Abstract
Objective
To conduct a cost-effectiveness analysis of screening strategies for identifying children with type 2 diabetes mellitus and dysglycemia (prediabetes/diabetes).Design
Cost simulation study.Setting
A one-time US screening program.Study participants
A total of 2.5 million children aged 10 to 17 years.Intervention
Screening strategies for identifying diabetes and dysglycemia.Main outcome measures
Effectiveness (proportion of cases identified), total costs (direct and indirect), and efficiency (cost per case identified) of each screening strategy based on test performance data from a pediatric cohort and cost data from Medicare and the US Bureau of Labor Statistics.Results
In the base-case model, 500 and 400 000 US adolescents had diabetes and dysglycemia, respectively. For diabetes, the cost per case was extremely high ($312 000-$831 000 per case identified) because of the low prevalence of disease. For dysglycemia, the cost per case was in a more reasonable range. For dysglycemia, preferred strategies were the 2-hour oral glucose tolerance test (100% effectiveness; $390 per case), 1-hour glucose challenge test (63% effectiveness; $571), random glucose test (55% effectiveness; $498), or a hemoglobin A1c threshold of 5.5% (45% effectiveness; $763). Hemoglobin A1c thresholds of 5.7% and 6.5% were the least effective and least efficient (ranges, 7%-32% and $938-$3370) of all strategies evaluated. Sensitivity analyses for diabetes revealed that disease prevalence was a major driver of cost-effectiveness. Sensitivity analyses for dysglycemia did not lead to appreciable changes in overall rankings among tests.Conclusions
For diabetes, the cost per case is extremely high because of the low prevalence of the disease in the pediatric population. Screening for diabetes could become more cost-effective if dysglycemia is explicitly considered as a screening outcome.Free full text

Cost-effectiveness of Screening Strategies for Identifying Pediatric Diabetes Mellitus and Dysglycemia
Abstract
Objective
To conduct a cost-effectiveness analysis of screening strategies for identifying children with type 2 diabetes mellitus and dysglycemia (prediabetes/diabetes).
Design
Cost simulation study.
Setting
A one-time US screening program.
Study Participants
A total of 2.5 million children aged 10 to 17 years.
Intervention
Screening strategies for identifying diabetes and dysglycemia.
Main Outcome Measures
Effectiveness (proportion of cases identified), total costs (direct and indirect), and efficiency (cost per case identified) of each screening strategy based on test performance data from a pediatric cohort and cost data from Medicare and the US Bureau of Labor Statistics.
Results
In the base-case model, 500 and 400 000 US adolescents had diabetes and dysglycemia, respectively. For diabetes, the cost per case was extremely high ($312 000–$831 000 per case identified) because of the low prevalence of disease. For dysglycemia, the cost per case was in a more reasonable range. For dysglycemia, preferred strategies were the 2-hour oral glucose tolerance test (100% effectiveness; $390 per case), 1-hour glucose challenge test (63% effectiveness; $571), random glucose test (55% effectiveness; $498), or a hemoglobin A1c threshold of 5.5% (45% effectiveness; $763). Hemoglobin A1c thresholds of 5.7% and 6.5% were the least effective and least efficient (ranges, 7%–32% and $938– $3370) of all strategies evaluated. Sensitivity analyses for diabetes revealed that disease prevalence was a major driver of cost-effectiveness. Sensitivity analyses for dysglycemia did not lead to appreciable changes in overall rankings among tests.
Conclusions
For diabetes, the cost per case is extremely high because of the low prevalence of the disease in the pediatric population. Screening for diabetes could become more cost-effective if dysglycemia is explicitly considered as a screening outcome.
Given reports of increasing levels of type 2 diabetes mellitus (T2D) in children and adolescents during the late 1990s, the American Diabetes Association (ADA) established population-based pediatric screening guidelines for T2D in 2000, which were also endorsed by the American Academy of Pediatrics (AAP).1 The ADA guidelines were based on the best evidence available at the time, recommending that asymptomatic high-risk adolescents (i.e., those with a body mass index 85th percentile for age and sex and 2 additional risk factors, including positive family history of T2D, nonwhite ethnic origin, signs of insulin resistance, or maternal history of diabetes or gestational diabetes) be screened for T2D with either a fasting plasma glucose test or a fasting 2-hour oral glucose tolerance test (OGTT).
Despite the guidelines, only a fraction of pediatric health care professionals (4%–21%) in the primary care setting report screening practices consistent with ADA guidelines, in large part due to the inconvenience of the fasting requirement.2,3 As part of an effort to lower screening barriers, the ADA updated its diabetes diagnostic guidelines in 2010, advocating the use of hemoglobin A1c (HbA1c) for the diagnosis of diabetes and prediabetes for adults and children, which could lead to increased uptake of this test for screening purposes.4
One-third of the US pediatric population is classified as overweight or obese,5 and the Centers for Disease Control and Prevention has estimated that up to 2.5 million US adolescents potentially qualify for T2D screening.6 Ideally, screening strategies should be valid and reliable, and their overall costs and cost effectiveness are also important considerations,7,8 particularly given the low prevalence of undiagnosed diabetes in the pediatric population (0.02%).9–11 Although previous investigators have reported on the cost-effectiveness of various screening strategies for identifying diabetes and dysglycemia (prediabetes or diabetes) in adults,12,13 we are unaware of similar studies in children. Therefore, our objective was to examine the total costs, effectiveness, and efficiency of different screening strategies for identifying children with diabetes and dysglycemia. We considered dysglycemia as an outcome given that it has a relatively high prevalence in the US pediatric population (16%–23%)14,15 and its early detection could lead to prevention or delay of the development of diabetes.16
METHODS
STUDY POPULATION
On the basis of estimates of the number of overweight or obese US children eligible for diabetes screening,6 our study population consisted of a hypothetical cohort of 2.5 million children aged 10 to 17 years.6 For our base-case analyses, we assumed a 16% prevalence of dysglycemia and a 0.02% prevalence of diabetes based on national estimates.11,15,17 Weassumed 100% adherence to the screening strategies, including the initial and confirmatory screens.
COST-EFFECTIVENESS ANALYSIS
We evaluated a variety of different screening strategies for identifying children with diabetes and dysglycemia.
Identifying Diabetes
The initial strategy we considered was the 2-hour OGTT, with the assumption that all eligible participants were tested. No confirmatory screen was required for this strategy because it is considered the criterion standard and has been used to define diabetes and prediabetes in landmark trials, such as the Diabetes Prevention Program.4,16 We assumed a test performance of 100% sensitivity and specificity, similar to previous studies.12,18 Wethen tested 3 additional strategies using HbA1c, including thresholds of 6.5% (ADA definition of diabetes), 5.7% (ADA definition of prediabetes),19 and 5.5% (a threshold suggested as an optimal cutoff for detecting prediabetes in children).20 For all the HbA1c strategies, we assumed a 2-step screening strategy in which only individuals with a positive initial test result (ie, above the defined threshold) receive a confirmatory 2-hour OGTT. Assumptions of test performance for HbA1c for the base case were based on a previously studied clinical cohort of children for whom the outcome of dysglycemia was studied.21 Because the diabetes test performance data were not previously published in that study, we conducted the analyses with the same pediatric cohort for the outcome of diabetes (see test performance results in the eTable; http://www.jamapeds.com), for which 3 children were identified as having diabetes.
Identifying Prediabetes
For prediabetes, we also evaluated a 2-hour OGTT, assuming 100% sensitivity and specificity. For the additional strategies, we assumed a 2-step screening strategy (confirmatory 2-hour OGTT after a positive initial test result) to evaluate HbA1c at thresholds of 5.5%, 5.7%, and 6.5%; random glucose test results (blood glucose level in a nonfasting state) at thresholds of 100 and 110 mg/dL (to convert to millimoles per liter, multiply by 0.0555); and a 1-hour glucose challenge test (GCT) result (blood glucose measurement 1 hour after ingestion of 50 g of Glucola in a nonfasting state) at thresholds of 110 and 120 mg/dL. We acknowledge that the random glucose test and the 1-hour GCT are not endorsed by the ADA; however, we thought that cost-effectiveness evaluation of these strategies was warranted given their improved test performance over HbA1c in a previously published study.21
Our analyses were conducted from the health care system perspective (direct costs) and the societal perspective (direct plus indirect costs). Table 1 gives the test performance assumptions used for the base-case analysis and sensitivity analyses. The direct and indirect cost assumptions for each screening strategy are listed in Table 2. Direct costs were calculated using Medicare reimbursement rates22 and included costs of the initial screening test, the follow-up 2-hour OGTT, and health care provider time.
Table 1
Test Performance Assumptions for Various Screening Strategies (at Selected Thresholds) for Identifying Children With Diabetes Mellitus and Dysglycemia for the Base-Case Analysis and Sensitivity Analyses
| Screening Strategy and Cutoff Value | Sensitivity, % | Specificity, % |
|---|---|---|
| Base-Case Analysis | ||
| Diabetes mellitus | ||
 2-h OGTT (200 mg/dL) 2-h OGTT (200 mg/dL) | 100 | 100 |
 HbAlc HbAlc | ||
  5.5% 5.5% | 33 | 56 |
  5.7% 5.7% | 33 | 71 |
  6.5% 6.5% | 33 | 96 |
| Dysglycemia (prediabetes and diabetes) | ||
  2-h OGTT (140 mg/dL) 2-h OGTT (140 mg/dL) | 100 | 100 |
  Random glucose test Random glucose test | ||
   100 mg/dL 100 mg/dL | 55 | 67 |
   110 mg/dL 110 mg/dL | 30 | 88 |
 HbAlc HbAlc | ||
  5.5% 5.5% | 45 | 57 |
  5.7% 5.7% | 32 | 74 |
  6.5% 6.5% | 7 | 98 |
 1-h Glucose challenge test 1-h Glucose challenge test | ||
  110 mg/dL 110 mg/dL | 63 | 63 |
  120 mg/dL 120 mg/dL | 44 | 81 |
|
| ||
| Sensitivity Analyses | ||
| Diabetes mellitus | ||
 HbAlc HbAlc | ||
  5.7% 5.7% | 71 | 79 |
  6.5% 6.5% | 32 | 99 |
| Dysglycemia (prediabetes and diabetes) | ||
  HbAlc HbAlc | ||
   5.7% 5.7% | 34 | 83 |
   6.5% 6.5% | 4 | 100 |
Abbreviations: HbA1c hemoglobin A1c; OGTT, oral glucose tolerance test.
Table 2
Cost Assumptions for the Base-Case Analysisa
| Screening Strategy | Cost Per Screen, $ | Additional Time for Testing, min |
|---|---|---|
| 2-h OGTT | 18.44 | 135 |
| Random glucose test | 5.62 | 15 |
| HbA1c | 13.90 | 15 |
| 1-h Glucose challenge test | 6.80 | 75 |
Abbreviations: HbA1c hemoglobin A1c; OGTT, oral glucose tolerance test.
Provider time for identification of high-risk patients and interpretation of laboratory results was valued at one fifth the cost ($20) of a full primary care visit (25 minutes of face-to-face time) for all screening strategies. Indirect costs were calculated using wage data from the US Bureau of Labor Statistics23 and accounted for the value of the patients’ time, which was calculated as the lost wages of the parent/guardian (who must accompany their child to the screening). As a conservative estimate, parent/guardian time was valued at half the mean hourly wage for all occupations in 2010.24 All cost data were expressed in 2010 US dollars.
We conducted sensitivity analyses to assess the effect of differing levels of (1) adherence (75% adherence to the nonfasting screening strategies and 75% and 50% adherence to the 2-hour OGTT), (2) changes in the prevalence of dysglycemia or diabetes (±25% and adult prevalence estimates), (3) costs (doubling of provider time costs and reduction in HbA1c costs by half), and (4) alternate estimates of test performance for HbA1c from an additional pediatric study.20
OUTCOME VARIABLES
Our main outcome of interest was the cost per identified case of diabetes or dysglycemia (efficiency) from the health care (direct medical costs associated with testing) and societal (direct medical costs plus indirect costs associated with parent/guardian time) perspectives. To derive this estimate, we had to assess total costs from the health care and societal perspectives and the percentage of cases of diabetes and dysglycemia identified (effectiveness). All analyses were conducted using Excel 2010 (Microsoft Inc). Results for the sensitivity analyses from the health care system perspective are available on request.
RESULTS
Table 3 gives the results of the base-case analysis from the societal and health care system perspectives. In the base-case model, there were 500 adolescents with diabetes and 400 000 adolescents with dysglycemia.
Table 3
Proportion of Cases and Costs Identified for the Different Screening Strategies for the Base-Case Analysis
| Screening Strategy and Cutoff Value | Cases Identified, % | Total Costs (Direct), $ | Total Costs (Direct and Indirect), $ | Cost per Case Identified (Direct), $ | Cost per Case Identified (Direct and Indirect), $ |
|---|---|---|---|---|---|
| Diabetes mellitus | |||||
 2-h OGTT(200mg/dL) 2-h OGTT(200mg/dL) | 100 | 96 065 001 | 156 111 876 | 192 130 | 312 224 |
 HbAlc HbAlc | |||||
  5.5% 5.5% | 33 | 105 182 379 | 138 513 737 | 631 157 | 831 166 |
  5.7% 5.7% | 33 | 97 992 217 | 121 958 136 | 588 012 | 731 822 |
  6.5% 6.5% | 33 | 86 377 340 | 95 214 473 | 518 316 | 571 344 |
| Dysglycemia (prediabetes and diabetes) | |||||
  2-h OGTT(140mg/dL) 2-h OGTT(140mg/dL) | 100 | 96 065 001 | 156 111 876 | 240 | 390 |
  Random glucose test Random glucose test | |||||
   100mg/dL 100mg/dL | 55 | 80 850 720 | 109 451 714 | 368 | 498 |
   110mg/dL 110mg/dL | 30 | 70 874 680 | 86 481 530 | 591 | 721 |
 HbAlc HbAlc | |||||
  5.5% 5.5% | 45 | 104 685 520 | 137 369 701 | 582 | 763 |
  5.7% 5.7% | 32 | 97 143 559 | 120 004 072 | 759 | 938 |
  6.5% 6.5% | 7 | 86 005 799 | 94 358 987 | 3072 | 3370 |
 1-h Glucose challenge test 1-h Glucose challenge test | |||||
  110mg/dL 110mg/dL | 63 | 85 939 761 | 144 014 430 | 341 | 571 |
  120mg/dL 120mg/dL | 44 | 77 568 001 | 124 738 157 | 441 | 709 |
Abbreviations: HbAlc hemoglobin Alc; OGTT, oral glucose tolerance test.
Total costs for a one-time screening were in a similar range for dysglycemia and diabetes, between 94 million and 156 million US dollars, because the same pool of eligible children would need to be screened regardless of the disease prevalence. Total costs were highest for the 2-hour OGTT for both dysglycemia and diabetes, in part due to higher test costs and the patient time costs associated with a longer test (21/2 hours).
For diabetes, the 2-hour OGTT had the highest test effectiveness, detecting 100% of cases vs 33% for the HbA1c strategies. The cost per case was extremely high for all strategies because of the small number of children with diabetes: $312 000 per case for the 2-hour OGTT and $571 000 to $831 000 per case for the HbA1c strategies.
Figure 1 A shows effectiveness and efficiency for the base-case diabetes analysis from the societal perspective. The figure illustrates tradeoffs between 2 competing goals of screening efforts: identifying a greater proportion of cases and minimizing the cost per case identified. Preferred screening strategies are in the upper left, maximizing the percentage of cases identified and minimizing the total costs per case. In contrast, least preferred screening strategies are in the bottom right. On the basis of these criteria, the 2-hour OGTT was the most desirable strategy for diabetes. Effectiveness was similar across the various HbA1c thresholds; however, the cost per case was lowest for the HbA1c threshold of 6.5%.
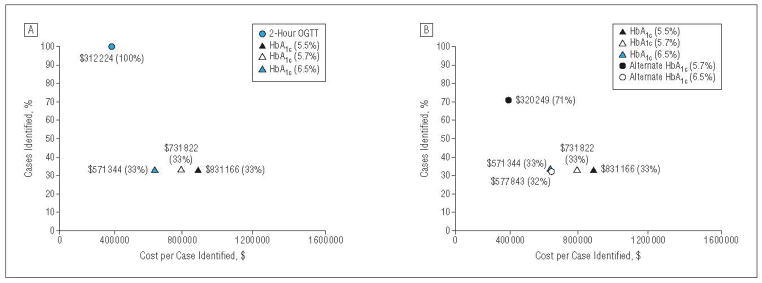
Screening effectiveness for diabetes (percentage of cases identified) plotted against screening efficiency (costs per case identified) from a societal perspective. A, Base-case analysis; B, alternative estimates of hemoglobin Alc (HbAlc) test performance. OGTT indicates oral glucose tolerance test.
For dysglycemia, the 2-hour OGTT strategy had the highest test effectiveness (100%) followed by a 1-hour GCT threshold of 110 mg/dL (63%), a random glucose threshold of 100 mg/dL (55%), and an HbA1c threshold of 5.5% (45%). The HbA1c thresholds of 5.7% and 6.5% had lower levels of effectiveness (32% and 7%, respectively). The 2-hour OGTT had the lowest total costs per case identified ($390), followed by a random glucose threshold of 100 mg/dL ($498), a 1-hour GCT threshold of 110 mg/dL ($571), and an HbA1c threshold of 5.5% ($763). In contrast, HbA1c thresholds of 5.7% and 6.5% had the highest costs per case identified, ranging from $938 to $3370.
Figure 2 A shows effectiveness plotted against efficiency for dysglycemia from the societal perspective. The 2-hour OGTT was the most desirable strategy, followed by a 1-hour GCT, a random glucose test, and an HbA1c level of 5.5%. The HbA1c thresholds of 5.7% and 6.5% were the least desirable strategies.
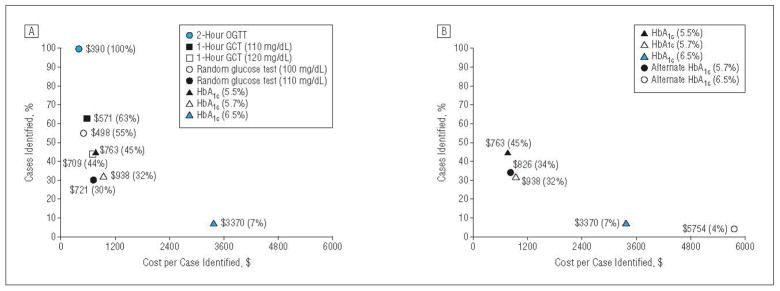
Screening effectiveness for dysglycemia (percentage of cases identified) plotted against screening efficiency (costs per case identified) from a societal perspective. A, Base-case analysis; B, alternative estimates of hemoglobin A1c (HbA1c) test performance. GCT indicates glucose challenge test; and OGTT, oral glucose tolerance test.
In our sensitivity analyses, similar trends were seen for diabetes and dysglycemia. Lower adherence to the nonfasting strategies and to the 2-hour OGTT decreased total costs and screening effectiveness (Table 4). Cost per case identified was unchanged for the 2-hour OGTT but increased for the nonfasting strategies given an increasing number of missed cases.
Table 4
Proportion of Cases and Costs Identified for the Different Screening Strategies for 75% Adherence to Initial Nonfasting Screening Strategies and 75% or 50% Adherence to 2-Hour OGTT
| Screening Strategy and Cutoff Value | 75% Adherence to Nonfasting Screening and 75% Adherence to 2-Hour OGTT
| 75% Adherence to Nonfasting Screening and 50% Adherence to 2-Hour OGTT
| ||||
|---|---|---|---|---|---|---|
| Cases Identified, % | Total Costs (Direct and Indirect), $ | Cost per Case Identified (Direct and Indirect), $ | Cases Identified, % | Total Costs (Direct and Indirect), $ | Cost per Case Identified (Direct and Indirect), $ | |
| Diabetes mellitus | ||||||
 2-h OGTT (200 mg/dL) 2-h OGTT (200 mg/dL) | 75 | 117 083 907 | 312 224 | 50 | 78 055 938 | 312 224 |
 HbAlc HbAlc | ||||||
  5.5% 5.5% | 19 | 94 810 233 | 1011 410 | 12 | 86 053 541 | 1376994 |
  5.7% 5.7% | 19 | 85 855 882 | 915 888 | 12 | 80 083 973 | 1281472 |
  6.5% 6.5% | 19 | 70 931 963 | 756 683 | 12 | 70 134 694 | 1122 267 |
| Dysglycemia (prediabetes and diabetes) | ||||||
 2-h OGTT (140 mg/dL) 2-h OGTT (140 mg/dL) | 75 | 117083 907 | 390 | 50 | 78 055 938 | 390 |
 Random glucose test Random glucose test | ||||||
  100 mg/dL 100 mg/dL | 31 | 74 820 378 | 605 | 21 | 67 551 971 | 819 |
  110 mg/dL 110 mg/dL | 17 | 61 899 650 | 917 | 11 | 58 938 152 | 1310 |
 HbAlc HbAlc | ||||||
  5.5% 5.5% | 25 | 94 405 496 | 932 | 17 | 85 783 716 | 1271 |
  5.7% 5.7% | 18 | 84 637 329 | 1176 | 12 | 79 271 605 | 1651 |
  6.5% 6.5% | 4 | 70 211 969 | 4458 | 3 | 69 654 698 | 6634 |
 1-h Glucose challenge test 1-h Glucose challenge test | ||||||
  110 mg/dL 110 mg/dL | 35 | 99 818 937 | 704 | 24 | 91 627 052 | 970 |
  120 mg/dL 120 mg/dL | 25 | 88 976 034 | 899 | 17 | 84 398 450 | 1279 |
Abbreviations: HbA1c hemoglobin Alc; OGTT, oral glucose tolerance test.
A higher prevalence of disease slightly increased total costs but resulted in a lower cost per case, whereas a lower prevalence slightly decreased total costs and resulted in a higher cost per case (Table 5). When we assumed a population prevalence of diabetes and dysglycemia similar to adult levels, the cost per case decreased markedly for both outcomes but particularly for diabetes, which decreased from $312,000 to $831,000 per case to $781 to $2064 per case.
Table 5
Proportion of Cases and Costs Identified for the Different Screening Strategies for a 25% Increase in Prevalence, a 25% Decrease in Prevalence, or Adult Prevalence Assumptions
| Screening Strategy and Cutoff Value | Cases Identified, % | 25% Increase in Prevalence
| 25% Decrease in Prevalence
| Adult Prevalence Assumptions (8% for Diabetes and 37% for Prediabetes)
| |||
|---|---|---|---|---|---|---|---|
| Total Costs (Direct and Indirect), $ | Cost per Case Identified (Direct and Indirect), $ | Total Costs (Direct and Indirect), $ | Cost per Case Identified (Direct and Indirect), $ | Total Costs (Direct and Indirect), $ | Cost per Case Identified (Direct and Indirect), $ | ||
| Diabetes mellitus | |||||||
 2-h OGTT(200mg/dL) 2-h OGTT(200mg/dL) | 100 | 156111876 | 249779 | 156111876 | 416298 | 156111876 | 781 |
 HbAlc HbAlc | |||||||
 5.5% 5.5% | 33 | 138513150 | 664 930 | 138514325 | 1108225 | 137576050 | 2064 |
 5.7% 5.7% | 33 | 121958377 | 585459 | 121957896 | 975 761 | 122 341851 | 1835 |
 6.5% 6.5% | 33 | 95216051 | 457 083 | 95 212895 | 761 779 | 97732 759 | 1466 |
| Dysglycemia (prediabetes and diabetes) | |||||||
 2-hOGTT(140mg/dL) 2-hOGTT(140mg/dL) | 100 | 156111876 | 312 | 156111876 | 520 | 156111876 | 169 |
| Random glucose test | |||||||
 100mg/dL 100mg/dL | 55 | 110385806 | 401 | 108517621 | 658 | 114 355 700 | 225 |
 110mg/dL 110mg/dL | 30 | 87245787 | 582 | 85 717272 | 952 | 90493 882 | 326 |
| HbA1c | |||||||
 5.5% 5.5% | 45 | 137454618 | 611 | 137284783 | 1017 | 137815518 | 331 |
 5.7% 5.7% | 32 | 120258824 | 752 | 119749319 | 1247 | 121341523 | 410 |
 6.5% 6.5% | 7 | 94 571280 | 2702 | 94146693 | 4483 | 95 473 529 | 1474 |
| 1-h Glucose challenge test | |||||||
 110mg/dL 110mg/dL | 63 | 145118357 | 461 | 142 910502 | 756 | 149810049 | 257 |
 120mg/dL 120mg/dL | 44 | 125 799626 | 572 | 123 676688 | 937 | 130310868 | 326 |
Abbreviations: HbA1c hemoglobin A1c; OGTT, oral glucose tolerance test.
Doubling of provider time resulted in higher total costs and higher cost per case identified (Table 6), and halving the price of HbA1c resulted in slightly lower total costs and lower cost per case, with no changes in the effectiveness estimates for either sensitivity analysis.
Table 6
Proportion of Cases and Costs Identified for the Different Screening Strategies for Doubling the Provider Costs or Cutting the HbA1c Test Costs in Half
| Screening Strategy and Cutoff Value | Cases Identified, % | Double Provider Costs, $
| 50%HbAlc Test Costs, $
| ||
|---|---|---|---|---|---|
| Total Costs (Direct and Indirect) | Cost per Case Identified (Direct and Indirect) | Total Costs (Direct and Indirect) | Cost per Case Identified (Direct and Indirect) | ||
| Diabetes mellitus | |||||
 2-hOGTT(200mg/dL0 2-hOGTT(200mg/dL0 | 100 | 206076 8 76 | 412154 | ||
 HbAlc HbAlc | |||||
  5.5% 5.5% | 33 | 188478737 | 1 130986 | 121138738 | 726905 |
  5.7% 5.7% | 33 | 171923136 | 1 031 642 | 104 583137 | 627562 |
  6.5% 6.5% | 33 | 145179473 | 871164 | 77 839474 | 467084 |
| Dysglycemia (prediabetes and diabetes) | |||||
 2-hOGTT(140mg/dL) 2-hOGTT(140mg/dL) | 100 | 206076876 | 515 | ||
 Random glucose test Random glucose test | |||||
  100mg/dL 100mg/dL | 55 | 159416714 | 725 | ||
  110mg/dL 110mg/dL | 30 | 136446530 | 1137 | ||
 HbAlc HbAlc | |||||
  5.5% 5.5% | 45 | 187334701 | 1041 | 119994 702 | 667 |
  5.7% 5.7% | 32 | 169969072 | 1328 | 102 629073 | 802 |
  6.5% 6.5% | 7 | 144 323987 | 5154 | 76983 988 | 2749 |
 1-h Glucose challenge test 1-h Glucose challenge test | |||||
  110mg/dL 110mg/dL | 63 | 193 979430 | 770 | ||
  120mg/dL 120mg/dL | 44 | 174 703157 | 993 | ||
Abbreviations: HbA1c hemoglobin A1c; OGTT, oral glucose tolerance test.
Finally, when we assumed alternate test performance characteristics for HbA1c for diabetes,20 the cost per case identified was lower ($320 000–$578 000) compared with the base case ($571 000–$831 000) due to improved test performance but still remained quite high (Figure 1B). For dysglycemia, the cost per case was lower for a threshold of 5.7% ($826 vs $938) because of improved test performance, and the cost per case was higher for a threshold of 6.5% ($5754 vs $3370) because of lower test performance (Figure 2B). Across the multiple sensitivity analyses for dysglycemia, the relative rankings of efficiency and effectiveness for the various screening strategies for dysglycemia were similar to the base case.
COMMENT
This study evaluated the total costs, effectiveness, and efficiency (cost per case identified) of a number of different screening strategies for identifying children with diabetes and dysglycemia. One of the most striking findings of our study was the very high cost per case of screening for T2D in adolescents regardless of test type, ranging from $312 000 to $831 000 per case identified. This finding is due to the low prevalence of T2D in the US pediatric population.11
The ADA formulated the first screening guidelines for T2D in children in 2000 in response to an epidemic of obesity and increasing reports of a T2D phenotype in tertiary care clinics25 and high-risk populations.26 At the time that the recommendations were made, empirical data on screening efficacy and costs for T2D were not available; therefore, the recommendations were based on expert opinion. Since that time, a number of population-based studies9–11,27 evaluating the epidemiology of pediatric diabetes have been conducted and suggest that the overall burden of T2D in children is still low, particularly compared with the burden among adults.
A similar study13 conducted in adults reported a cost per case identified of T2D, ranging from $600 to $850 per case across a variety of HbA1c thresholds and assuming a national T2D prevalence of 8%. When we assumed a similar prevalence for our pediatric population, the cost per case for children was reduced markedly, closer to the range reported in adults, suggesting that disease prevalence is the major determinant of cost-effectiveness for diabetes screening in children.
We did not explicitly calculate costs per quality adjusted life-year for our analysis, which makes determination of cost-effectiveness difficult, but the extremely high cost per case values would suggest that screening for T2D in children may not fall into the cost effective range. In contrast, our analyses for identification of dysglycemia resulted in much more reasonable cost-per-case ratios. A screening program for glucose abnormalities could therefore be considered more cost effective if dysglycemia were explicitly considered as a screening outcome. However, the ADA and AAP guidelines are focused on screening for diabetes as the primary outcome.
Screening for dysglycemia could be endorsed by the ADA and AAP because studies28,29 such as the Diabetes Prevention Program in adults have demonstrated that early identification and treatment of individuals with prediabetes is both effective and cost-effective for reducing the rates of diabetes. However, it is unclear whether these conclusions could be extrapolated to the pediatric population, especially given the accelerated progression of disease reported in adolescents vs adults30 and the fact that the benefits of early detection of dysglycemia in children are currently unknown.31
When comparing our cost-per-case ratios for identifying dysglycemia with adult studies,13 the cost-percase ratios were in a similar range but were higher for our pediatric population ($390–$3000 per case) compared with adults ($150–$500 per case). Again, this finding was likely in part due to the lower prevalence of prediabetes as shown by the results of our sensitivity analysis when we assumed a prevalence similar to the adult population (37%).
When comparing screening strategies for dysglycemia, the 2-hour OGTT (a fasting test) was the preferred strategy. However, previous studies2,3,32 have shown remarkably low adherence with ADA-recommended screening tests among providers (4%–21%) in pediatric settings, most likely due to their onerous fasting requirement and associated increase in nonadherence rates among patients. Given these findings, the nonfasting 1-hour GCT and random glucose test may represent convenient and preferred screening alternatives for health care professionals. If efficiency (lower cost per case) were prioritized, then the random glucose test (100 mg/dL) would be a preferred strategy. Alternatively, if effectiveness (percentage of cases identified) were prioritized, then the 1-hour GCT (110 mg/dL) would be preferred.
The HbA1c thresholds of 5.7% and 6.5% were the least preferred strategies because they had the highest cost per case and the highest proportion of cases missed—a finding that has important policy implications given the recent ADA guidelines recommending the exclusive use of HbA1c for the diagnosis of prediabetes and diabetes in children. The guidelines will likely lead to increased use of HbA1c as a screening test. Given our findings, reconsideration of the new HbA1c guidelines or a lowering of the HbA1c threshold to 5.5% may be warranted.
We are unaware of previous studies that have compared the cost-effectiveness of different nonfasting screening strategies for identifying diabetes and dysglycemia in children. Previous studies13 have focused exclusively on adults for whom there are notable differences in test performance and disease prevalence.33 Strengths of our study include the fact that our model simulation was based on empiric screening data in overweight and obese children20,21 and the fact that our findings were robust to a variety of sensitivity analyses that explored changes in dysglycemia prevalence, testing adherence, provider time costs, lower test costs, and alternate test performance estimates for HbA1c.
We acknowledge several limitations of our study. First, we assessed the cost-effectiveness of a one-time screening of the pediatric population, despite the ADA recommendation that children receive biannual screenings because of a lack of longitudinal data regarding pediatric screening outcomes. Second, we used Medicare estimates for our cost assumptions. Testing costs may vary by payer; however, our objective was to evaluate the relative costs of different screening strategies. Third, our analysis included only costs associated with the detection of diabetes and dysglycemia, which represents just one aspect of the total costs associated with screening programs and did not consider the costs associated with treatment, health care utilization, or downstream productivity costs. Fourth, we did not address the benefits of early detection, including improvements in length and quality of life. Last, our test performance data relied on a single 2-hour OGTT to classify children as having dysglycemia. Although most studies of children and adults with dysglycemia rely on this definition,9,18,34,35 we acknowledge that some studies36 have reported a lack of reproducibility of the 2-hour OGTT results in children.
We recognize that all overweight and obese children likely require aggressive lifestyle management, regardless of their glycemic status, to reduce the risk of comorbidities. However, given limited health care resources, early identification of children with dysglycemia may represent a reasonable strategy for targeting the children at highest risk.
Since the recent guidelines recommending HbA1c for diagnosis of diabetes in children and adults, commentaries have highlighted testing costs as an important issue for determining which tests should be prioritized.37 Total costs, efficiency (cost per case), and effectiveness (proportion of cases identified) of screening at-risk individuals are important criteria for determining optimal screening policy, and our findings highlight important tradeoffs to consider for the pediatric population. Future longitudinal studies are needed to evaluate the long-term outcomes (effectiveness and cost-effectiveness) of screening for pediatric glucose abnormalities, particularly for the most promising strategies (1-hour GCT and random glucose test). The high cost per case of screening for diabetes should inform future pediatric screening policy. The low effectiveness and high cost per case of current recommended HbA1c thresholds warrant reconsideration of the recent ADA guidelines recommending HbA1c measurement for the diagnosis of diabetes and dysglycemia in adolescents.
Acknowledgments
Funding/Support: Dr Lee was supported by grant K08 DK082386 from the National Institute of Diabetes and Digestive and Kidney Disorders and the Clinical Sciences Scholars Program at the University of Michigan. This project was supported by the following: grant UL1RR024986 from the Michigan Clinical Research Unit, Michigan Institute for Clinical and Health Research Pilot and Collaborative Grant UL1RR024986, Blue Cross Blue Shield Foundation of Michigan Investigator Initiated Research Grant, and grants from the University of Michigan (Elizabeth Kennedy Award, Elizabeth Crosby Funds, and Office of the Vice President for Research). This work used the laboratory core(s) of the Michigan Diabetes Research and Training Center funded by grant 5P60 DK20572 from the National Institute of Diabetes and Digestive and Kidney Disorders, National Institutes of Health.
Footnotes
Conflict of Interest Disclosures: None reported.
Additional Contributions: We thank Courtney Nelson, BS, for her assistance with the manuscript.
Author Contributions: Acquisition of data: Wu, Kazzi, and Lee. Analysis and interpretation of data: Wu, Kazzi, and Lee. Drafting of the manuscript: Wu, Kazzi, and Lee. Critical revision of the manuscript for important intellectual content: Kazzi and Lee. Statistical analysis: Wu, Kazzi, and Lee. Obtained funding: Lee. Administrative, technical, and material support: Kazzi and Lee. Study supervision: Lee.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamapediatrics.2013.419
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc3837695?pdf=render
Citations & impact
Impact metrics
Article citations
Perception and Awareness of Diabetes Risk and Reported Risk-Reducing Behaviors in Adolescents.
JAMA Netw Open, 6(5):e2311466, 01 May 2023
Cited by: 4 articles | PMID: 37133860 | PMCID: PMC10157422
14. Children and Adolescents: Standards of Care in Diabetes-2023.
Diabetes Care, 46(suppl 1):S230-S253, 01 Jan 2023
Cited by: 61 articles | PMID: 36507640 | PMCID: PMC9810473
Review Free full text in Europe PMC
Prospective Test Performance of Nonfasting Biomarkers to Identify Dysglycemia in Children and Adolescents.
Horm Res Paediatr, 96(3):316-324, 15 Nov 2022
Cited by: 3 articles | PMID: 36380614 | PMCID: PMC10183477
Heterogeneity of circulating CXCR5-PD-1hiTph cells in patients of type 2 and type 1 diabetes in Chinese population.
Acta Diabetol, 60(6):767-776, 06 Mar 2023
Cited by: 0 articles | PMID: 36879107
2. Classification and Diagnosis of Diabetes: Standards of Care in Diabetes-2023.
Diabetes Care, 46(suppl 1):S19-S40, 01 Jan 2023
Cited by: 524 articles | PMID: 36507649 | PMCID: PMC9810477
Review Free full text in Europe PMC
Go to all (21) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Screening adults for pre-diabetes and diabetes may be cost-saving.
Diabetes Care, 33(7):1484-1490, 01 Jul 2010
Cited by: 45 articles | PMID: 20587721 | PMCID: PMC2890345
Implementation of a screening program to detect previously undiagnosed dysglycemia in hospitalized patients.
Can J Diabetes, 38(2):79-84, 01 Apr 2014
Cited by: 7 articles | PMID: 24690501
Glucose challenge test screening for prediabetes and early diabetes.
Diabet Med, 34(5):716-724, 02 Nov 2016
Cited by: 13 articles | PMID: 27727467 | PMCID: PMC5388592
Screening for type 2 diabetes and dysglycemia.
Epidemiol Rev, 33:63-87, 30 May 2011
Cited by: 40 articles | PMID: 21624961
Review
Funding
Funders who supported this work.
NCRR NIH HHS (2)
Grant ID: UL1RR024986
Grant ID: UL1 RR024986
NHLBI NIH HHS (1)
Grant ID: T35 HL007690
NIDDK NIH HHS (5)
Grant ID: K08 DK082386
Grant ID: P30 DK020572
Grant ID: 5P60 DK20572
Grant ID: P60 DK020572
Grant ID: P30 DK092926





