Abstract
Free full text

Case Report
Pseudohypoaldosteronism without nephropathy masking salt-wasting congenital adrenal hyperplasia genetically confirmed
Abstract
Salt-losing crisis with hypoglycaemia and shock are the main manifestations of congenital adrenal hyperplasia (CAH) during the first weeks of life, while hyponatremia and hyperpotassemia alone are seen on mineralocorticoid deficiency or resistance. During the neonatal period, high blood levels of adrenal steroids may lead to confusing laboratory tests not being able to identify the real level of each hormone. A 33-day-old male baby was admitted at the emergency department with severe salt-losing crisis (Na+ 99 mEq/l and K+ 9.4 mEq/l) and mild acidosis. No hypoglycaemia or hypotension was seen. Urinary tract infection was excluded. Despite treatment with hydrocortisone and fludrocortisone, hyperpotassemia was hard to control. Laboratory tests could not differentiate between pseudohypoaldosteronism and CAH as both the aldosterone (2454 pg/ml) and 17-OH-progesterone (656.6 ng/ml) levels were high. Diagnosis was made, thanks to the genetic study that proved classical mutations in both alleles of the 21-hydroxylase gene.
Background
The clinical and genetic characteristics of congenital adrenal hyperplasia (CAH) have been widely described,1 and paediatricians think on it and urgently start replacement treatment when Na+ and K+ disorders are seen.2 Nevertheless, hormonal and genetic studies must be performed to get the final diagnosis.3 We present an unusual form of presentation of CAH in which a genetic study was essential.
Case presentation
We present a 33-day-old male baby (with palpable testicles in the scrotum) born at term with a birth weight of 3280 g who was brought to the emergency department because of vomiting, poor sucking and failure to thrive. The patient's general condition was poor. The physical examination revealed lethargy and bradycardia. The electrocardiogram revealed a cardiac frequency of 80 bpm, frequent extrasystoles, wide QRS complexes and peaked T waves. Laboratory results were as follows: Na+ 99 mEq/l; K+ 9.4 mEq/l; pH 7.3; HCO3 13.9 mmol/l; BE −10.9 mmol/l.
The infant was admitted to the intensive care unit where supplemental sodium was started immediately as well as potassium-lowering therapy (gluconate calcium, sodium bicarbonate, furosemide and endovenous salbutamol). After blood sampling for later analysis, substitutive treatment with hydrocortisone and fludrocortisone was started.
Investigations
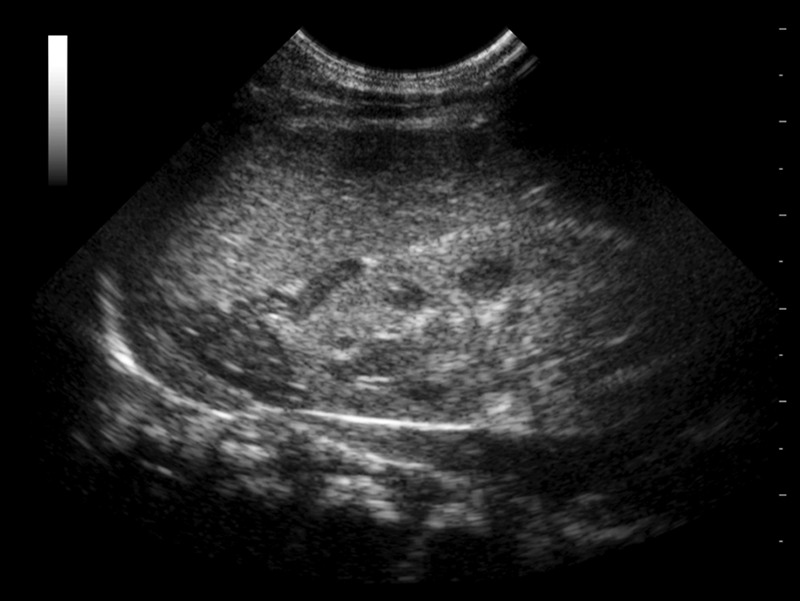
Urinary tract infection was excluded, and renal function and ultrasound were normal. Adrenal ultrasound showed enlarged adrenal glands with lobulated surface and cerebriform pattern (figure 1). Serum laboratory tests revealed high levels of aldosterone (Immunotech, Beckman Coulter Company, Prague, Czech Republic): 2454 pg/ml (normal: 17–130 pg/ml) and renin plasmatic activity: >40 ng/ml/h (normal: 2.35–37 ng/ml/h). Cortisol was 36 µg/dl. A case of pseudohypoaldosteronism (PHA) was suspected and treatment with hydrocortisone was stopped (with no clinical consequences on the patient) until very high levels of 17-OH-progesterone 656.6 ng/ml (normal: 0–4.5 ng/ml) were known. The presumptive explanation for high aldosterone levels was a cross-reactivity of other adrenal steroids as radioimmunoassay (RIA) was performed without previous chromatographic separation.
Differential diagnosis
The main difficulty was to differentiate between PHA (primary or secondary) and classic CAH to choose the correct therapy. CAH was suspected because of the severity of the symptoms and the cerebriform pattern found in adrenal ultrasound, but as the laboratory tests were not conclusive, replacement treatment was maintained until a genetic test was obtained.
Treatment
The patient was discharged 22 days after admission and his regimen consisted of supplemental sodium (3 g/day), hydrocortisone (25 mg/m2/day) and fludrocortisone (0.1 mg/day). He was followed on an outpatient basis, keeping electrolyte levels within the normal range.
Outcome and follow-up
The sample for the genetic test was obtained when the baby was 53 days old, and 3 weeks later, the genetic study revealed a classical genotype of 21-hydroxylase deficiency, which gave the diagnosis of CAH. The patient was hemizygous for the frequent splicing mutation c.293–13 Ao C>G (maternal allele) due to a gene deletion/conversion involving the 8 bp deletion at exon 3, c.332–339del (paternal allele).
Discussion
The singularity of this case relapses on the incomplete spectrum of symptoms despite having a classic severe mutation of CYP21. In contrast to the important salt-losing crisis, glycaemia and arterial blood pressure were always within the normal range and acidosis was mild. On the other hand, a false high level of aldosterone led to an initial misdiagnosis of pseudohypoaldosteronism.
To make the correct diagnosis of CAH, it is extremely important to know the exact level of each steroid. There are many techniques available to determine 17-hydroxyprogesterone levels, and RIA may be the most common one. Some studies point that chromatography should be performed before RIA when profiling adrenal steroid as cases of cross-reactivity have already been described when levels are high.1 4 Mass spectrometry linked to liquid chromatography (MS-LC) is being used and developed for neonatal screening of CAH. This method competes with RIA as it will probably minimise adrenal steroid cross-reactions,2 thanks to its higher sensitivity and lower detection limit. Another advantage of MS-LC over RIA is the need for small sample volumes, which is especially useful for the paediatric population. For those reasons, some laboratories already use MS-LC to quantify steroid hormones.
Due to laboratory difficulties in identifying adrenal steroids levels and the need for a definitive diagnosis, the genotype of possible mutations acquires importance3 not only for genetic counselling but also in a clinical context.
Adrenal ultrasound was useful as an adjunct diagnosis tool. The sensitivity and specificity of this technique in CAH were described to be high by Al-Alwan et al,5 although it requires the services of an experienced operator who must be aware not only of the adrenal shape but also of its echogenicity and surface contours.1 Furthermore, the cerebriform pattern of the adrenal gland appears to be a sonography-specific sign of CAH. False-negative cases have been reported if other characteristics but shapes are not considered.6
Classical CAH presents as a salt-wasting crisis with dehydration, hypoglycaemia and shock as both mineralocorticoid and glucocorticoid syntheses are impaired. Its phenotypical expression is variable and depends on the residual enzymatic activity according to its mutation. This phenotype–genotype relationship regarding to salt-loss has already been described.2 7 In contrast, the severity of salt-wasting does not correlate with the degree of virilisation.2 In our case, while the patient presented with severe dyselectrolitemia due to aldosterone impairment, he did not present with clinical or biochemical cortisol deficiency. This may be a problem of onset time not related to the clinical expression of his disease. Probably if he had been treated as a PHA with supplemental sodium exclusively, the patient would have presented a life-threatening cortisol deficiency later on. As the certain diagnosis is already known, we cannot ethically prove his cortisol deficiency.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
Articles from BMJ Case Reports are provided here courtesy of BMJ Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1136/bcr-2012-008281
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3603811?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Salt-Losing Syndrome in a Girl with Type I and II Pseudohypoaldosteronism.
Am J Case Rep, 23:e937536, 28 Oct 2022
Cited by: 1 article | PMID: 36303414 | PMCID: PMC9623541
Molecular Diagnosis of Steroid 21-Hydroxylase Deficiency: A Practical Approach.
Front Endocrinol (Lausanne), 13:834549, 29 Mar 2022
Cited by: 8 articles | PMID: 35422767 | PMCID: PMC9001848
Review Free full text in Europe PMC
Secondary Pseudohypoaldosteronism Associated With Mild Hydronephrosis in a Newborn.
Cureus, 13(2):e13462, 20 Feb 2021
Cited by: 2 articles | PMID: 33777551 | PMCID: PMC7985187
A Case of Salt-Wasting 21-Hydroxylase Deficiency With Resistance to Aldosterone due to Urinary Tract Infection.
Cureus, 12(11):e11763, 29 Nov 2020
Cited by: 0 articles | PMID: 33409011 | PMCID: PMC7779137
False elevation of serum cortisol in chemiluminescence immunoassay by Siemens Advia Centaur XP system in 21-hydroxylase deficiency: an 'endocrine laboma'.
BMJ Case Rep, 13(9):e235450, 07 Sep 2020
Cited by: 2 articles | PMID: 32900728 | PMCID: PMC7477984
Go to all (7) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Does pseudohypoaldosteronism mask the diagnosis of congenital adrenal hyperplasia?
J Clin Res Pediatr Endocrinol, 3(4):219-221, 01 Jan 2011
Cited by: 7 articles | PMID: 22155467 | PMCID: PMC3245498
High aldosterone and cortisol levels in salt wasting congenital adrenal hyperplasia: a clinical conundrum.
J Pediatr Endocrinol Metab, 30(12):1327-1331, 01 Nov 2017
Cited by: 4 articles | PMID: 29127765
Molecular testing in congenital adrenal hyperplasia due to 21α-hydroxylase deficiency in the era of newborn screening.
Clin Genet, 82(1):64-70, 03 Jun 2011
Cited by: 8 articles | PMID: 21534945
[Salt wasting syndrome caused by congenital, insufficient synthesis or aldosterone function--etiology, diagnosis and management].
Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw, 11(2):103-108, 01 Jan 2005
Cited by: 0 articles | PMID: 15996340
Review