Abstract
Free full text

Selective changes in mu opioid receptor properties induced by chronic morphine exposure.
Abstract
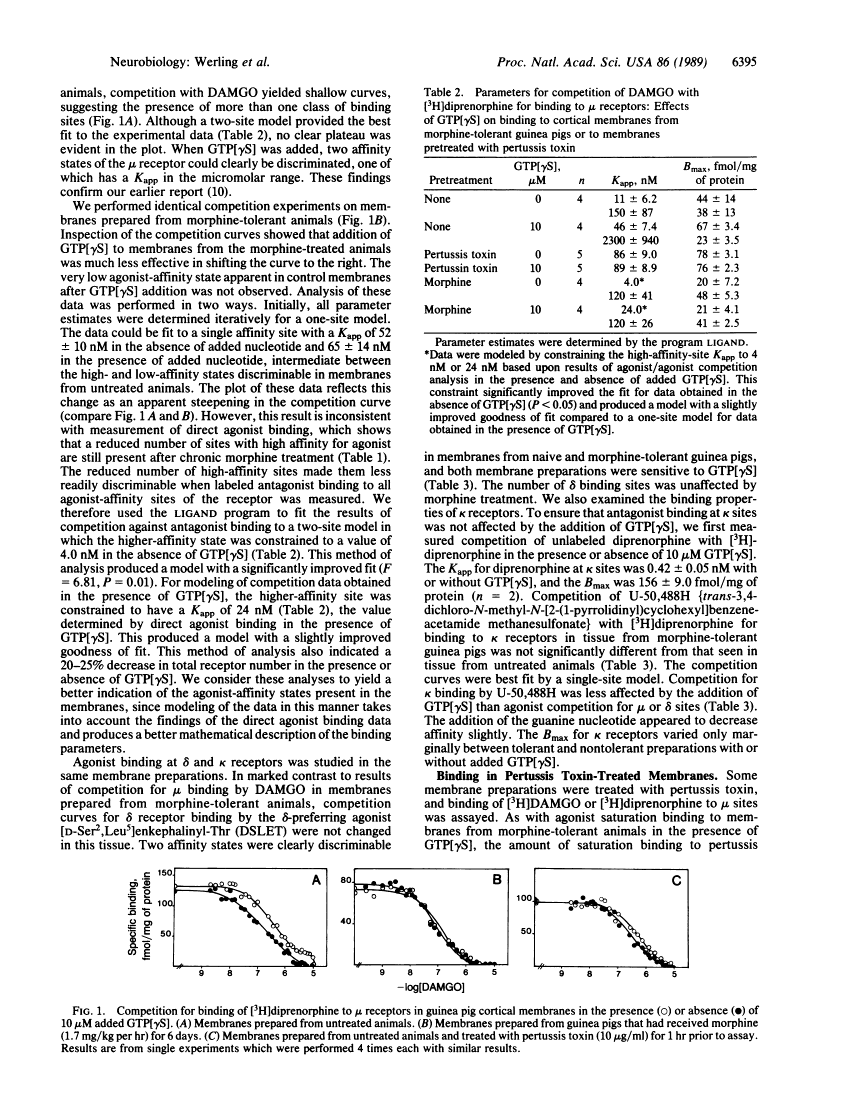
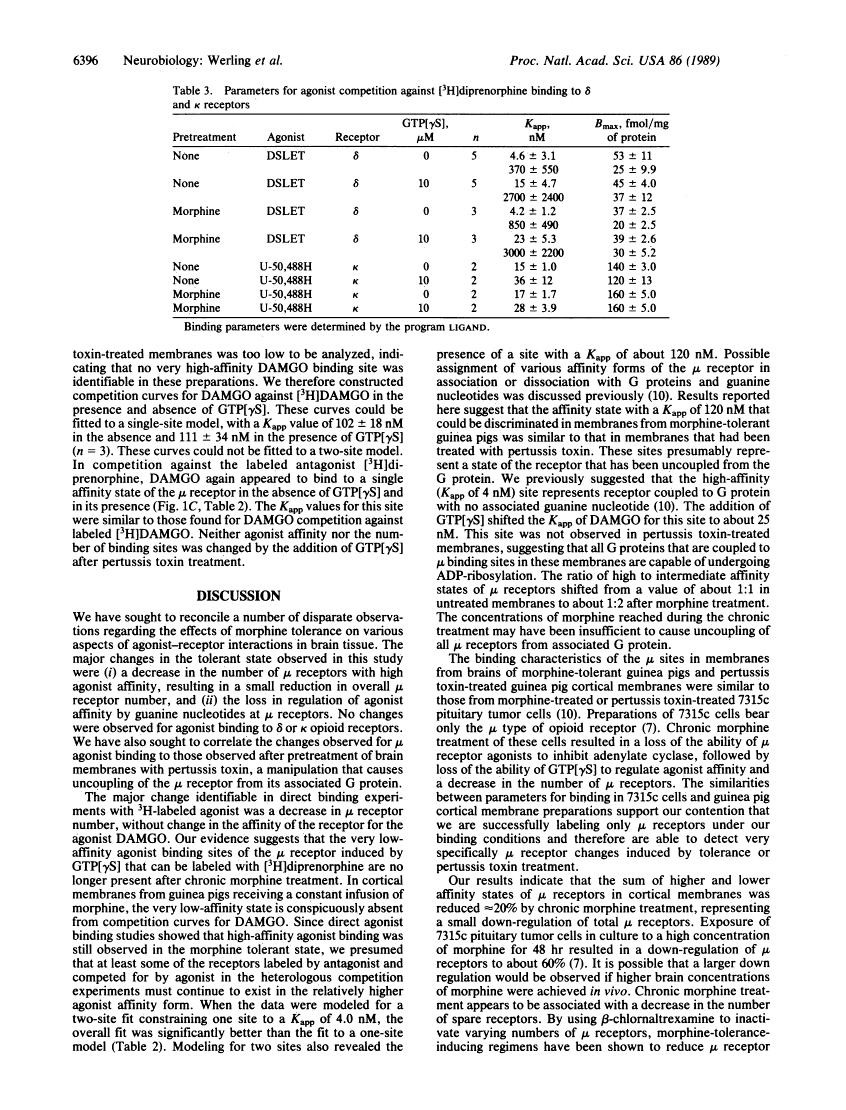
Chronic infusion of morphine to guinea pigs produced selective changes in mu agonist binding properties in cerebrocortical membrane preparations. Employing the mu-selective opioid agonist [D-Ala2,MePhe4,Gly-ol5]enkephalin (DAMGO) in direct binding studies and in competition of labeled antagonist binding, we found that the major changes were a decrease in the number of sites with high affinity for agonist, a small reduction in total receptor number, and a loss in the ability of guanosine 5'-[gamma-thio]triphosphate to regulate binding. A fraction of high-affinity mu receptors appeared to retain their high affinity for agonist and their sensitivity to guanine nucleotide analogue after the induction of morphine tolerance, possibly because the morphine concentrations achieved in brain were insufficient to uncouple all mu receptors from associated guanine nucleotide-binding regulatory proteins. Some membrane preparations were treated with pertussis toxin, which has been shown to functionally uncouple mu opioid receptors from their effector systems. In these preparations, a single agonist-affinity state of the receptor was observed. The apparent dissociation constant for this affinity state in pertussis toxin-treated membranes was similar to the lower-affinity state observed in preparations from morphine-tolerant animals. In contrast to the changes observed at mu opioid binding sites, no significant changes in agonist affinity or binding density were observed for selective delta or kappa agonists, consistent with the development of selective tolerance at mu receptors.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.1M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ORAHOVATS PD, WINTER CA, LEHMAN EG. The effect of N-allylnormorphine upon the development of tolerance to morphine in the albino rat. J Pharmacol Exp Ther. 1953 Dec;109(4):413–416. [Abstract] [Google Scholar]
- Mucha RF, Kalant H. Naloxone prevention of morphine LDR curve flattening associated with high-dose tolerance. Psychopharmacology (Berl) 1981;75(2):132–133. [Abstract] [Google Scholar]
- Schulz R, Wüster M, Krenss H, Herz A. Lack of cross-tolerance on multiple opiate receptors in the mouse vas deferens. Mol Pharmacol. 1980 Nov;18(3):395–401. [Abstract] [Google Scholar]
- Schulz R, Wüster M, Herz A. Differentiation of opiate receptors in the brain by the selective development of tolerance. Pharmacol Biochem Behav. 1981 Jan;14(1):75–79. [Abstract] [Google Scholar]
- Werling LL, McMahon PN, Cox BM. Selective tolerance at mu and kappa opioid receptors modulating norepinephrine release in guinea pig cortex. J Pharmacol Exp Ther. 1988 Dec;247(3):1103–1106. [Abstract] [Google Scholar]
- Law PY, Hom DS, Loh HH. Opiate receptor down-regulation and desensitization in neuroblastoma X glioma NG108-15 hybrid cells are two separate cellular adaptation processes. Mol Pharmacol. 1983 Nov;24(3):413–424. [Abstract] [Google Scholar]
- Puttfarcken PS, Werling LL, Cox BM. Effects of chronic morphine exposure on opioid inhibition of adenylyl cyclase in 7315c cell membranes: a useful model for the study of tolerance at mu opioid receptors. Mol Pharmacol. 1988 May;33(5):520–527. [Abstract] [Google Scholar]
- Klee WA, Streaty RA. Narcotic receptor sites in morphine-dependent rats. Nature. 1974 Mar 1;248(5443):61–63. [Abstract] [Google Scholar]
- Höllt V, Dum J, Bläsig J, Schubert P, Herz A. Comparison of in vivo and in vitro parameters of opiate receptor binding in naive and tolerant dependent rodents. Life Sci. 1975 Jun 15;16(12):1823–1828. [Abstract] [Google Scholar]
- Werling LL, Puttfarcken PS, Cox BM. Multiple agonist-affinity states of opioid receptors: regulation of binding by guanyl nucleotides in guinea pig cortical, NG108-15, and 7315c cell membranes. Mol Pharmacol. 1988 Apr;33(4):423–431. [Abstract] [Google Scholar]
- Carroll JA, Shaw JS, Wickenden AD. The physiological relevance of low agonist affinity binding at opioid mu-receptors. Br J Pharmacol. 1988 Jun;94(2):625–631. [Europe PMC free article] [Abstract] [Google Scholar]
- Goldstein A, Schulz R. Morphine-tolerant longitudinal muscle strip from guinea-pig ileum. Br J Pharmacol. 1973 Aug;48(4):655–666. [Europe PMC free article] [Abstract] [Google Scholar]
- Rogers NF, el-Fakahany EE. Intact brain cells: a novel model system for studying opioid receptor binding. Life Sci. 1985 Jul 29;37(4):307–314. [Abstract] [Google Scholar]
- Rogers NF, el-Fakahany E. Morphine-induced opioid receptor down-regulation detected in intact adult rat brain cells. Eur J Pharmacol. 1986 May 27;124(3):221–230. [Abstract] [Google Scholar]
- Tao PL, Law PY, Loh HH. Decrease in delta and mu opioid receptor binding capacity in rat brain after chronic etorphine treatment. J Pharmacol Exp Ther. 1987 Mar;240(3):809–816. [Abstract] [Google Scholar]
- Kurose H, Katada T, Amano T, Ui M. Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction via alpha-adrenergic, cholinergic, and opiate receptors in neuroblastoma x glioma hybrid cells. J Biol Chem. 1983 Apr 25;258(8):4870–4875. [Abstract] [Google Scholar]
- Sullivan KA, Miller RT, Masters SB, Beiderman B, Heideman W, Bourne HR. Identification of receptor contact site involved in receptor-G protein coupling. Nature. 1987 Dec 24;330(6150):758–760. [Abstract] [Google Scholar]
- Aghajanian GK, Wang YY. Pertussis toxin blocks the outward currents evoked by opiate and alpha 2-agonists in locus coeruleus neurons. Brain Res. 1986 Apr 23;371(2):390–394. [Abstract] [Google Scholar]
- Crain SM, Crain B, Makman MH. Pertussis toxin blocks depressant effects of opioid, monoaminergic and muscarinic agonists on dorsal-horn network responses in spinal cord-ganglion cultures. Brain Res. 1987 Jan 1;400(1):185–190. [Abstract] [Google Scholar]
- Zarr GD, Werling LL, Brown SR, Cox BM. Opioid ligand binding sites in the spinal cord of the guinea-pig. Neuropharmacology. 1986 May;25(5):471–480. [Abstract] [Google Scholar]
- Munson PJ, Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. [Abstract] [Google Scholar]
- Chavkin C, Goldstein A. Opioid receptor reserve in normal and morphine-tolerant guinea pig ileum myenteric plexus. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7253–7257. [Europe PMC free article] [Abstract] [Google Scholar]
- Christie MJ, Williams JT, North RA. Cellular mechanisms of opioid tolerance: studies in single brain neurons. Mol Pharmacol. 1987 Nov;32(5):633–638. [Abstract] [Google Scholar]
- Aub DL, Frey EA, Sekura RD, Cote TE. Coupling of the thyrotropin-releasing hormone receptor to phospholipase C by a GTP-binding protein distinct from the inhibitory or stimulatory GTP-binding protein. J Biol Chem. 1986 Jul 15;261(20):9333–9340. [Abstract] [Google Scholar]
Associated Data
Articles from Proceedings of the National Academy of Sciences of the United States of America are provided here courtesy of National Academy of Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1073/pnas.86.16.6393
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc297846?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Hunting for the high-affinity state of G-protein-coupled receptors with agonist tracers: Theoretical and practical considerations for positron emission tomography imaging.
Med Res Rev, 39(3):1014-1052, 18 Nov 2018
Cited by: 14 articles | PMID: 30450619 | PMCID: PMC6587759
Review Free full text in Europe PMC
Reverse of Acute and Chronic Morphine Tolerance by Lithocholic Acid via Down-Regulating UGT2B7.
Front Pharmacol, 7:404, 01 Nov 2016
Cited by: 4 articles | PMID: 27847477 | PMCID: PMC5088436
Cellular, molecular, and genetic substrates underlying the impact of nicotine on learning.
Neurobiol Learn Mem, 107:108-132, 22 Aug 2013
Cited by: 37 articles | PMID: 23973448 | PMCID: PMC3873373
Review Free full text in Europe PMC
Tolerance and sensitization to chronic escalating dose heroin following extended withdrawal in Fischer rats: possible role of mu-opioid receptors.
Psychopharmacology (Berl), 225(1):127-140, 25 Jul 2012
Cited by: 10 articles | PMID: 22829433 | PMCID: PMC3494815
Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A μ-opioid receptor knock-in mice.
J Neurosci, 31(39):13890-13896, 01 Sep 2011
Cited by: 45 articles | PMID: 21957251 | PMCID: PMC6633166
Go to all (50) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Multiple agonist-affinity states of opioid receptors: regulation of binding by guanyl nucleotides in guinea pig cortical, NG108-15, and 7315c cell membranes.
Mol Pharmacol, 33(4):423-431, 01 Apr 1988
Cited by: 51 articles | PMID: 2833686
Effects of chronic morphine exposure on opioid inhibition of adenylyl cyclase in 7315c cell membranes: a useful model for the study of tolerance at mu opioid receptors.
Mol Pharmacol, 33(5):520-527, 01 May 1988
Cited by: 80 articles | PMID: 2835651
Continuous intrathecal opioid treatment abolishes the regulatory effects of magnesium and guanine nucleotides on mu opioid receptor binding in rat spinal membranes.
J Pharmacol Exp Ther, 262(1):317-326, 01 Jul 1992
Cited by: 19 articles | PMID: 1320689
Opioid-receptor (OR) signaling cascades in rat cerebral cortex and model cell lines: the role of plasma membrane structure.
Physiol Res, 63(suppl 1):S165-76, 01 Jan 2014
Cited by: 4 articles | PMID: 24564656
Review