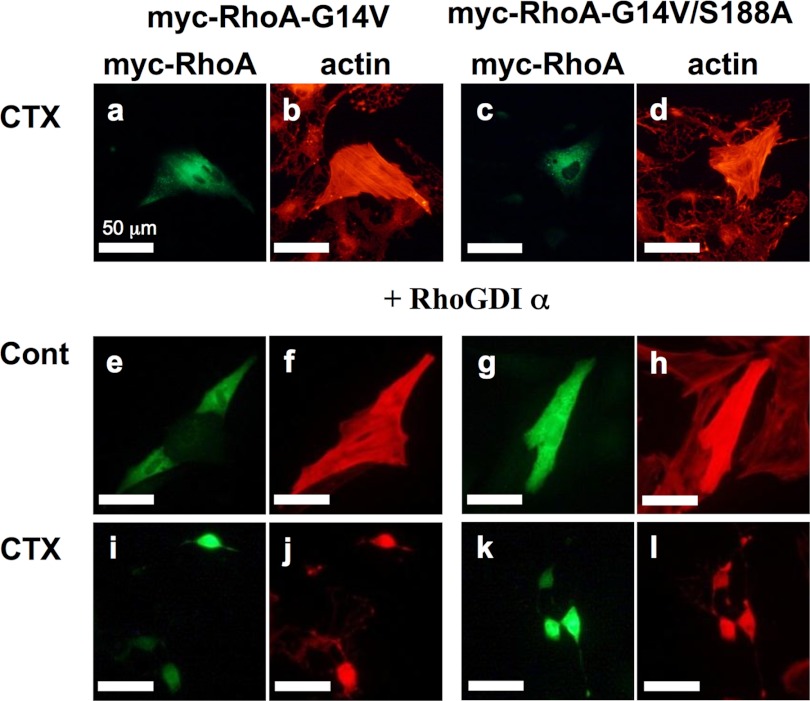
Expression of a constitutively active phospho-resistant mutant of RhoA is not sufficient to maintain the normal morphology of cardiac fibroblasts following PKA stimulation in the presence of co-expressed RhoGDIα. Cardiac fibroblasts transiently expressing Myc-tagged RhoA-G14V or RhoA-G14V/S188A without or with B. taurus HA-RhoGDIα were incubated with anti-Myc antibody (1:2000 dilution) and Texas Red X-phalloidin (1:60 dilution) as described under “Experimental Procedures.” Cholera toxin (100 ng/ml) was added to the medium 3 h before the assay (a–d and i–l). Each set of results is representative of at least two additional experiments.
According to the reports by Michaelson et al. (41) the molar sum of RhoA, Rac1, and Cdc42 is approximately equal to the molar amount of RhoGDIα, and the RhoGDIα/RhoA molar ratio is between 3:1 and 8:1. Moreover, it has been reported that about 2–3% of the endogenous RhoA is located at the plasma membrane and that constitutively active RhoA, which is less than 10% of the total endogenous RhoA, is sufficient to induce changes in the actin cytoskeleton (42–45). Based on these stoichiometric ratios of RhoA and RhoGDIα, we speculated that the transient expression of RhoA-G14V increases RhoA localization at the plasma membrane, which exceeds the capacity of endogenous RhoGDIα to sequester it in complex form. To avoid any stoichiometric imbalance, we next transiently expressed a RhoA mutant with RhoGDIα. Surprisingly, however, cells expressing RhoA-G14V/S188A showed cAMP-induced cell rounding as prominently as cells expressing RhoA-G14V (Fig. 2). To resolve the question of why the PKA-dependent morphological change occurs in cells expressing RhoA-G14V/S188A, we postulated that the PKA-induced phosphorylation of RhoGDIα at Ser-174 (26, 28) might inhibit activated RhoA and play an important role in the onset of morphological changes in cardiac fibroblasts.
siRNA of Rat RhoGDIα Inhibits the Endogenous Expression of RhoGDIα and Inhibits PKA-induced Morphological Change
To test the hypothesis that phosphorylated RhoGDIα at Ser-174 sequesters RhoA at the plasma membrane and inactivates it, we first transiently expressed the RhoGDIα-S174A mutant or RhoGDIα-WT in cardiac fibroblasts and investigated the impact of this on cAMP-induced morphological changes. However, we observed no differences between the cells expressing RhoGDIα-WT and those expressing RhoGDIα-S174A. We thus speculated that endogenous phosphorylated RhoGDIα may cause cell rounding even in cells expressing RhoGDIα-S174A. Our negative data contrast with the positive data reported from transient expression experiments in COS7 cells in another study (28).
To avoid any interference from endogenous RhoGDIα, we next tried to deplete endogenous RhoGDIα by siRNA and then express RhoGDIα-WT or its mutant exogenously. Two siRNAs targeting rat RhoGDIα (20 nm) were co-transfected with or without a HA-tagged B. taurus RhoGDIα expression construct into rat cardiac fibroblasts and successfully depleted the expression of endogenous rat RhoGDIα (Fig. 3A). In contrast, neither siRNA molecule disrupted the exogenous expression of HA-tagged B. taurus RhoGDIα (Fig. 3A) or other proteins such as MAPK (data not shown), confirming their specificity.
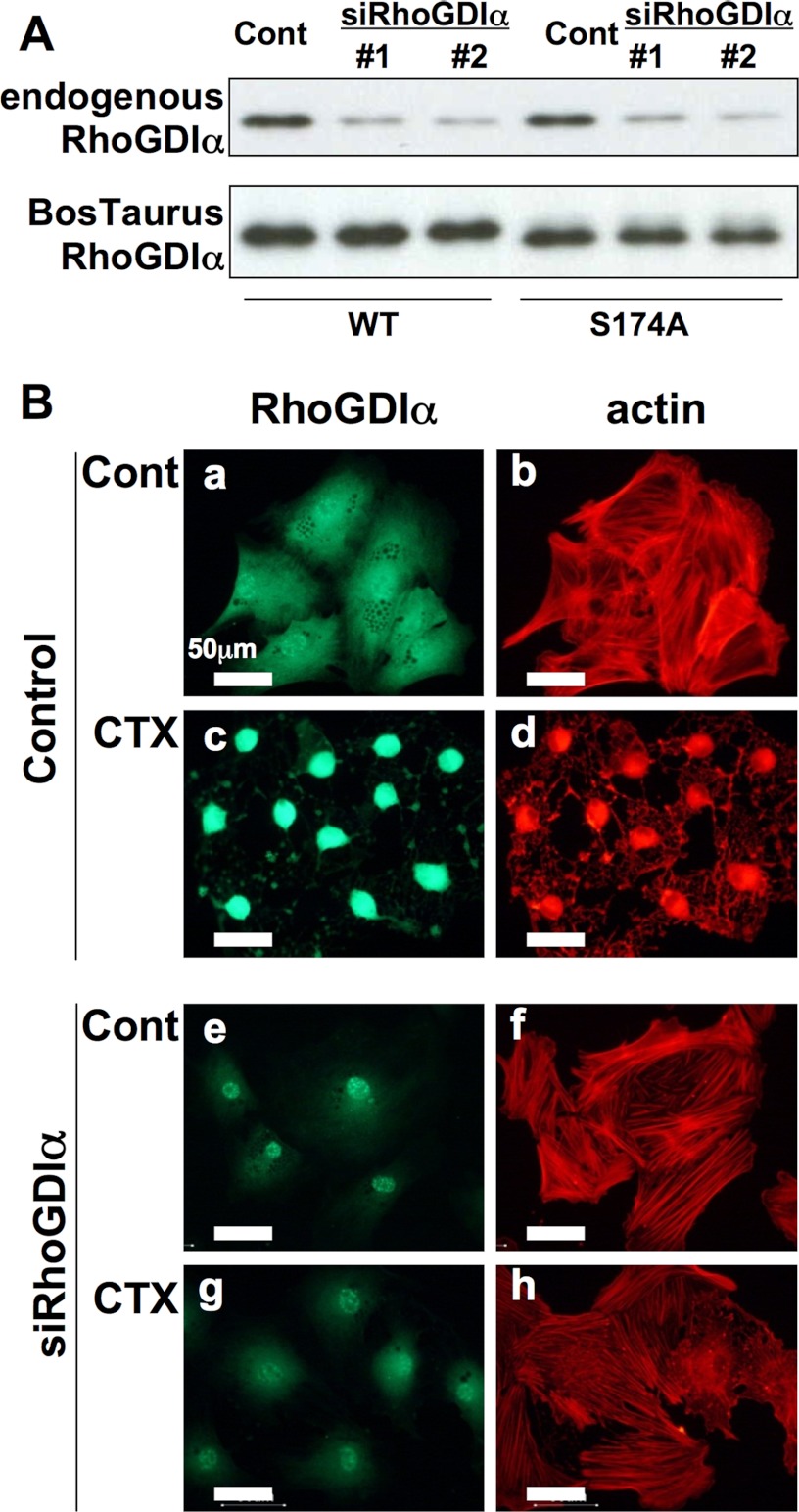
Effects of a knockdown of rat RhoGDIα by siRNA and B. taurus HA-RhoGDIα expression on cAMP-induced cell morphological changes. A, immunoblotting of RhoGDIα. Cardiac fibroblasts were transfected with siRNA against rat RhoGDIα (20 nm) without (upper) or with (lower) HA-tagged B. taurus RhoGDIα construct (0.4 μg/0.2 × 105 cells/24 well) and the proteins were detected using anti-RhoGDIα antibody (1:500 dilution) or anti-HA antibody (1:2000 dilution). B, immunofluorescence staining. Cardiac fibroblasts were transfected with a combination of two siRNAs (#1 and #2, 20 nm each) targeting rat RhoGDIα and incubated with anti-RhoGDIα antibody (1:500 dilution) and Texas Red X-phalloidin (1:60 dilution) as described under “Experimental Procedures.” Cholera toxin (100 ng/ml) was added to the medium 3 h before the assay (c and d and g and h). Each set of results is representative of at least two additional experiments.
The effectiveness of the two RhoGDIα siRNAs was demonstrated in a rat RhoGDIα immunoblotting assay (Fig. 3A, upper lanes) and by immunofluorescent analysis of the expression of this protein (Fig. 3B, a and e). Most cells transfected with this siRNA mixture showed no morphological changes following CTX treatment (Fig. 3B, f and h). This observation, however, does not contradict the well established hypothesis that phosphorylated RhoA is sequestered by RhoGDIα in the cytosol.
Exogenously Transfected RhoGDIα-WT, but Not Its S174A Mutant, Reverses the cAMP-induced Morphological Changes in Cells Depleted of Endogenous RhoGDIα
We transfected rat cardiac fibroblasts with a wild type or S174A mutant HA-tagged B. taurus RhoGDIα together with the aforementioned siRNA mixture. We found that the expression of HA-tagged RhoGDIα-WT or its S174A mutant was observed in about 10% of the cells, whereas transfection efficiency of the siRNAs was more than 90%. Therefore, almost all of the cells expressing HA-tagged RhoGDIα were co-transfected with siRNA (data not shown). The cells expressing HA-tagged RhoGDIα-WT, but not those expressing RhoGDIα-S174A, showed morphological changes following CTX treatment (Fig. 4A). The ratio of cells showing cAMP-induced morphological changes was quantitatively evaluated (Fig. 4B). The expression levels of these HA-RhoGDIα proteins were almost equal (Fig. 4B). These data suggest that the phosphorylation of RhoGDIα-S174 by PKA is likely to play an important role in the PKA-induced morphological change of cardiac fibroblasts.
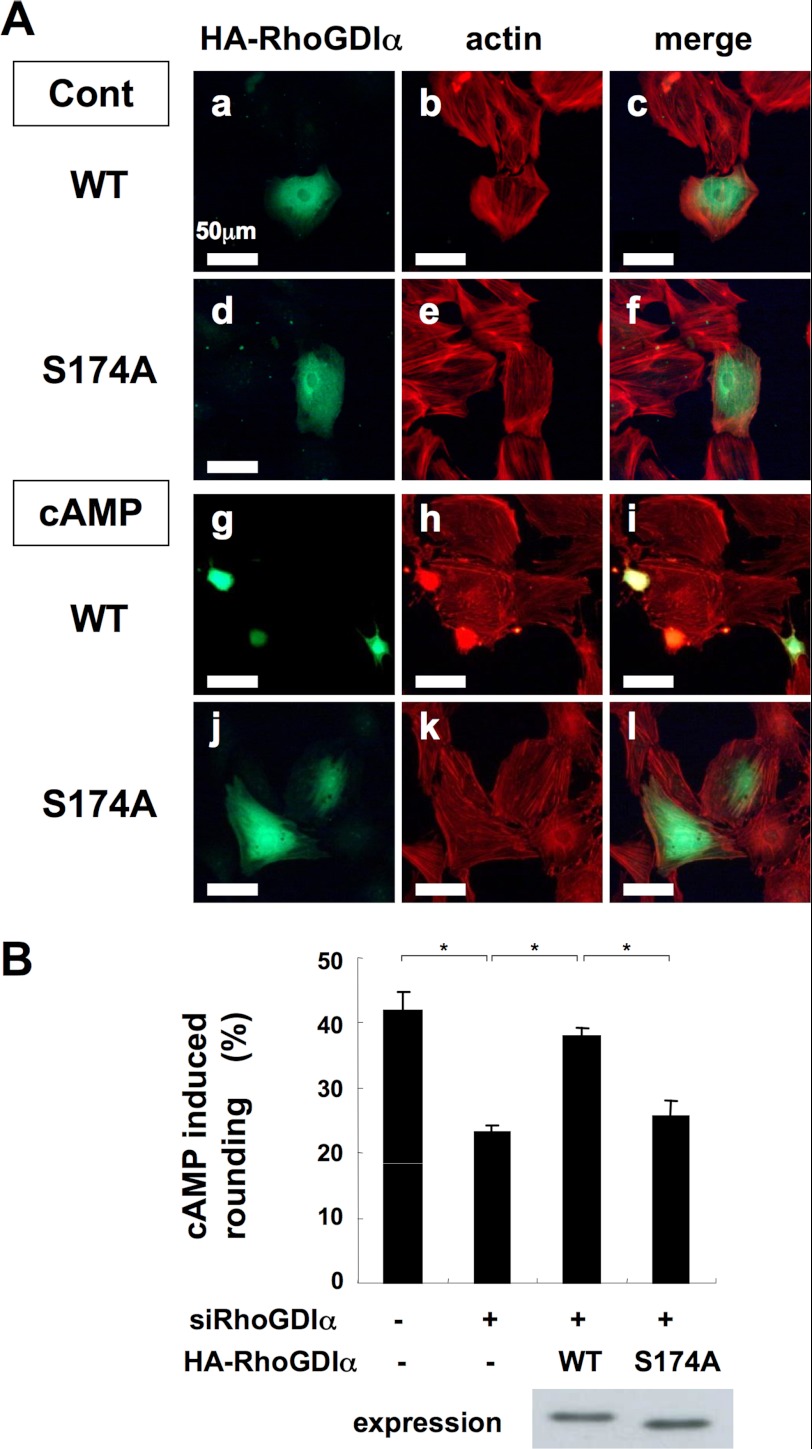
Rescue of rat RhoGDIα-knockdown by HA-tagged B. taurus RhoGDIα. A, immunofluorescence staining of B. taurus RhoGDIα. Rat cardiac fibroblasts were transfected with a combination of two siRNAs (#1 and #2, 20 nm each) targeting rat RhoGDIα and with wild type or S174A mutant HA-tagged B. taurus RhoGDIα (as described in Fig. 3A). The cells were immunostained with anti-HA antibody (1:2000 dilution) and Texas Red X-phalloidin (1:60 dilution). Cholera toxin was added to the medium 3 h before the assay. B, the ratio of cAMP-induced metamorphoses. Cardiac fibroblasts were transfected with the siRNA mix targeting rat RhoGDIα and with wild type or S174A mutant HA-tagged B. taurus RhoGDIα as described in A. After stimulation with 500 nm isoproterenol for 30 min, the cells were incubated with anti-HA antibody (1:2000 dilution) and Texas Red X-phalloidin (1:60 dilution). The ratio of the cAMP-induced metamorphosis of cardiac fibroblasts was quantified as described under “Experimental Procedures.” No difference between the expression level of RhoGDIα WT and S174A was shown by Western blotting. Data are the mean ± S.D. of three independent experiments. *, p < 0.02. Each set of results is representative of at least two additional experiments.
To confirm our hypothesis, we next made HEK293 cell lines stably expressing Myc-RhoA-G14V or Myc-RhoA-G14V/S188A and investigated the correlation between cell rounding, active RhoA distribution, and MYPT1 phosphorylation. siRNA-induced knockdown of endogenous RhoGDIα and its rescue by HA-tagged B. taurus RhoGDIα operated successfully in HEK293 cells (Fig. 5A). We then transfected HEK293 cells stably expressing RhoA-G14V or RhoA-G14V/S188A with a wild type or S174A mutant HA-tagged B. taurus RhoGDIα together with the siRNA. We found that transfection efficiency of HA-tagged RhoGDIα or siRNA was more than 90% (data not shown). Without CTX treatment, neither of the two HEK293 stable cell lines showed cell rounding (Fig. 5B, a–d). After CTX treatment, the cells (in both cell lines) expressing HA-tagged RhoGDIα-WT, but not those expressing RhoGDIα-S174A, showed morphological changes (Fig. 5B, e–h). These paralleled with decreases in membrane Myc-RhoA proteins and MYPT1 phosphorylation (Fig. 5C). These data further support the notion that phosphorylation of RhoGDIα-Ser-174 by PKA may play a key role in the PKA-induced morphological change and RhoA inhibition.
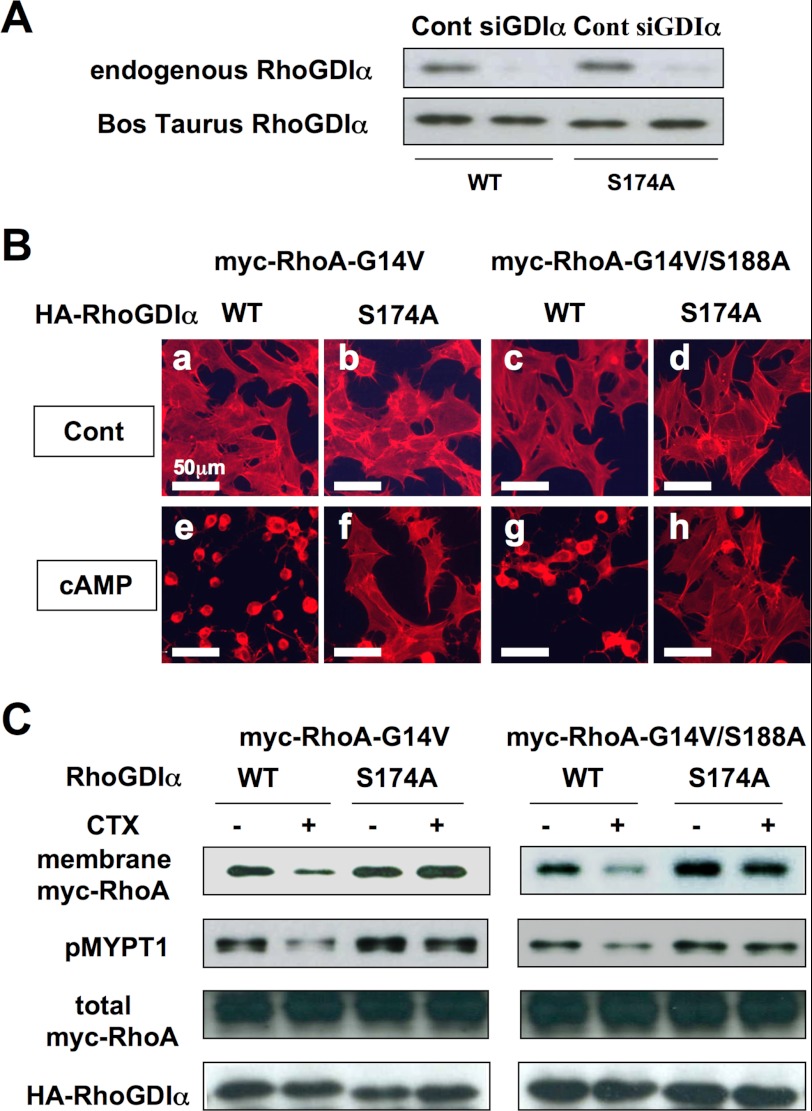
Knockdown of RhoGDIα by siRNA and rescue by HA-tagged B. taurus RhoGDIα in HEK293 cells. A, immunoblotting of RhoGDIα. HEK293 were transfected with siRNA against human RhoGDIα (5 nm) without (upper) or with (lower) the HA-tagged B. taurus RhoGDIα (with silent mutations) construct (0.02 μg/1.5 × 105 cells/24 well) and the proteins were detected using anti-RhoGDIα antibody (1:2000 dilution) or anti-HA antibody (1:2000 dilution). B, immunofluorescence staining. HEK293 cells stably expressing Myc-RhoA-G14V or Myc-RhoA-G14V/S188A were transfected with siRNA targeting human RhoGDIα and with wild type or S174A mutant HA-tagged B. taurus RhoGDIα (as described in A). The cells were immunostained with Texas Red X-phalloidin (1:60 dilution) as described under “Experimental Procedures.” Cholera toxin (1 mg/ml) was added to the medium 3 h before the assay (e–h). C, membrane localization of RhoA and phosphorylation of MYPT1. HEK293 stably expressing Myc-RhoA-G14V or Myc-RhoA-G14V/S188A transfected with siRNA targeting human RhoGDIα and with wild type or S174A mutant HA-tagged B. taurus RhoGDIα (as described in B) was harvested as a whole cell lysate for phospho-MYPT1(T696), total Myc-RhoA, and total HA-RhoGDIα or fractionated for membrane Myc-RhoA as described in the legend to Fig. 1C. Myc-RhoA (in 5 μg of membrane protein) and phosphorylation of MYPT1 (Thr-696), total Myc-RhoA, and total HA-RhoGDIα were detected by immunoblotting against a specific antibody as described under “Experimental Procedures.” Cholera toxin (1 μg/ml) was added to the medium 3 h before the assay. Each set of results is representative of at least two additional experiments.
The Phosphorylation of RhoGDIα at Ser-174, but Not That of RhoA at Ser-188, Is Detectable in Intact Cells
We next examined whether endogenous RhoGDIα is phosphorylated at Ser-174 by PKA in intact cardiac fibroblasts. The phosphorylation of RhoGDIα at Ser-174 or RhoA at Ser-188 by CTX treatment was analyzed in whole cell lysates of cardiac fibroblasts using the respective anti-phosphoprotein antibodies, but none was detected. Each phosphorylation event was then investigated in immunoprecipitated protein preparations. The phosphorylation of endogenous RhoGDIα at Ser-174 was detected by immunoprecipitation with a RhoGDIα antibody followed by immunodetection with an anti-phospho-RhoGDIα (Ser-174) antibody. The phosphorylation of RhoA on Ser-188 was not detected in the same way using the corresponding antibodies (Fig. 6A, left panel). In addition, similar results were obtained when 32P phosphorylation of endogenous RhoGDIα and RhoA was investigated (Fig. 6A, left panel). These results were confirmed using exogenously tagged RhoGDIα and RhoA in COS7 cells (Fig. 6A, right panel).
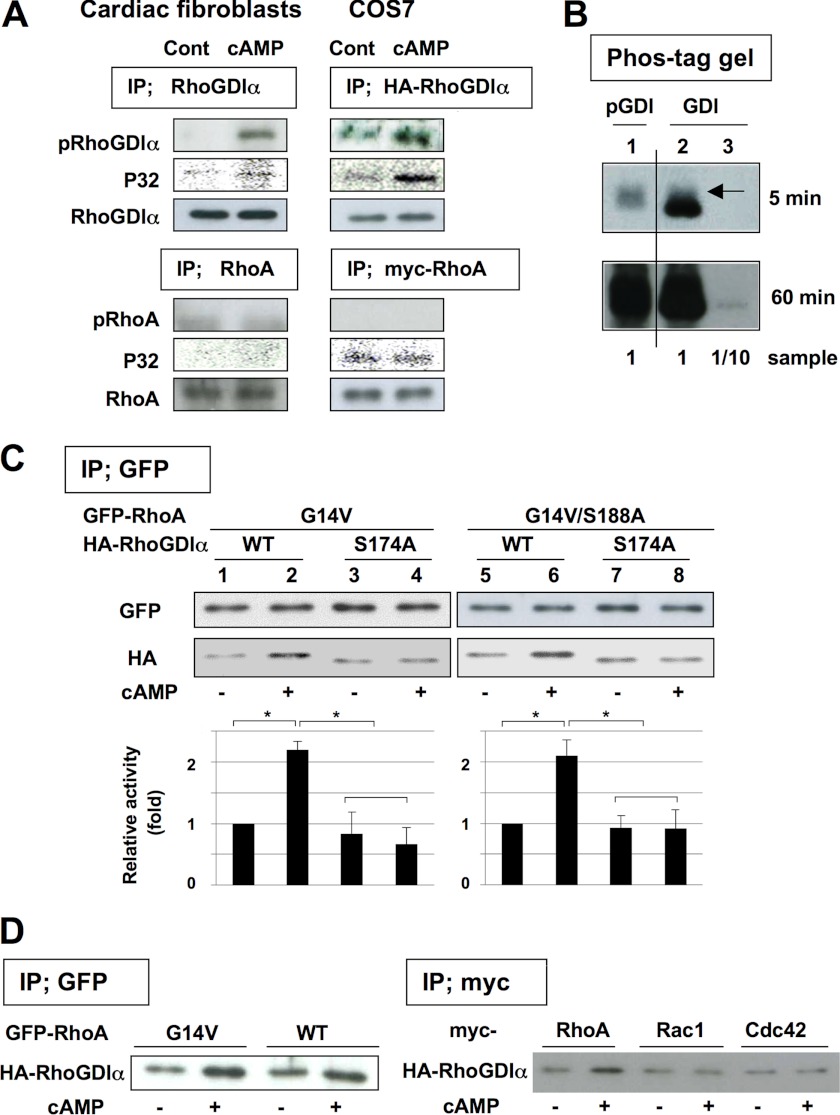
Phosphorylation of RhoGDIα and coimmunoprecipitation of RhoGDIα with RhoA. A, cAMP-induced phosphorylation of RhoGDIα in cardiac fibroblasts (left panel) and COS7 cells (right panel). The cells were incubated without or with cholera toxin (1 μg/ml) for 3 h (for cardiac fibroblasts) or with 200 μm forskolin for 1 h (for COS7 cells transfected with HA-RhoGDIα or myc-RhoA) and lysed. In cardiac fibroblasts, RhoGDIα or RhoA was immunoprecipitated (IP) using anti-RhoGDIα antibody or anti-RhoA antibody (1 μg each), respectively. In COS7 cells, HA-RhoGDIα or Myc-RhoA were immunoprecipitated using anti-HA antibody or anti-Myc antibody (1 μg each), respectively. Phosphorylated proteins were then detected using anti-phospho-RhoGDIα-S174 antibody (1:1000 dilution) or anti-phospho-RhoA-S188 antibody (1:1000 dilution), respectively. For 32P phosphorylation experiments, the cells were loaded with 100 μCi/ml of 32P for 20 h before the experiments and 32P-labeled phosphorylated proteins were detected by autoradiography. B, stoichiometric analysis of RhoGDIα phosphorylation. The phosphorylated and nonphosphorylated RhoGDIα in cardiac fibroblasts was separated by using Phos-tag gel as described under “Experimental Procedures.” Phosphorylated RhoGDIα or RhoGDIα were detected using anti-phospho-RhoGDIα-S174 antibody (1:1000 dilution, lane 1) or anti-RhoGDIα antibody (1:30,000 dilution, lanes 2 and 3), respectively. Arrow in lane 2 corresponds phosphorylated RhoGDIα. C, cAMP regulates the coimmunoprecipitation of RhoGDIα with active RhoA. COS7 cells expressing GFP-RhoA (constitutively active G14V or G14V/S188A) and HA-RhoGDIα (wild type or S174A) were incubated with 200 μm forskolin for 1 h and lysed. Then GFP-RhoA was immunoprecipitated using 1 μg of anti-GFP antibody and the immunoprecipitated proteins were detected using anti-GFP antibody (1:2000 dilution) or anti-HA antibody (1:2000 dilution, for detecting co-immunoprecipitated HA-RhoGDIα). The relative activity of coimmunoprecipitated HA-RhoGDIα was quantified as described under “Experimental Procedures.” Data are the mean ± S.D. of three independent experiments. *, p < 0.02. Each set of results is representative of at least two additional experiments. D, specificity for phosphorylated RhoGDIα interaction. COS7 cells expressing GFP-RhoA (G14V or WT (left panel)) or Myc-RhoA-G14V, Myc-Rac1-G12V, or Myc-Cdc42-G12V (right panel) together with HA-RhoGDIα (wild type) were incubated with 200 μm forskolin for 1 h and lysed. Then GFP-RhoA or Myc-RhoGTPase was immunoprecipitated using anti-GFP antibody or anti-Myc antibody (1 μg each), respectively, and co-immunoprecipitated HA-RhoGDIα was quantified as described in C. Each set of results is representative of at least two additional experiments.
The Stoichiometic Analysis of RhoGDIα Phosphorylation
To determine what percentage of endogenous RhoGDIα of cardiac fibroblasts can be phosphorylated, we analyzed the CTX-treated RhoGDIα sample in Fig. 6A using Phos-tag gel. The phosphorylated RhoGDIα (Fig. 6B, lane 1 and arrow in lane 2) was barely separated from nonphosphorylated RhoGDIα. 6B). At 5 min exposure, phosphorylated RhoGDIα (arrow in lane 2) but not nonphosphorylated RhoGDIα of 1/10 sample was detected, suggesting that at least more than 10% (likely 20–30%) of endogenous RhoGDIα can be phosphorylated by PKA in cardiac fibroblasts.
The Phosphorylation of RhoGDIα at Ser-174 Increases Its Affinity for RhoA
To verify the effects of phosphorylation of RhoGDIα at Ser-174 on activation of the RhoA-RhoGDIα complex, the affinity between the RhoA-G14V mutant and RhoGDIα was examined in co-immunoprecipitation experiments. We co-expressed GFP-tagged RhoA-G14V or RhoA-G14V/S188A with HA-tagged B. taurus RhoGDIα-WT or RhoGDIα-S174A in COS7 cells and then immunoprecipitated GFP-RhoA with an anti-GFP antibody and detected co-immunoprecipitated HA-RhoGDIα using an anti-HA antibody.
The HA-RhoGDIα co-immunoprecipitated by anti-GFP antibody was then examined by immunoblotting. The levels of RhoGDIα-WT co-immunoprecipitated with RhoA-G14V were found to be increased by forskolin treatment, whereas those of RhoGDIα-S174A co-immunoprecipitated with RhoA-G14V were unchanged by the presence of forskolin (Fig. 6C, lanes 1–4). The level of RhoGDIα-WT co-immunoprecipitated with RhoA-G14V/S188 was also found to be increased by forskolin treatment, but not that of RhoGDIα-S174A co-immunoprecipitated with RhoA-G14V/S188A (Fig. 6C, lanes 5–8). These findings indicate that the cAMP-induced phosphorylation of RhoGDIα-S174, but not that of RhoA-S188, is a likely determinant of the increased affinity between active RhoA and RhoGDIα. This may underlie the molecular mechanism of cAMP-dependent RhoA translocation from the membrane to the cytosol.
Last, we investigated whether the interaction of phosphorylated RhoGDIα is specific in two ways (Fig. 6D). First, to investigate the specificity between RhoA-GTP and RhoA-GDP, we co-expressed HA-RhoGDIα with GFP-RhoA-G14V or GFP-RhoA-WT in COS7 cells and analyzed HA-RhoGDIα co-immunoprecipitated with GFP-RhoA. The forskolin-induced increase of HA-RhoGDIα co-immunoprecipitated with GFP-RhoA-G14V appeared to be more prominent than that co-immunoprecipitated with GFP-RhoA-WT. This was likely because the basal interaction of RhoA-WT and RhoGDIα is more prominent than that of RhoA-G14V and RhoGDIα at least in part.
To investigate the specificity among RhoGTPases, we co-expressed HA-RhoGDIα with Myc-RhoA-G14V, Myc-Rac1-G12V, or Myc-Cdc42-G12V in COS7 cells and analyzed HA-RhoGDIα co-immunoprecipitated with each Myc-RhoGTPase. The forskolin-induced increase of coimmunoprecipitated HA-RhoGDIα was observed with RhoA but not with Rac1 or Cdc42, suggesting that the cAMP-induced regulation is likely to be specific for RhoA.
DISCUSSION
In our present study, we have elucidated the principal molecular mechanism underlying Gs/cAMP-induced cross-talk with the Gq/G13/RhoA signaling pathway. Our findings reveal that the cAMP-induced phosphorylation of RhoGDIα at Ser-174 is likely to play a key role in the cAMP-induced sequestration of RhoA in the cytosol and in the consequent cell rounding of cardiac fibroblasts. Our current findings show that: 1) the knockdown of endogenous RhoGDIα by siRNA inhibits cAMP-induced morphological changes (Fig. 3); 2) the replacement of RhoGDIα-WT in cells in which endogenous RhoGDIα is knocked down recovers the potency of the cAMP-induced morphological change mechanism, whereas the replacement of RhoGDIα-S174A does not (Figs. 4 and and55B); 3) PKA phosphorylates RhoGDIα at Ser-174 in intact cells (Fig. 6A); and 4) the phosphorylation of RhoGDIα is likely to increase its affinity to the GTP-form of RhoA (Fig. 6C). It must be noted, however, that our data do not rule out the possibility of an additional role of RhoA phosphorylation in intact cells (46). We first discuss the detailed molecular mechanism underlying cAMP-dependent inactivation, and elaborate on our novel working model, its structural implications, and the possibility that there is a dual regulation of RhoA by RhoGDIα.
Molecular Mechanism of cAMP-dependent Inactivation of RhoA
It has been hypothesized that PKA-induced morphological changes are caused by phosphorylation of RhoA at Ser-188, which increases the affinity of RhoA-GTP for RhoGDIα and causes GTP-bound RhoA to be sequestered from the membrane to the cytosol in a complex with RhoGDIα. However, this has been mainly concluded from in vitro data using cell-free systems (14–16, 20, 21, 47, 48). Many groups have reported in cell-free systems that PKA can phosphorylate RhoA at Ser-188 and some of these laboratories have identified an almost stoichiometric phosphorylation. In contrast, only a few groups have observed the phosphorylation of RhoA in intact cells (17, 18). This can be partly explained by the fact that the geranylgeranylation of RhoA in intact cells diminishes its phosphorylation by PKA. Yet, based on the previous finding that PKA seems to phosphorylate active RhoA specifically because RhoGDIα binding to RhoA inhibits the phosphorylation of RhoA in vitro (14), it has been postulated that, even in intact cells, RhoA in its GTP form (2–3% of the total RhoA and therefore possibly below the threshold of detection) is phosphorylated by PKA and sequestered away from the membrane in its GTP-bound form by RhoGDIα (14).
Two groups have reported that RhoGDIα is phosphorylated at its Ser-174 residue by PKA in vitro, and one of these laboratories has demonstrated the Ser-174 phosphorylation of expressed RhoGDIα in intact cells and speculated that this may inhibit RhoA activity (26, 28). Bolz et al. (22) reported that they were unable to show that a RhoA-S188A mutant was resistant to PKG-induced RhoA inactivation, and in a later review report, Puetz and co-workers (22, 23) referred to the necessity of examining the phosphorylation of RhoGDIα in the context of RhoA inactivation.
Based on these reports, we and others (22, 23) have hypothesized that phosphorylation of RhoGDIα at Ser-174 may regulate RhoA activity and PKA-induced morphological changes. At least in rat cardiac fibroblasts and HEK293 cells, we have found in our present study that phosphorylation of RhoGDIα at Ser-174 by PKA is likely to increase the affinity of GTP-RhoA for RhoGDIα, which may explain the cAMP-dependent sequestration of active RhoA away from the membrane to the cytosol and the consequent induction of cellular morphological changes.
A Novel Working Model of the cAMP-induced Regulation of the RhoA-RhoGDIα Cycle
Our findings in intact cells, combined with previous findings of others, suggest a biochemical basis for RhoA cycle regulation by Gs/cAMP pathways (Fig. 7). In the resting state, geranylgeranylated and GDP-bound RhoA forms a complex with RhoGDIα via hydrophobic and charge-charge interactions (please note the discussed implications of this in the structure section) and accumulates in the cytosol. When extracellular stimuli facilitate the dissociation of RhoA from RhoGDIα, RhoA anchors to the membrane. The Gq/G13-coupled receptor (such as thrombin receptor, endothelin receptor, and angiotensin II type 1 receptor)-triggered activation of Rho-GEF promotes the RhoA activation step, i.e. GDP-GTP exchange, and the GTP-bound RhoA activates effector proteins such as ROCK or mDia. The RhoA inactivation step, i.e. GTP hydrolysis, is accelerated by GAP and the resulting GDP-RhoA is sequestered by RhoGDIα in the cytosol once more. The cAMP-induced phosphorylation of RhoGDIα at Ser-174 increases its affinity for the GTP-bound active form of RhoA, sequestering it in the cytosol and thereby inhibiting RhoA signaling. Morphologically, this cAMP-induced RhoA inactivation event produces cell rounding.
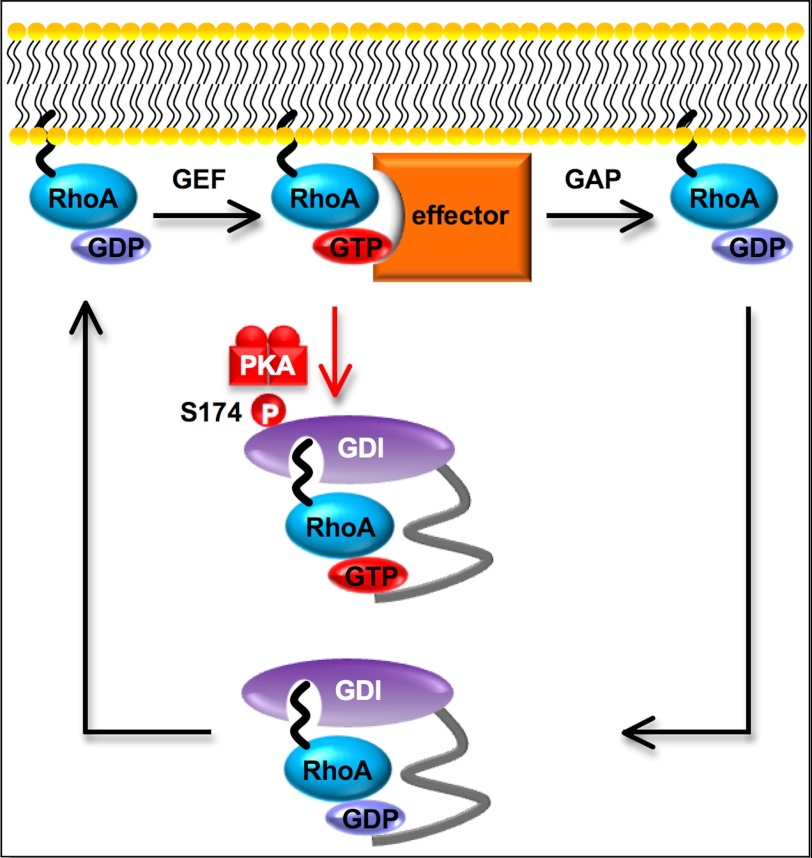
Model of cAMP-induced regulation of RhoA-RhoGDIα cycle. At resting state, geranylgeranylated and GDP-bound RhoA forms a complex with RhoGDIα and accumulates in the cytosol. When extracellular stimuli facilitate the dissociation of RhoA from RhoGDIα, RhoA anchors to the membrane. Receptor-triggered activation of Rho-GEF promotes an activation step, i.e. GDP-GTP exchange for RhoA and GTP-bound RhoA activates effector proteins (e.g. ROCK or mDia). The RhoA inactivation step, i.e. GTP hydrolysis, is accelerated by GAP and inactivated RhoA is sequestered by RhoGDIα to the cytosol once more. The cAMP-induced phosphorylation of RhoGDIα at Ser-174 increases its affinity for the GTP-bound active form of RhoA, and sequesters it in the cytosol. RhoA signaling is thereby inhibited. Morphologically, this cAMP-induced RhoA inactivation event produces cell rounding.
We have shown that at least more than 10% of RhoGDIα can be phosphorylated in cardiac fibroblasts (Fig. 6B). Previously, the RhoGDIα/RhoA molar ratio was reported to be between 3:1 and 8:1 (41), and about 2–3% of RhoA seems to be located at the plasma membrane as active RhoA (42–45). Therefore, phosphorylation of more than 10% of RhoGDIα would be enough to inhibit active RhoA, causing cell rounding.
Structure Aspects of the Association between RhoA and RhoGDIα
The association between RhoA and RhoGDIα has been postulated to result at least on part from both hydrophobic interactions and charge-charge interactions between the C-terminal domains of the two proteins. The geranylgeranyl moiety bound to a cysteine within the C-terminal domain of RhoA, which is composed of a positively charged polybasic region (in the case of RhoA, RRGKKKSGC) can be inserted into a geranylgeranyl binding pocket of the C-terminal domain of RhoGDIα, which has a negative charge. Ser-174 of RhoGDIα is located in a portion of the β sheet of the geranylgeranyl binding pocket (49).
It is speculated that free RhoA (uncoupled from RhoGDIα) is anchored at the plasma membrane. It is feasible that dual interactions (hydrophobic and charge-charge interactions) assist with the anchoring of free RhoA to the membrane (50). Because RhoGDIα binding masks the geranylgeranyl moiety and positive charges of the C-terminal domain of RhoA, a RhoA-RhoGDIα complex can remain stable in the cytosol.
At present, we do not know how the phosphorylation of RhoGDIα at Ser-174 impacts upon the RhoA-RhoGDIα complex. We may speculate that a conformational change in RhoGDIα induced by phosphorylation at Ser-174 may indirectly increase the affinity between RhoA and RhoGDIα and enable RhoA-GTP to bind to RhoGDIα. An alternative but less likely possibility is that the added negative charge on RhoGDIα via phosphorylation may promote the charge-charge interactions. It has been reported that phosphorylation of the geranylgeranyl binding pocket of RhoGDIα by other kinases, such as at Ser-96 by PKCa (51), Ser-101 and Ser-174 by Pak1 (26), and Tyr-156 by SRC (52), promotes the dissociation of Rac despite the increasing negative charge at the geranylgeranyl binding pocket of RhoGDIα.
Potential Dual Regulation of RhoA by RhoGDIα
We have shown here that the cAMP-dependent phosphorylation of RhoGDIα at Ser-174 is likely to increase the affinity between RhoA-GTP and RhoGDIα. If this mechanism underlies the inhibition of RhoA leading to morphological changes in cells, it may be possible that overexpression of RhoGDIα even without cAMP stimulation may mimic phosphorylation of RhoGDIα. Indeed, we have observed that overexpression of RhoGDIα at high levels alone can cause cell rounding in HEK293 cells (data not shown). Furthermore, this phenomenon has been observed not only when wild type RhoGDIα is overexpressed but also when RhoGDIα-S174A is overexpressed. Miura et al. (53) have also reported that overexpression of RhoGDIα in Swiss 3T3 cells causes cell rounding accompanied by the disappearance of stress fibers.
In conclusion, the inhibition of RhoA signal can be induced by: 1) increases in RhoGDIα expression or 2) phosphorylation of RhoGDIα. These dual regulatory events, and instances where they are defective, may therefore have physiological and pathophysiological implications.
Acknowledgments
We thank members of the Iiri lab and Masaomi Nangaku for assistance and helpful suggestions. We thank Tatyana voyno-Yasenetskaya and Richard Cerione for generously donating constructs for RhoA and RhoGDIα, respectively.
*This work was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture, Japan.
3The abbreviations used are:
- CTX
- cholera toxin
- PKA
- protein kinase A
- PKG
- protein kinase G
- MYPT1
- myosin phosphatase target subunit 1
- ROCK
- Rho-associated kinase.
REFERENCES
Articles from The Journal of Biological Chemistry are provided here courtesy of American Society for Biochemistry and Molecular Biology
Full text links
Read article at publisher's site: https://doi.org/10.1074/jbc.m112.401547
Read article for free, from open access legal sources, via Unpaywall:
http://www.jbc.org/content/287/46/38705.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Article citations
Actomyosin-mediated inhibition of synaptic vesicle release under CB<sub>1</sub>R activation.
Transl Psychiatry, 14(1):335, 21 Aug 2024
Cited by: 0 articles | PMID: 39168993 | PMCID: PMC11339458
NPRC deletion attenuates cardiac fibrosis in diabetic mice by activating PKA/PKG and inhibiting TGF-β1/Smad pathways.
Sci Adv, 9(31):eadd4222, 02 Aug 2023
Cited by: 9 articles | PMID: 37531438 | PMCID: PMC10396312
High extracellular glucose promotes cell motility by modulating cell deformability and contractility via the cAMP-RhoA-ROCK axis in human breast cancer cells.
Mol Biol Cell, 34(8):ar79, 17 May 2023
Cited by: 1 article | PMID: 37195739 | PMCID: PMC10398875
Unleashing Spinal Cord Repair: The Role of cAMP-Specific PDE Inhibition in Attenuating Neuroinflammation and Boosting Regeneration after Traumatic Spinal Cord Injury.
Int J Mol Sci, 24(9):8135, 02 May 2023
Cited by: 3 articles | PMID: 37175842 | PMCID: PMC10179671
Review Free full text in Europe PMC
Targeting the DP2 receptor alleviates muscle atrophy and diet-induced obesity in mice through oxidative myofiber transition.
J Cachexia Sarcopenia Muscle, 14(1):342-355, 16 Dec 2022
Cited by: 4 articles | PMID: 36527201 | PMCID: PMC9891918
Go to all (35) article citations
Data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA.
Am J Physiol Cell Physiol, 295(5):C1161-8, 03 Sep 2008
Cited by: 37 articles | PMID: 18768928 | PMCID: PMC2584978
TROY interacts with Rho guanine nucleotide dissociation inhibitor α (RhoGDIα) to mediate Nogo-induced inhibition of neurite outgrowth.
J Biol Chem, 288(47):34276-34286, 15 Oct 2013
Cited by: 10 articles | PMID: 24129566 | PMCID: PMC3837168
Rho GDP dissociation inhibitor α silencing attenuates silicosis by inhibiting RhoA/Rho kinase signalling.
Exp Cell Res, 380(2):131-140, 25 Apr 2019
Cited by: 10 articles | PMID: 31029634
Role of Rho-specific guanine nucleotide dissociation inhibitor α regulation in cell migration.
Acta Histochem, 119(3):183-189, 08 Feb 2017
Cited by: 13 articles | PMID: 28187905
Review




