Abstract
Free full text

Cytokine production associated with smallpox vaccine responses
Abstract
Smallpox was eradicated 34 years ago due to the success of the smallpox vaccine; yet, the vaccine continues to be studied because of its importance in responding to potential biological warfare and the adverse events associated with current smallpox vaccines. Interindividual variations in vaccine response are observed and are, in part, due to genetic variation. In some cases, these varying responses lead to adverse events, which occur at a relatively high rate for the smallpox vaccine compared with other vaccines. Here, we aim to summarize the cytokine responses associated with smallpox vaccine response to date. Along with a description of each of these cytokines, we describe the genetic and adverse event data associated with cytokine responses to smallpox vaccination.
Vaccinia virus (VV) has been used for centuries to immunize humans against infection by variola virus, the causal agent of smallpox. Because of genetic and antigenic similarities shared between these poxviruses [1], vaccination with VV elicits humoral and cellular immune responses capable of protecting vaccine recipients against the deadly variola virus infection, as well as other orthopox virus infections.
Although smallpox was eradicated by VV-based vaccines in 1980, recent concerns regarding smallpox as a weapon of biological warfare, the possible emergence of new orthopox diseases in humans by way of zoonosis [2,3] and the relatively high rates of adverse events (AEs) associated with the current smallpox vaccines have stimulated continued research on smallpox vaccines [4]. Approximately 20–30% of smallpox vaccine recipients experience one or more AEs that range in prevalence and severity [5–7]. Minor, more common AEs include low-grade fever, headache, lymphadenopathy, folliculitis and malaise, whereas substantially fewer vaccinees experience more serious AEs (SAEs), including acneiform rash, eczema vaccinatum, generalized or progressive vaccinia infection, myopericarditis and encephalitis [8–12]. SAEs only occur in a few hundred patients per million vaccine doses, with death resulting in one to two patients per million doses [8,13]. Because of smallpox eradication, as well as the risk of vaccine-induced AEs, routine smallpox vaccination eventually ceased.
Despite recent advancements made in smallpox vaccine development, data on the cellular response elicited by the smallpox vaccine is limited compared with humoral response data. Understanding the role of the cytokine-mediated cellular immune response after smallpox vaccination is important, as it is suggested to play an integral role in wholly eliminating orthopox virus infections from the body [9,14,15]. In addition, orthopox viruses, including VV, are able to influence cytokine-mediated cellular immune response to infection and vaccination through the actions of virally encoded cytokines, cytokine receptors and cytokine binding proteins. These proteins may mimic or compete with the host immune system, allowing for viral evasion of an effective host immune response [16–18].
In this review, we provide a comprehensive overview of systemic cytokine secretion in post smallpox vaccination subjects, primarily through studies of peripheral blood mononuclear cells (PBMCs) restimulated with VV in vitro. We subsequently expand on each cytokine’s importance in mediating an antiviral response, discuss the immunogenetic data available in relation to cytokine secretion, and highlight the role of each cytokine in the development of mild AEs after vaccination. Although there are several murine studies available, this review focuses mainly on human subjects. See Table 1 for a brief summary of the main studies used in this review.
Table 1
Brief overview of human studies used in the review.
| Author (year) | Subjects (n) | Vaccine | Primary vaccinees or revaccinees | Sample collection time in relation to receipt of vaccine | Methods/assays of interest | Major findings | Ref. |
|---|---|---|---|---|---|---|---|
| Cohen et al. (2010) | 42 | 36 Dryvax recipients, 6 ACAM2000 recipients | 27 primary vaccinees, 15 revaccinees | Every other day post vaccination for 2 weeks and 1 month post vaccination | Pierce SearchLight Protein Array multiplexed sandwich-ELISA† system (ThermoFischer) | Primary vaccinees significantly more likely to experience symptoms than revaccinees Primary vaccinees more likely to have elevated cytokine levels; significantly higher levels of G-CSF | [19] |
| Combadiere et al. (2004) | 79 | Not specified | 62 primary vaccinees, 17 revaccinees | 14+ years post vaccination | ELISpot‡ assays and flow cytometry | Proliferative memory T-cell responses detectable in 72.5% of subjects vaccinated at least 20 years prior to study | [20] |
| Ennis et al. (2002) | 8 | Connaught | Primary vaccinees | 27 days post vaccination | ELISpot assays | Statistically significant increase in IFN-γ secreting T-cells post smallpox vaccination | [21] |
| Frey et al. (2002) | 60 | Dryvax | Primary vaccinees | 45 days post vaccination | ELISA | Vaccination ‘take’ sites correlate with antibody and T-cell response Statistically significant increase in IFN-γ secreting T-cells post smallpox vaccination | [22] |
| Hammarlund et al. (2003) | 306 vaccinated, 26 unvaccinated controls | Not specified | Primary vaccinees and revaccinees | 1 month to 75 years post vaccination | ICCS | 90% of subjects maintained humoral and/or cellular immunity against VV after 25–75 years | [23] |
| Haralambieva et al. (2011) | 1076 | Dryvax | Primary vaccinees | 1 month to 4 years post vaccination | Vaccinia neutralizing antibody assay, SNP genotyping (Illumina) | Significant associations between IL18R1 and IL18 genes and humoral immune response – racial differences shown in these associations | [24] |
| Haralambieva et al. (2012) | 197 | Dryvax | Primary vaccinees | 1 month to 4 years post vaccination | Microarray | Upregulation of cytokine production by Th17 pathway and PGE2 signaling in immune response pathway >20 genes differentially expressed with significance in response to VV stimulation | [25] |
| Haralambieva et al. (2013) | 1071 | Dryvax | One dose; primary vaccinees | 1 month to 4 years post vaccination | ELISA and ELISpot assays | Higher IFN-α and IL-2 in Caucasians than in African–Americans or Hispanics | [26] |
| Judkowski et al. (2011) | in vitro studies on PBMCs; number of patients not specified | Dryvax or MVA | Primary vaccinees and revaccinees | 1–4 months post vaccination | Cytokine multiplex assay and intracellular staining | GM-CSF is a good marker of vaccinia-specific CD4 T cells Significantly higher levels of GM-CSF, TNF-α, IL-13 and IL4 detected at 48 h than at 6 h | [27] |
| Kennedy et al. (2012) | 1076 | Dryvax | Primary vaccinees | 1 month to 4 years post vaccination | ELISA, SNP genotyping (Illumina) | SNPs significantly associated with secreted TNF-α levels (data discussed in this review) and secreted IL-1β, IL-6, IL-10 and IL-12p40 levels (data not discussed in this review) – racial differences shown in these associations | [28] |
| McKinney et al. (2006) | 74 | APSV® | Primary vaccinees | 6–9 days post vaccination | Dual antibody sandwich immunoassay arrays | Statistically significant increases in the following cytokines post smallpox vaccination in the presence of adverse events: sICAM-1, G-CSF, TIMP-2, SCF, eotaxin and TIMP-2 | [29] |
| Ovsyannikova et al. (2011) | 1071 | Dryvax | Primary vaccinees | 1 month to 4 years post vaccination | ELISA | HLA associations with vaccinia-induced antibody levels Significant HLA associations with IFN-γ, IL-1β, TNF-α, IL-12p40 and IL-6 levels | [30] |
| Ovsyannikova et al. (2012) | 1076 | Dryvax | Primary vaccinees | 1 month to 4 years post vaccination | ELISA | Significant IL18R1 allele–VV dose associations | [31] |
| Ovsyannikova et al. (2013) | 1076 | Dryvax | Primary vaccinees | 1 month to 4 years post vaccination | ELISpot; SNP genotyping (Illumina) | IL18R1 gene haplotype correlations with IFN-γ ELISpot responses | [32] |
| Reif et al. (2009) | 148 | APSV | Vaccinated as part of the study; primary vaccinees | 6–9 days post vaccination | Rolling circle amplification technology-enhanced custom dual antibody sandwich immunoassay arrays | Three IL4 gene SNPs associated with adverse event development | [33] |
| Rock et al. (2004) | 107 | APSV | Primary vaccinees | 6–9 days (acute) and 3–5 (convalescent) weeks post vaccination | CBA | Primarily a IFN-γ dominated response Subjects with fever had elevated IFN-γ, TNF-α and IL-5 Subjects with rash had elevated TNF-α, IL-10, IL-4 and IL-2, which lasted through convalescent phase Statistically significant increases in the following cytokines during acute phase post smallpox vaccination: IFN-γ, TNF-α and IL-10 Statistically significant increases in the following cytokines in the presence of adverse events during acute phase post smallpox vaccination: IFN-γ, TNF-α, IL-5, IL-10, IL-2 and IL-4 | [7] |
| Umlauf et al. (2011) | 1076 | Dryvax | Primary vaccinees | 1 month to 4 years post vaccination (median: 15.3 months) | ELISA | TH1-like cytokine response pattern Correlation between NAb and secreted IFN-γ and IL-2 | [9] |
CBA: Cytometric bead arrays; ICCS: Intracellular cytokine staining; MVA: Modified vaccinia Ankara; NAb: Neutralizing antibody; PBMC: Peripheral blood mononuclear cell; VV: Vaccinia virus.
Smallpox vaccine variations
In the past 36 years, three generations of smallpox vaccines have been developed worldwide [4,34]. Yet, efforts are currently underway to develop smallpox vaccines with fewer associated AEs. The so-called first-generation of smallpox vaccines includes Dryvax®, the Aventis Pasteur Smallpox Vaccine® (APSV®) and Lister strain vaccines (L-IVP® and Lancy Vaxina®). Both Dryvax and APSV are derived using the NYCBH VV strain, while L-IVP and Lancy Vaxina are derived using the Lister VV strain. NYCHB strain vaccines have been shown to result in fewer AEs and deaths than Lister strain vaccines [34,35]. The Dryvax vaccine contains several vaccinia strains harvested from lesions on infected cows. Limited supply and safety concerns with the Dryvax smallpox vaccine sparked the development of second-generation vaccines, including Acambis’ ACAM2000® [4]. ACAM2000, derived from one plaque-purified vaccinia virus from the Dryvax vaccine, replaced Dryvax vaccines in the US in February 2008 [36].
ACAM2000 produces similar T-cell responses and frequency of AEs as the Dryvax vaccine in animal [37] and human [38] studies. Frey et al. reported that the duration of viral shedding tended to be longer in Dryvax recipients and that fourfold increases of neutralizing antibody (neutralizing Ab) titers from day 0 to day 45 were similar between Dryvax and ACAM2000 recipients (all primary vaccinees); however, geometric mean titers were lower in the ACAM2000 vaccine recipients [38]. Artenstein et al. also showed no significant differences in neutralizing Ab titers between the two vaccines in their study [39].
With further room for improvement, third- generation vaccines, including modified vaccinia Ankara based vaccines, emerged with a focus on using attenuated viruses and are currently in clinical trials. Next-generation vaccines using protein and DNA subunits are already in development. In order for the development of a more effective smallpox vaccine with fewer side effects to be accomplished, a more thorough understanding of the cellular and humoral immune response to the smallpox vaccine must be obtained.
Cytokine secretion post smallpox vaccination
IFN-γ
IFN-γ, produced largely by activated NK cells and T cells in response to viral infection, plays an integral role in inflammation processes, febrility and immune function [40]. It also has antiviral properties and subsequent abilities to inhibit VV infection, viral protein synthesis and virion replication by a variety of mechanisms, including the stimulation of innate and adaptive immunity through NK cells, activation of effector cells such as macrophages and induction of nitric oxide (shown in in vitro studies) [41,42]. The importance of IFN-γ in controlling the replication of various viruses has also been demonstrated in studies of IFN-γ and IFN-γ receptor knockout mice [43–47].
Several studies have shown that both the number of IFN-γ-secreting cells and the concentrations of serum IFN-γ increase significantly from baseline after smallpox vaccination [9]. Data from Ennis et al. demonstrate a significant increase in the number of IFN-γ producing cells, primarily CD8+ cytotoxic T lymphocytes, in ELISpot assays of PBMCs obtained from primary smallpox vaccinees with subsequent in vitro VV stimulation [21]. Frey et al. reported similar results. In their study, 31 primary vaccinees displayed a significant increase in IFN-γ-producing cells, as measured by by ELISAs of PBMCs stimulated with VV in vitro. All 31 subjects developed a prominent ‘take’ site after vaccination, indicated by the formation of a vesicle and surrounding erythema. This suggests that the formation of a ‘take’ site may be correlated with a robust IFN-γ response [22].
Previous studies have analyzed the relationship between secreted IFN-γ and vaccinia-specific neutralizing Ab levels to determine whether the humoral and cellular immune responses are correlated. Umlauf et al. identified a positive, but modest, correlation between neutralizing Ab titers and IFN-γ (IFN- γ levels were detected by ELISAs of PBMCs retrieved from primary vaccine recipients and stimulated in vitro with VV) [9].
It has been demonstrated that the IFN-γ response to viral replication occurs more quickly than the vaccinia-specific neutralizing Ab response after VV exposure [48]. It has also been demonstrated that VV-specific antiviral T-cell responses wane slowly over time with a half-life of 8–15 years post vaccination, while the antiviral antibody responses exhibit an initial decline in the first several years after vaccination, followed by a longer period (typically, decades) of relative stability [23,49,50]. Hammarlund et al. demonstrated a tenfold increase in the number of IFN-γ-producing cells after in vitro VV stimulation of PBMCs from individuals vaccinated 1–61 years prior to the study when compared with an unvaccinated control group. The vaccinated group contained 160 primary vaccinees and 81 revaccinees [23]. These results are also supported by Combadiere et al. who found that proliferative memory T-cell responses to VV remained detectable in 72.5% of subjects vaccinated at least 20 years prior to the start of the study. These studies were performed on PBMCs obtained from 62 primary and 17 secondary vaccine recipients and stimulated in vitro with VV [20]. The results of these two studies demonstrate the presence of vaccinia-specific T-cell memory responses long after primary smallpox vaccination.
Despite the relative overall increase in IFN-γ-producing cells after smallpox vaccination, inter-individual variations exist in both the number of IFN-γ secreting cells and serum concentrations of IFN-γ. This response may be explained, in part, by genetic differences between study subjects. A study by Kennedy et al. identified several SNPs correlated with IFN-γ secreting cells and concentrations of secreted IFN-γ using genome-wide analysis of PBMCs obtained from 1076 primary smallpox vaccine recipients and subsequently stimulated in vitro with VV. Eleven SNPs were correlated with CD8+ IFN-γ ELISpot assay results in Caucasian subjects, while four SNPs had the same correlation for African–American subjects. Lastly, six and five SNP correlations with secreted levels of IFN-γ, as detected on the previously mentioned PBMCs via ELI-SAs, were found in the Caucasian and African–American cohorts, respectively. Three associations found in the African–American subjects correlated increased levels of total IFN-γ secreting cells with the presence of the FAS gene, which is responsible for mediating the cytotoxicity of T cells and NK cells. SNPs in the FAS gene may influence the survival of cytotoxic T cells, thereby altering IFN-γ secretion. The same study demonstrated race-specific relationships between gene expression and VV-specific IFN-γ response. Although no significant associations existed between SNP and total PBMC IFN-γ ELISpot results among the Caucasian subjects of the studied population, several significant associations were identified among the African–American subjects, including two SNPs (in NFIB and GNAI1 genes) correlated with a decrease in total IFN-γ ELISpot responses, and four SNPs (in FAS and OPRMI genes) correlated with an increase in total IFN-γ ELISpot responses. Additionally, one SNP near the DAOA gene was correlated with a decrease in serum IFN-γ levels, as measured by IFN-γ ELISAs) in both Caucasian and African–American populations [51].
The effect of race on cell-mediated vaccine response was analyzed using PBMCs from a cohort of 1071 primary smallpox vaccine recipients that were stimulated in vitro with VV. Results showed that Caucasians had significantly higher total IFN-γ ELISpot responses than African–Americans and Hispanics. Additionally, Caucasians exhibited higher CD8+ IFN-γ ELISpot responses than both African–Americans and Hispanics, although this trend was not significant, likely owing to sample size. This study also showed Caucasians to have significantly higher levels of secreted IL-2 and IFN-α than all other races [26]. These data suggest that genetic diversity may be responsible for the racial and interindividual differences in response to smallpox vaccination.
A study by Ovsyannikova et al. examined genetic variations and correlations with IFN-γ ELISpot assays that were carried out on PBMCs isolated from the previously mentioned cohort of 1076 smallpox vaccinees and stimulated in vitro with VV. Two haplotypes were identified in the IL-18R1 gene that resulted in a decreased IFN-γ response, while two haplotypes in the same gene resulted in a high IFN-γ response. Evaluation of the influence of IL-18)on IFN-γ production suggests that SNPs in the IL18 gene may significantly affect the secretion of IFN-γ post smallpox vaccination [32].
Several HLA allelic associations with IFN-γ production were identified in a study of PBMCs, which were stimulated in vitro with VV after retrieval from the abovementioned cohort of 1071 vaccinees. Five associations were identified between total PBMC IFN-γ ELISpot and specific HLA-DRB1 alleles, three associations between CD8+ IFN-γ ELISpot and HLA-DQB1 alleles, six associations between secreted IFN-γ and HLA-DRB1 alleles, three associations between secreted IFN-γ and HLA-DQA1 alleles, and two associations between secreted IFN-γ and HLA-DQB1 alleles [30]. These results signify the importance of HLA genes on IFN-γ production in response to VV stimulation post vaccination.
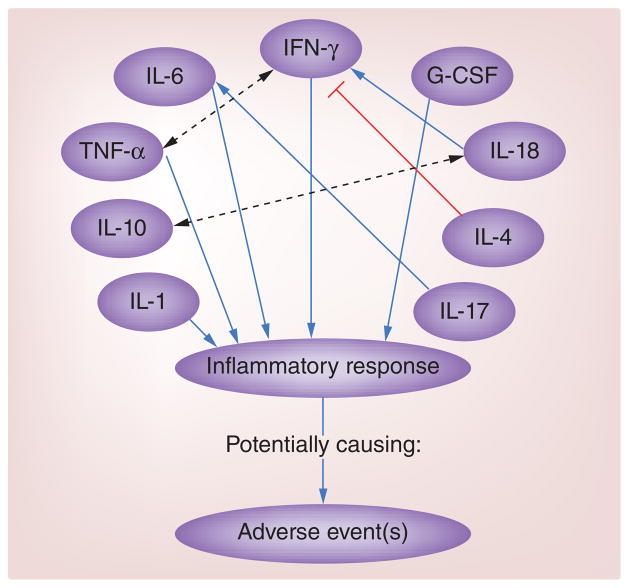
Studies also suggest that robust IFN-γ responses after vaccination may increase the likelihood of AE development [7,19]. While no SNPs or haplotypes have been shown to associate the IFNG gene with AE development [52], two SNPs in the IRF1 gene have been linked to AEs [12]. To analyze the role of IFN-γ in AE occurrences after smallpox vaccination, Rock et al. conducted a study on serum from 107 primary vaccinees who received APSV. Of the 107 subjects, 41 developed at least one AE (characterized by localized rash, generalized rash, fever and/or lymphadenopathy). Statistical analysis of cytokine responses, measured via cytometric bead array, showed that IFN-γ concentrations increased in all subjects during the acute phase after smallpox vaccination, but were significantly higher in subjects with one or more AE, particularly fever, than in subjects with no reported AEs [7]. Cohen et al. yielded similar findings. In their study, 27 primary vaccinees received either Dryvax or ACAM2000, and 15 revaccinees received Dryvax, resulting in a cohort of 42 subjects. Of these subjects, 13 reported having at least one AE, signified by fever and/or lymphadenopathy. Analysis of cytokines present in blood serum samples revealed significant associations between the level of systemic IFN-γ (measured by ELISAs) and reported fatigue, lymphadenopathy and myalgia. Higher concentrations of IFN-γ in primary vaccinees correlated with higher incidences of AEs for this group of subjects [19]. The direct administration of IFN-γ has been shown to result in fever and flu-like symptoms [53], supporting the claim that excessive serum concentrations of IFN-γ elicit inflammatory-related AEs after smallpox vaccination, specifically fever and rash. See Figure 1 for a summary of cytokine interactions that have been shown to result in AE development.
TNF-α
TNF-α, secreted primarily by macrophages and CD4+ lymphocytes, is a proinflammatory cytokine responsible for inducing a pyrogenic response to infection by working cooperatively with other cytokines, such as IFN-γ. The dual production of IFN-γ and TNF-α by 90% of vaccinia-specific CD4+ lymphocytes illustrates the important relationship between these cytokines, making TNF-α an important factor in vaccine response [23]. After primary smallpox vaccination, serum concentrations of TNF-α appear to elevate >50% from baseline. TNF-α has also been observed to be secreted in concert with many other proinflammatory cytokines, such as IL-6 and IFN-γ, suggesting that VV inoculation elicits a strong inflammatory response within vaccine recipients [9].
Variations in TNF-α secretion post smallpox immunization are also explained, in part, by interindividual genetic variation. A study by Kennedy et al. of PBMCs from 1076 primary smallpox vaccine recipients identified eight SNPs (by way of a genome-wide analysis) that were significantly associated with secreted levels of TNF-α (detected by ELISAs of PBMCs obtained from smallpox vaccinees and stimulated with VV in vitro after obtention). An association between TNF-α and the IRAK4 gene in African–American individuals is noteworthy, as IRAK4 mediates viral invasion via stimulation of TLRs, resulting in cytokine secretion and activation of T cells. Therefore, it is plausible that a SNP in the IRAK4 gene would result in differential production of TNF-α [28].
A study by Ovsyannikova et al. revealed 60 significant SNP associations with TNF-α secretion, including several allele–dose dependent SNPs in the IL18R1 gene (TNF-α secretion was measured in PBMCs isolated from 1076 primary smallpox vaccinees and stimulated in vitro with VV) [31]. Additionally, Ovsyannikova et al. identified an association between HLA-B*5701 and increased levels of TNF-α secretion [30]. HLA-B*5701 has historically been shown to slow HIV/AIDS disease progression and lead to enhanced cytotoxic T-cell responses [54–57]. These two studies also showed an increase in TNF-α production post smallpox vaccination that commonly causes fever in humans. For this reason, elevated levels of TNF-α have also been associated with the development of AEs after smallpox immunization.
As mentioned, Rock et al. analyzed the relationships between systemic cytokine levels and AEs. Results showed that, similar to IFN-γ production, TNF-α production increased in all subjects immediately following smallpox vaccination. Furthermore, subjects who reported at least one AE had significantly higher serum levels of TNF-α than those who reported no AEs. Of note, although systemic IFN-γ concentrations returned to homeostatic levels after the acute phase, serum concentrations of TNF-α remained elevated by 21% throughout the convalescent phase in subjects who developed a localized rash after vaccination [7]. Cohen et al. published results coinciding with these findings. Higher serum concentrations of TNF-α in first-time vaccine recipients were positively associated with a greater likelihood of AE development, especially rash, in that population [19]. Thus, a robust proinflammatory cytokine response to smallpox immunization provides lasting immunity against future infection; however, complications associated with highly elevated levels of TNF-α and other proinflammatory cytokines still exist.
IL-17
IL-17 is a proinflammatory cytokine secreted by T-helper 17 (Th17) cells, which functions to enhance the recruitment of effector cells (e.g., neutrophils) to sites of infection by inducing chemokine production by epithelial and stromal cells [58]. This cytokine has also been shown to induce the production of IL-6 and IL-10 (discussed below), which can inhibit NK cell activity [59–61]. This is significant with regards to the immune response following smallpox vaccination, especially because of the importance of NK cell proliferation in recovery from VV infections [62].
A study of 197 subjects, who represented the high and low extremes of neutralizing Ab and IFN-γ ELISpot responses to primary smallpox vaccine in the previously described cohort of 1076 subjects, was conducted by Haralambieva et al. Microarrays performed on PBMCs stimulated in vitro with VV showed upregulation of the pathway of cytokine production by Th17 cells and the prostaglandin E2 (PGE2) signaling in immune response pathway [25]. Both of these pathways are linked to IL-17. As Yao et al. demonstrated, one mechanism by which PGE2 promotes inflammation is through driving Th17 expansion [63]. PGE2 has also been shown to decrease NK cell activity [64]. Thus, these studies suggest a potential overall increase of IL-17 and decrease of NK cell activity after smallpox vaccination.
IL-17 has been linked to eczema vaccinatum, a condition known to occur in some patients with atopic dermatitis after receipt of the smallpox vaccine, in murine studies. Eczema vaccinatum is a contraindication to the smallpox vaccine [65], and results in an increased viral load at the vaccine take site, causing the formation of satellite lesions and potential systemic infection [66]. Oyoshi et al. identified IL-17 as a potential mediator of the progression, spread, and increased viral load of the primary lesion following smallpox vaccination in mice that had undergone epicutaneous sensitization with ovalbumin [67]. When these mice were injected with anti-IL-17, the viral load was reduced in the sites of skin inflammation. Yuko et al. reported similar findings after anti-IL-17 treatment in their eczema vaccinatum mouse models that received the smallpox vaccine. This group also showed that VV is able to cause eczema vaccinatum because of the inhibition of NK cell activity by IL-17, which was upregulated in their eczema vaccinatum mouse model [68].
Further confirmation of IL-17’s role in AEs after smallpox vaccination was given by Patera et al. who infected mice with recombinant VV containing IL-17 (VV-IL17). The length of survival of mice infected with VV-IL17 was remarkably shorter compared with that of mice infected with wild-type VV (VV-WT). During the first 3 days after infection, virus titers in the ovaries of mice infected with VV-IL17 were significantly higher than those of mice infected with VV-WT. Unlike mice infected with the VV-WT, weight loss also occurred during the first 3 days post infection in VV-IL17 mice, while most of the deaths of the VV-IL17 mice occurred within 5 days post infection. All of these results indicate that IL-17 may inhibit early stages of the immune response to VV. Similar to the above eczema vaccinatum studies, Patera et al. also showed that mice infected with VV-IL17 have decreased NK cell activity. These results indicate that IL-17 plays a significant role in the modulation of the immune response to vaccinia virus infection [62].
Taken together, the above studies indicate that IL-17 is upregulated after smallpox vaccination, and this upregulation of IL-17 causes a decrease in NK cell activity. Both of these consequences seem to be magnified in patients who experience AEs. It should be noted, however, that murine VV-IL17 studies by Kohyama et al. have produced results conflicting with those previously mentioned. Their studies show that the presence of IL-17 results in a decreased viral load and more successful outcomes in vaccinia-infected mice [69].
IL-1 & IL-18
IL-1 is a proinflammatory cytokine known to cause fever in humans. Genes in the IL-1 complex have been linked to atopy [70] and other conditions [71,72], several of which are autoimmune in nature. A blockade of IL-1 secretion decreases inflammatory symptoms and reduces mortality, as demonstrated using a murine model of myocarditis [73,74], which is a potential SAE of smallpox vaccination. There is little information regarding IL-1’s role during the immune response to vaccinia infection, but genetic information indicates a role may, indeed, be present.
In a previously described study, Ovsyannikova et al. performed ELISAs on VV-stimulated PBMCs from primary smallpox vaccinees to quantify cytokines. IL-1β, along with other Th1 cytokines including IFN-γ, IL-12P40, IL-1β and TNF-α, was observed to be upregulated. The presence of these cytokines is indicative of the important role innate immune responses have in generating protective immunity after smallpox vaccination. In the same study, 79 IL-1β SNPs, one IL18 SNP and one IL6 SNP were identified as being associated with variations in IL-1β secretion. They also showed a linkage between an IL1RN SNP and the dose-dependent decrease of IL-2 secretion and between an IL12RB2 SNP and a dose-dependent decrease in IL-1β secretion [31]. In another study using the same cohort, Ovsyannikova et al. used ELISAs to detect cytokine levels and UniTray® typing assays to perform HLA genotyping. The HLA-B locus had several associations with IL-1β secretion, with some alleles associated with increased IL-1β secretion and some associated with decreased IL-1β secretion. This study suggests that variations in IL-1β immune responses post smallpox vaccination are partially controlled by HLA genes [30].
Immunogenetic data linking IL-1 to AEs is also available. Stanley et al. conducted a study analyzing the immunogenetics of 346 subjects who experienced AEs after smallpox vaccination. DNA was obtained from whole blood samples and genotyped using a high-throughput template-directed dye-incorporation assay with fluorescence polarization detection SNP genotyping assay. Results suggested that several haplotypes in the IL1 gene complex and the IL18 gene (discussed more below) predict the likelihood of developing a fever after smallpox immunization. Several haplotypes in the IL1 gene complex were identified as being associated with both increased and decreased risks of fever after smallpox vaccination. Of the three haplotypes identified in the IL1A gene, each presents a different risk in fever development; one appears to reduce the risk, one has no effect, and one increases the risk. Two haplotypes in the IL1B gene have been shown to increase the chance of developing fever, and one haplotype in the IL1R1 gene has been correlated with an increased risk; however, this trend is only observed in subjects with a previous smallpox vaccination history [52]. It is important to note that the transcription of IL-1 depends on NF-κB [75], which is also important in the regulation of the cellular immune response to viral infections [76,77]. Gene polymorphisms could affect binding of transcription factors such as NF-κB to regulatory regions of cytokine genes, potentially resulting in AEs.
IL-18, also a member of the IL-1 cytokine superfamily, is known to induce IFN-γ production by T cells and NK cells [78], enhance Th1- and Th2-driven immune responses [79], and cause anti-vaccinia virus effects in mouse models of infection [80–83]. Although ELISAs performed on PBMCs in a previously discussed study detected extremely low levels of IL-18 [30], there seem to be several significant findings regarding polymorphisms of the IL18 gene post smallpox vaccination. In a study by Haralambieva et al., 1076 subjects who received one dose of Dryvax vaccine within 4 years prior to recruitment were genotyped for 785 known immune-related SNPs. Sixty-three significant associations between SNPs and vaccinia-specific antibody levels, 31 of which were located within the IL18R1 and IL18 genes, were identified. A total of seven SNPs had highly significant associations with vaccinia-specific antibody levels in both Caucasian and African–American subjects, the two most prevalent races in this study. Five of these seven SNPs were in the IL18R1 gene and were also associated with virus-specific neutralizing Ab levels [24]. Ovsyannikova et al. identified several of the same IL18R1 haplotypes as being associated with IFN-γ ELISpot responses from the same subject cohort [32], as described previously.
Several haplotypes in the IL18 gene were also found to be associated with AEs after smallpox vaccination by Stanley et al. with one haplotype predicting a reduced risk of fever and two haplotypes predicting an increased risk of fever. This trend was shared between primary vaccinees and revaccinees, lending support to the notion that IL-18, as well as IL-1, may be a factor in smallpox vaccine AE development [52]. In addition, levels of both IL-1 and IL-18 have been shown to be elevated in mouse models of coxsackie virus myocarditis [84]. Although potential IL-1 and IL-18 links to myopericarditis after smallpox vaccination have not been studied, this may be an area for further investigation, as several cases of myopericarditis in smallpox vaccinees have been reported [13,85].
sICAM-1
ICAM-1, also known as CD54, is a transmembrane intercellular adhesion molecule mainly expressed on endothelial cells and many APCs. It allows for migration through blood vessels by binding to LFA and Mac-1 [58]. It is involved in cell–cell adhesion, signal transduction and monocyte differentiation into macrophages. Although ICAM-1 is not considered a cytokine, it can also be present in a soluble form (sICAM-1) and exerts many cytokine-like effects, such as macrophage recruitment, especially in the presence of disease [86]. Studies show that sICAM-1 seems to be involved in AE development after smallpox vaccination.
McKinney et al. quantified serum cytokines in 74 individuals pre- and 1 week post vaccination with APSV using custom dual antibody sandwich immunoassay arrays. Twenty-two of these subjects experienced systemic AEs. This study concluded that subjects who experienced AEs had significantly upregulated secretion of six serum cytokines/chemokines: sICAM-1, G-CSF, eotaxin, TIMP-2, MIG and SCF. A decision tree was constructed for this study, with sICAM-1 being the root node. The effect of the other cytokines included in the decision tree (G-CSF, eotaxin and TIMP-2) on the presence of AEs post smallpox vaccine were shown to be conditionally dependent on the presence of sICAM-1. AEs were seen in patients with the following cytokine secretion profiles relative to their baseline (prevaccination) cytokine profile: sICAM-1 > 11%, G-CSF > 97% and TIMP-2 ≤ 51%; and sICAM-1 > 11%, G-CSF ≤ 97%, eotaxin > −10%, and TIMP-2 > 37% [29].
Reif et al. also created a decision tree with sICAM-1 as the root node to summarize their studies that quantify serum cytokines and identify genetic polymorphisms from a group of 61 healthy adults who received APSV. Serum cytokine levels and genetic polymorphisms were measured at baseline (before vaccination) and 6–9 days post vaccination. The cytokines in this decision tree include IL-10, an IL-4 gene SNP, and G-CSF. AEs were detected in the following two conditions (relative to baseline levels): the presence of increased sICAM-1 and increased IL-10 secretion; and in the presence of increased sICAM-1, decreased IL-10 and increased G-CSF secretion [33].
Two of the cytokines described by McKinney [29] and Reif’s [33] groups – G-CSF and ICAM-1 – are secreted by fibroblasts and stimulate neutrophil proliferation and differentiation in the proinflammatory IL-17 pathway. IL-17 has been shown to increase the expression of ICAM-1 on fibroblasts [87], and ICAM-1 has been shown to assist in T-cell recruitment during contact hypersensitivity. This information could be helpful in future studies explaining the mechanism behind sICAM-1’s involvement in the occurrence of AEs post smallpox vaccination.
G-CSF
G-CSF plays an integral role in the inflammatory process by evoking neutrophil proliferation after its release from activated T cells, macrophages and endothelial cells at infection sites. Limited information is available regarding G-CSF’s presence post smallpox vaccination in general, but, because of the involvement of G-CSF in inflammation, it has been identified as a target for AE research. In addition to the aforementioned studies by McKinney and Reif [29,33], studies by Cohen et al. [19] have shown that high serum concentrations of G-CSF were significantly correlated with chills, myalgia and fatigue after smallpox vaccination. Not surprisingly, primary vaccinees were more likely to have higher systemic concentrations of G-CSF than subjects with a prior smallpox vaccine history, which may contribute to the higher prevalence of AEs among first-time smallpox vaccine recipients [19]. These results are supported by findings from Reif et al. in which AEs developed when there was a greater than 78% change from baseline in G-CSF concentrations [33]. All of these results are confirmative of G-CSF’s association with the development of AEs after smallpox vaccination.
GM-CSF
GM-CSF is a cytokine that works with IL-4 to induce the differentiation of monocytes into macrophages [58] and stimulates the production of monocytes and granulocytes in bone marrow [58]. In vitro studies by Judkowski et al. on PBMCs collected from vaccinated patients (primary vaccinees and revaccinees) and then infected with VV indicated that GM-CSF is a good marker of vaccinia-specific CD4 T cells. GM-CSF is produced by T cells (mainly CD4+ T cells) in response to vaccinia infection, and its production requires very low antigen concentrations in comparison to the concentrations necessary to stimulate the production of other cytokines during vaccinia infection. Additionally, the concentration of GM-CSF had a higher fold increase from 6- to 48-h post infection than the other cytokines evaluated in this study (IL-13, IL-2, IL-5, IFN-γ and TNF-α) [27]. These results imply that GM-CSF may play a protective role in the early stages of the immune response to VV infection.
IL-4
IL-4 is a Th2 cytokine responsible for the differentiation of CD4+ T-cells into Th2 cells, the differentiation of B cells into plasma cells, isotype switching to IgE and IgG4 in humans and the suppression of IFN-γ producing cells, such as CD8+ cytotoxic T lymphocytes [88,89]. Although it has been reported that smallpox vaccination typically generates a Th1-like cytokine response pattern in human hosts [9], IL-4 has been studied with respect to VV infection. Because of the inhibitory effect of IL-4 on IFN-γ production, and therefore the suppression of the host antiviral cell-mediated immune system, poxviruses modified to express IL-4 are much more virulent than wild-type strains [90]. Knowing this, it is not surprising that negligible levels of IL-4 have been detected upon VV stimulation post smallpox vaccination [9,25].
Based on the role of IL-4 in immune regulation, it can be surmised that variations in IL-4 production may have detrimental effects on the immune system and could, therefore, initiate an abnormal inflammatory response, resulting in AE development after smallpox vaccination [33,91]. Thus, several studies have analyzed the effects of IL-4 production on immune response post smallpox vaccination. One study identified an IL4 haplotype associated with decreased likelihood of fever after smallpox vaccination, suggesting that an increase in IL-4 production reduces inflammatory effects owing to the influence of IL-4 on IFN-γ production [52]. Another study showed that subjects who developed a rash after smallpox vaccination experienced an increase in serum concentrations of IL-4 during the convalescent phase, while this was not observed in subjects who did not develop an AE [7].
Studies by Reif et al. have identified three SNPs in the IL4 gene that appear to be associated with AE development after smallpox vaccination; however, the functionality of these genetic variants is not yet understood. Based on the results of these studies, it is probable that SNPs in the IL4 gene initiate the production of functionally different IL-4 proteins, or alter its bio-availability, resulting in an overstimulation or under-stimulation of the inflammatory response, leading to abnormal responses to smallpox vaccination [91].
IL-10
IL-10 is an anti-inflammatory cytokine that inhibits both activated macrophages and cytokine synthesis by cytotoxic T lymphocytes and NK cells. Some pox-viruses, including parapoxviruses, capripoxviruses and an avipoxvirus, have been shown to encode IL-10 orthologs to assist in suppressing the immune system and allow for viral replication [92]. A more robust increase of IL-10 has also been seen in VV-infected mice with a pre-existing airway condition than in infected mice without a pre-existing airway condition. Blocking IL-10 in the mice with a pre-existing airway condition led to increased pulmonary CD4+ T-cell IFN-γ production and serum levels of virus-specific IgG1 [93]. IL-10 has also been described to exhibit proinflammatory effects by enhancing the ability of IL-18 to induce IFN-γ secretion by NK cells [94]. Because of the roles this cytokine plays in both innate and adaptive immunity, fluctuations in IL-10 levels, such as those that may occur after smallpox vaccination, can offset sensitive homeostatic conditions in the body and result in AEs [95].
Haralambieva et al. found extremely low IL-10 levels by ELISA in their previously described study in which PBMCs from subjects who received the Dryvax vaccine within 4 years prior to recruitment were infected with VV [25]. However, other studies have shown that all subjects experience an initial increase in serum IL-10 levels after smallpox vaccination, but that excessive increases in serum IL-10 allow for discrimination between subjects who develop an AE and subjects who do not [7,29,33]. Additionally, data shows that those who presented with a localized rash after vaccination had serum IL-10 levels at least 18% above baseline [7], giving indication that IL-10 may play a role in AE development.
Other cytokines
Although the following cytokines have undergone few studies with regard to their relationship with the immune response following the smallpox vaccine, the studies they have been included in showed notable results.
IL-13
IL-13 is a Th2 cytokine that has been suggested to increase pathogen-induced inflammation [96,97]. It has also been shown to be upregulated in atopic dermatitis skin cells and play a role in increased viral replication in VV-infected keratinocytes in immunocompromised patients, including those with atopic dermatitis [65,96,97]. IL-13 production by vaccinia-specific T cells following VV infection was observed in the in vitro study carried out by Judkowski et al. [27].
IL-2
IL2 is a cytokine secreted by CD4 T cells that is important for CD8 T-cell production [98]. Ovsyannikova et al. found polymorphisms in the IL1 (mentioned above) and IL2 genes that correlate with vaccinia-specific IL-2 secretion in an allele–dose dependent fashion [31]. Moreover, Umlauf et al. detected a correlation between vaccinia-specific neutralizing Ab levels and IL-2 secretion in subjects who had been vaccinated with one dose of Dryvax [9]. Both of these studies are previously described. They suggest that IL-2 could be involved in the immune response to VV infection.
IL-12p40
IL-12p40 aids in cellular immunity by inducing IFN-γ secretion by T cells and NK cells [99,100]. It has been found to be slightly upregulated in PBMCs from vaccinated subjects that were subsequently stimulated with VV [25]. In addition, several alleles of the HLA-B locus, including B*5701 (described in more detail above), were found to be associated with IL-12p40 secretion after smallpox vaccination [30]. IL-12p40 could play a role in the cellular immune response – perhaps with respect to IFN-γ secretion – after VV infection.
IL-6
IL-6 is a mediator of fever and the acute phase response found to be present at high levels after VV infection of PBMCs from vaccinated subjects [25]. It has also been shown to play a role in enhancing IgA synthesis [101]. A handful of HLA-B alleles, including B*5701, were also found to be associated with IL-6 secretion after smallpox vaccination [30]. IL-6 may be involved in the induction of fever in some smallpox vaccine recipients.
Future perspective
The smallpox vaccine contains live vaccinia virus and is a strong immunogen, eliciting robust Th1 and pro-inflammatory cytokine responses. While these cytokines are an essential part of the development of protective immunity, they are a two-edged sword, as many have also been associated with AEs following vaccination.
The current smallpox vaccine has several contra-indications that include up to 30% of the US population, making widespread use of this vaccine problematic. Safer, attenuated and subunit smallpox vaccines (third-generation smallpox vaccines) [102,103] are in various stages of research and development. A deeper understanding of the role that cytokines play in immune responses to poxviruses will allow us to more readily find the delicate balance between immunogenicity and reactogenicity.
The currently available studies have a number of limitations that require further research, including: lack of functional studies of cytokines that have been shown to be immunogenetically linked to the cytokine response to smallpox vaccination; a narrow focus on common cytokines that could be overcome using high dimensional techniques such as Luminex bead-based assays; studies focused solely on cytokine secretion in the absence of data regarding the cell types responsible for that secretion or the tissue-types affected; and limited data available on cytokine responses to third-generation vaccines. In order to develop a smallpox vaccine with fewer associated AEs, we must have a deeper understanding of both the humoral and cellular immune responses to the vaccine. This information could be obtained by applying systems biology approaches to the study of poxvirus biology and vaccine response. Furthermore, the identified immunogenetic correlations with AEs (or lack thereof) post smallpox vaccination must be rigorously tested to identify the mechanistic underpinnings of the associations. Results of these studies have the potential to inform the design of next-generation smallpox vaccines that take into account our increased understanding of interindividual differences among vaccine recipients, and may lead to generalized smallpox vaccines with fewer side effects.
Acknowledgments
The authors thank the Mayo Clinic Vaccine Research Group in Rochester, MN, USA, the Naval Health Research Centerin San Diego, CA, USA and the subjects who participated in our studies. The authors thank CL Vitse for her editorial assistance.
Footnotes
Financial & competing interests disclosure
This project was funded by federal funds from the National Institute of Allergies and Infectious Diseases, NIH, Department of Health and Human Services, under Contract No. HH-SN266200400065C (N01AI40065). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. GA Poland is the chair of a Safety Evaluation Committee for novel investigational vaccine trials being conducted by Merck Research Laboratories. GA Poland offers consultative advice on vaccine development to Merck & Co. Inc., CSL Biotherapies, Avianax, Sanofi Pasteur, Dynavax, Novartis Vaccines and Therapeutics, PAXVAX Inc., Emergent Biosolutions, Adjuvance and Vaxness. GA Poland and IG Ovsyannikova hold two patents related to vaccinia peptide research. These activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and are conducted in compliance with Mayo Clinic Conflict of Interest policies. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•• of considerable interest
Full text links
Read article at publisher's site: https://doi.org/10.2217/imt.14.72
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4263415?pdf=render
Citations & impact
Impact metrics
Article citations
Prior cycles of anti-CD20 antibodies affect antibody responses after repeated SARS-CoV-2 mRNA vaccination.
JCI Insight, 8(16):e168102, 22 Aug 2023
Cited by: 1 article | PMID: 37606046 | PMCID: PMC10543713
Discovery of New States of Immunomodulation for Vaccine Adjuvants via High Throughput Screening: Expanding Innate Responses to PRRs.
ACS Cent Sci, 9(3):427-439, 23 Feb 2023
Cited by: 5 articles | PMID: 36968540 | PMCID: PMC10037445
Comparison of COVID-19 Vaccine-Associated Myocarditis and Viral Myocarditis Pathology.
Vaccines (Basel), 11(2):362, 05 Feb 2023
Cited by: 0 articles | PMID: 36851240 | PMCID: PMC9967770
Review Free full text in Europe PMC
Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines.
Biomedicines, 11(2):451, 03 Feb 2023
Cited by: 6 articles | PMID: 36830987 | PMCID: PMC9953067
Review Free full text in Europe PMC
Alu RNA Structural Features Modulate Immune Cell Activation and A-to-I Editing of Alu RNAs Is Diminished in Human Inflammatory Bowel Disease.
Front Immunol, 13:818023, 20 Jan 2022
Cited by: 4 articles | PMID: 35126398 | PMCID: PMC8813004
Go to all (13) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Recommendations for using smallpox vaccine in a pre-event vaccination program. Supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Healthcare Infection Control Practices Advisory Committee (HICPAC).
MMWR Recomm Rep, 52(rr-7):1-16, 01 Apr 2003
Cited by: 79 articles | PMID: 12710832
Smallpox vaccines for biodefense: need and feasibility.
Expert Rev Vaccines, 7(8):1225-1237, 01 Oct 2008
Cited by: 23 articles | PMID: 18844596 | PMCID: PMC9709930
Review Free full text in Europe PMC
Third-generation smallpox vaccines: challenges in the absence of clinical smallpox.
Future Microbiol, 5(9):1367-1382, 01 Sep 2010
Cited by: 8 articles | PMID: 20860482
Review
Smallpox vaccination and adverse reactions. Guidance for clinicians.
MMWR Recomm Rep, 52(rr-4):1-28, 01 Feb 2003
Cited by: 94 articles | PMID: 12617510
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: N01AI40065
Grant ID: N01 AI040065
National Institute of Allergies and Infectious Diseases, NIH, Department of Health and Human Services (1)
Grant ID: N01AI40065