Abstract
Free full text

Magnetic nanoparticle hyperthermia induced cytosine deaminase expression in microencapsulated E. coli for enzyme-prodrug therapy
Abstract
Engineered bacterial cells that are designed to express therapeutic enzymes under the transcriptional control of remotely inducible promoters can mediate the de novo conversion of non-toxic prodrugs to their cytotoxic forms. In situ cellular expression of enzymes provides increased stability and control of enzyme activity as compared to isolated enzymes. We have engineered Escherichia coli (E. coli), designed to express cytosine deaminase at elevated temperatures, under the transcriptional control of thermo-regulatory λpL-cI857 promoter cassette which provides a thermal switch to trigger enzyme synthesis. Enhanced cytosine deaminase expression was observed in cultures incubated at 42 °C as compared to 30 °C, and enzyme expression was further substantiated by spectrophotometric assays indicating enhanced conversion of 5-fluorocytosine to 5-fluorouracil. The engineered cells were subsequently co-encapsulated with magnetic iron oxide nanoparticles in immunoprotective alginate microcapsules, and cytosine deaminase expression was triggered remotely by alternating magnetic field-induced hyperthermia. The combination of 5-fluorocytosine with AMF-activated microcapsules demonstrated tumor cell cytotoxicity comparable to direct treatment with 5-fluorouracil chemotherapy. Such enzyme-prodrug therapy, based on engineered and immunoisolated E. coli, may ultimately yield an improved therapeutic index relative to monotherapy, as AMF mediated hyperthermia might be expected to pre-sensitize tumors to chemotherapy under appropriate conditions.
1.0 Introduction
Enzyme-prodrug therapy synthesizes chemotherapeutic drugs within the tumor microenvironment, thereby reducing systemic toxicity. In this approach, an exogenous enzyme is targeted to the tumor, a non-toxic prodrug is administered systemically, and the tumor-localized enzyme converts the prodrug to its toxic form (Rooseboom et al., 2004). Enzyme-prodrug combinations of cytochrome P450 2B1 (CYP2B1); ifosfamide/acrolein and isophosphoramide mustard, Herpes simplex virus-thymidine kinase; ganciclovir/ganciclovir triphosphate drug (HSV-TK/GCV/GCV triphosphate) and cytosine deaminase; 5-fluorocytosine/5-fluorouracil (CD;5-FC/5-FU) have been evaluated in humans (Cunningham and Nemunaitis, 2001; Salmons et al., 2003; Sangro et al., 2010). 5-FU is widely used in the clinical management of various cancers including that of the pancreas, breast, colon and the brain (Longley et al., 2003). Cytosine deaminase is a nucleotide modifying enzyme, predominantly of bacterial and fungal origin, which converts the non-toxic prodrug 5-FC to the potent cytotoxic drug 5-FU (Ichikawa et al., 2000). 5-FU is a pyrimidine analog, which inhibits cell proliferation through inhibition of DNA replication and RNA processing, leading to cell death (Altaner, 2008). However, systemic administration of 5-FU is associated with toxicities of the liver, kidney and the heart (Sanoff et al., 2012). Targeting of cytosine deaminase enzyme to cancer cells for the intra-tumoral localization of the prodrug activating enzyme is achieved by various approaches including antibody-directed enzyme prodrug therapy (ADEPT), involving guided delivery of the enzyme to the tumors through antibodies, and gene-directed enzyme prodrug therapy (GDEPT), involving tumor cell modification via transfection and virus-directed enzyme prodrug therapy (VDEPT) (Tietze and Schmuck, 2011; Vajda et al., 2011). These strategies are limited by the availability of the catalytic enzyme in the tumor, and they require high doses of the prodrug to achieve significant conversion to the cytotoxic drug (Xu and McLeod, 2001).
A variety of approaches to enhance the therapeutic efficacy of enzyme-prodrug therapy have been attempted. These include design of improved prodrugs, with greater tumor diffusivity and more favorable activation profiles (Denny and Wilson, 1998; Hay et al., 1999), and better enzymes having improved specificities and higher catalytic efficiencies (Encell et al., 1999; Hamstra et al., 1999). Infusion of non-autologous cells, which can efficiently express therapeutic enzymes within the tumor microenvironment, is another attractive strategy. One example is the use of engineered mammalian cells designed to treat pancreatic cancer (Lohr et al., 2002; Salmons et al., 2003). Bacterial cells can also be engineered to express therapeutic enzymes, and importantly, induction of recombinant enzyme expression can be controlled using external triggers (Fuchita et al., 2009; Valdez-Cruz et al., 2010). However, such non-autologous cells are prone to immune rejection. Immunogenicity can be overcome by encapsulating the cells in immunoprotective matrices that can support cell survival and function, such as alginate microcapsules (Zimmermann et al., 2007). Immunoisolation of bacterial cells is especially important because of their adverse pyrogenic and allergic reactions to the host (Cirone et al., 2006; Prakash and Jones, 2005). Such microencapsulation of live bacterial cells has received considerable research interest as it has broad therapeutic applications (Chang and Prakash, 1998, 2001). Here, we report on triggering enzyme-prodrug therapy with magnetic nanoparticle hyperthermia (MNPHT) by immunoisolative microencapsulation of an engineered Escherichia coli (E. coli) strain that overexpresses the cytosine deaminase enzyme upon thermal induction. We show that in situ cytosine deaminase expression, enzymatic conversion of 5-FC to 5-FU, and subsequent killing of various cancer cells lines can be initiated via remote activation of magnetic iron oxide nanoparticles (MNP) that have been co-encapsulated with the bacterial expression host.
2.0 Materials and methods
2.1 Materials
Sodium alginate, calcium chloride, 5-FC and 5-FU were obtained from Sigma-Aldrich (www.sigmaaldrich.com). Magnetic iron (III) oxide nanopowder (~ 30 nm diameter) was procured from Alfa Aesar (www.alfa.com). Phusion high fidelity polymerase chain reaction (PCR) amplification kit, restriction enzymes (EcoRV, ClaI, BstBI) and Quick ligation kit were procured from New England Biolabs Inc. (www.neb.com). PCR primers were obtained from Integrated DNA technologies (www.idtdna.com). QIAquick PCR purification kit, QIAquick gel extraction kit and QIAprep spin miniprep plasmid isolation kit were procured from Qiagen (www.qiagen.com). Bugbuster protein extraction reagent was obtained from EMD Millipore (www.emdmillipore.com). NM522 (supE, thi, Δ(lac-proAB), hsd5 (r−, m−), [F′, proAB, lacIqZΔM15]) competent cells were obtained from Promega (www.promega.com). pLDR20 vector was obtained from ATCC (www.atcc.org). pBCD540FT vector is a kind gift from Prof. Martin Brown, Stanford University. The PC-3 human prostate cancer cells, MCF-7 human breast cancer cells and 9L rat brain glioma cells were obtained from the American Type Culture Collection (www.atcc.org). PC-3 cells were cultured in Ham’s F-12K culture medium. MCF-7 and 9L cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM). Media were supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin antibiotics (www.thermoscientific.com). Luria-Bertani (LB) and LB-Agar media for bacterial cell culture were procured from BD Biosciences (www.bd.com).
2.2 Plasmid construction
The bacterial cytosine deaminase gene was amplified by PCR from the pbCD540FT vector using 5′-CAGGATATCTATCCGCTCACAATTCCA-3′(forward primer with an EcoRV site) and 5′-GCATTCGAATCAACGTTTGTAATC-3′(reverse primer with a BstBI site). The PCR product was purified, digested using the EcoRV and BstBI restriction enzymes and verified using agarose gel electrophoresis. Separately, the pLDR20 vector was digested with EcoRV and ClaI (compatible with BstBI). This digested product was ligated with the cytosine deaminase gene using T4 DNA ligase to generate the pLDR20-cytosine deaminase vector.
2.3 NM522 cell transformation and heat induced protein expression
The transformation mixture obtained from the previous step, with an ampicillin resistant gene present in the pLDR20 vector, was plated at various volumes onto a LB-Agar plate supplemented with ampicillin (10 μg/ml). A fresh colony of engineered NM522 was selected from the LB-Agar-ampicillin selection plate and LB medium (5 ml) containing ampicillin (100 μg/ml) was inoculated with it. The culture was grown overnight at 30 °C with vigorous shaking (~200 rpm). Plasmid DNA was isolated from the engineered cells and the cloned sequence verified by PCR amplification and DNA sequencing. Two lots of LB medium (50 ml) were then inoculated with overnight culture (0.5 ml) and further incubated for 4–5 h at 30 °C with vigorous shaking. Once the bacterial cultures reached mid-log phase (OD600~0.6), one flask was heated at 42–43 °C for 30 min in a water bath to induce protein expression while the other was not. Cells were harvested from both flasks by centrifugation 4,000 × g for 15 min at 4°C and the total protein was isolated using Bugbuster reagent (www.emdmillipore.com) following manufacturer recommended protocol. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed in 10% w/v polyacrylamide gels containing 0.1% w/v SDS. Gels were stained with Coomassie Brilliant Blue R250 and protein expression was compared between the two sets of cultures grown at 30°C and 42–43°C.
2.4 Spectrophotometric assay of cytosine deaminase activity in engineered E. Coli
Thermoregulated cytosine deaminase expression was also evaluated by a functional assay based on the conversion of 5-FC to 5-FU, spectrophotometrically over a period of 24 h. Various concentrations of 5-FC solutions (0.125, 0.25 and 0.5 mM in Tris-HCl buffer 50 mM, pH 7.4) were incubated with the engineered E. coli (0.5 and 1 mg of cells) cultured at either 30°C or 42–43°C. The conversion was monitored over a period of 24 h and quantified by the ratio of absorbance at 267 (λmax of 5-FC) and 276 nm (λmax of 5-FU).
2.5 Co-encapsulation of magnetic iron oxide particles and engineered E. Coli in alginate microcapsules
Co-encapsulation of E. coli with MNP was carried out by re-suspending the cells cultured at 30 °C in a solution of sodium alginate (2% w/v) containing magnetic iron(III) oxide powder (0.2 % w/v). A homogenous suspension of the iron (III) oxide particles in alginate was formed overnight through gentle stirring on a shaking platform and the E. coli cells re-suspended in the mixture (~10 mg/ml of E. coli in the solution). Microcapsules were formed by extrusion of the alginate-iron (III) oxide- E. coli mixture (Alg-IO-EC) into a CaCl2 gelling solution (100 mM in PBS) using a 30G needle. The Alg-IO-EC microcapsules were washed twice with PBS and re-suspended in LB-Amp medium.
Morphology of the alginate microcapsules containing MNP and E. coli were characterized by scanning electron microscopy (SEM). Microcapsules were critical point dried and coated with a gold/palladium alloy (60:40). Images were acquired using a FEI XL-30 field emission gun environmental scanning electron microscope (www.fei.com) at 20 kV. Microcapsules were also characterized by tunneling electron microscopy (TEM). Thin sections were mounted on 400HH Cu grids (www.emsdiasum.com) and stained with methanolic uranyl acetate. Images were acquired using a JEOL 1010 transmission electron microscope equipped with an XR-41B AMT digital camera (www.jeolusa.com).
2.6 Magnetic nanoparticle hyperthermia induced cytosine deaminase expression in Alg-IO-EC microcapsules
Cytosine deaminase expression in Alg-IO-EC microcapsules was triggered through magnetic nanoparticle induced hyperthermia using an AMF generator. The AMF generator comprises a frequency generator and a power ampli er generating up to 27 A at60 kHz. The induction coil was built from brass tubes that are cooled using deionized water (20 °C). Alg-IO-EC microcapsules were transferred to a sterile 5 ml tube. The tube was placed in the induction coil and heating was performed at 450 Oe till the temperature reached 42–43 °C in the center of the tube – three fiber optic temperature probes were placed at the bottom, center and the top of the tube to monitor the temperature. Subsequently the power was reduced so that the temperature was maintained at 42–43 °C for 30 min. Cytosine deaminase activity in E. coli subjected to magnetic nanoparticle heating was compared with Alg-IO-EC microcapsules heated in a water bath at 42–43 °C for 30 min.
2.7 Cytotoxicity of magnetic nanoparticle hyperthermia induced cytosine deaminase expression in Alg-IO-EC microcapsules
Tumor cells from the 3 cell lines (PC-3, MCF-7 and 9L) were seeded at 5 × 104 cells per well in 12-well microtiter culture plates. Following overnight incubation, cells were supplemented with fresh medium supplemented with either (i) 5-FC (0.5 mM), (ii) 5-FU (0.5 mM), (iii) Alg-IO-EC microcapsules, (iv) 5-FC (0.5 mM) /Alg-IO-EC microcapsules pre-heated to 42–43 °C for 30 min using a water bath and (v) 5-FC (0.5 mM) / Alg-IO-EC microcapsules preheated to 42–43 °C using AMF for 30 min, ad (vi) controls with no supplements. Following incubation at 37 °C for 72 h, the culture medium was removed along with the microcapsules, and MTT solution (0.5 ml, 5mg/ml in PBS) was added to each well and further incubated for 4 h. Then the supernatant was removed, and the formazan precipitate formed by the viable cells was dissolved in 0.5 ml of dimethyl sulfoxide. Samples were transferred to a 96-well clear bottom plate and the absorbance measured at 570 nm using a microplate reader (www.moleculardevices.com) and SOFTmax 4.8 software. Cytotoxicity was determined for each condition and all 3 cell lines.
2.8 Statistical analysis
All values reported are means ± standard deviation. For comparison between the groups, a one-way analysis of variance (ANOVA) with Dunnett’s post-hoc test was used to determine statistically significant difference between the groups at a P value of 0.05.
3.0 Results and discussion
3.1 Cloning and thermoregulated expression of cytosine deaminase
Recombinant protein expression using E. coli facilitates large scale and low-cost expression of a variety of proteins and peptides (Baneyx, 1999; Valdez-Cruz et al., 2010). Protein expression can be triggered using externally inducible transcriptional promoters, which confer temporal control (Jana and Deb, 2005; Palomares et al., 2004). Of all the commonly used promoters, thermoregulators are preferred because of strong transcriptional control without the use of external chemicals or expensive specialized media (Makrides, 1996). Thermoregulated protein expression using the mutant cI857 repressor and the pL and/or pR phage λ promoters have been used to engineer recombinant prokaryotic cells (Mieschendahl and Muller-Hill, 1985; Valdez-Cruz et al., 2010). The gene of interest cloned downstream of the λ promoters can then be efficiently regulated by the mutant thermolabile cI857 repressor of bacteriophage λ (Mieschendahl and Muller-Hill, 1985; Villaverde et al., 1993). At temperatures below 37 °C, cI857 binds to the oL or oR regions of the pR promoter and blocks transcription by RNA polymerase. At higher temperatures, the functional cI857 dimer is destabilized, binding to the oL or oR DNA sequences is abrogated, and mRNA transcription is initiated (Valdez-Cruz et al., 2010). pLDR20 contains the thermo-regulated expression system driven by the major rightward (pR) promoter. A variety of proteins have been produced using this heat inducible protein expression system (Chen et al., 2002; Zhang et al., 2003). We have adopted a similar strategy to achieve heat induced cytosine deaminase expression in E. coli strain NM522 for enzyme-prodrug therapy.
The cytosine deaminase gene was PCR amplified from pbCD540FT and ligated downstream of the λ promoter of pLDR20 vector to provide temporal control over cytosine deaminase expression (Fig. 1). The ampicillin resistance enabled positive selection in LB-Amp media. From SDS-PAGE analysis of total protein extracts we observed higher levels of protein expression in cells cultured at 42–43 °C as compared to cells cultured at 30 °C (Fig. 2). These results confirm enhanced heat induced expression of cytosine deaminase in the engineered NM522 cells containing the cloned pLDR20-cytosine deaminase gene.
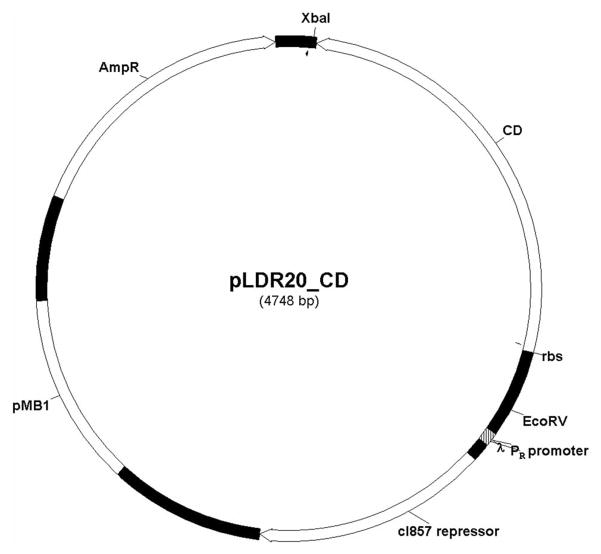
Vector map of the engineered pLDR20-cytosine deaminase plasmid designed to express cytosine deaminase under thermal stress. The plasmid vector comprises cytosine deaminase gene, under the transcriptional control of the λPR – cI857 thermorepressive promoter cassette, pMB1 origin of replication and an ampicillin resistance marker (AmpR).
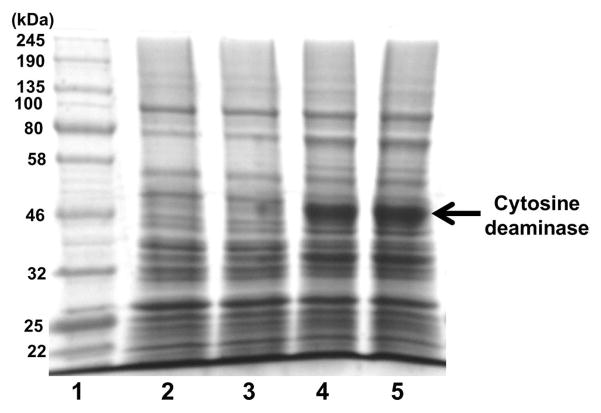
SDS-PAGE analysis of cytosine deaminase expression in cell lysates of transformed NM522 E. coli cells, at 30 and 42 °C. Lane 1 is a standard protein molecular weight marker ladder. Lanes 2,3 are total protein extracts from cells cultured at 30 °C and lanes 4,5 are protein extracts from cells cultured at 42°C.
3.2 Spectrophotometric analysis of cytosine deaminase activity
The thermoregulated expression of cytosine deaminase was further verified using spectrophotometric analysis of enzyme-catalyzed 5-FC to 5-FU conversion by whole cells. Engineered cells cultured at 42–43 °C catalyzed conversion of 5-FC to 5-FU, at all concentrations of 5-FC employed, over a 24 hour time period (Fig. 3), even when the cell concentration was reduced to 0.5 mg/ml, albeit at a slower rate (Supplementary Fig. S1). In contrast, cells cultured at 30 °C yielded little to no 5-FC conversion (Supplementary Fig. S2). For the heat induced cells, rates of conversion were found to be proportional to both the density of cellular catalyst and the concentration of 5-FC substrate.
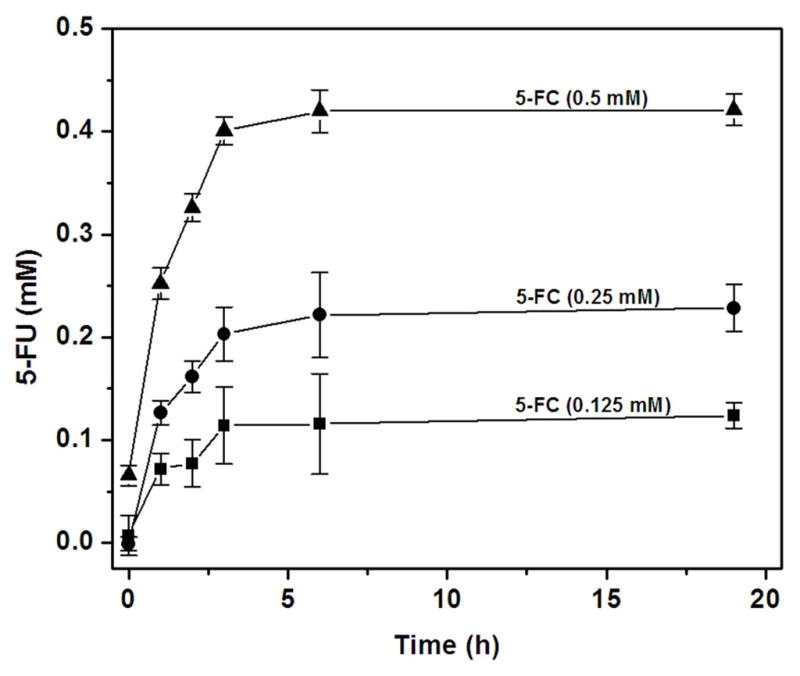
Engineered NM522 E. coli cells cultured at 42 °C (1mg/ml) efficiently convert 5-FC to 5-FU. NM522 cells cultured at 42 °C (1mg/ml) were incubated with (a) 0.125 mM (■), (b) 0.25 mM (●) and (c) 0.5 mM (▲)of 5-FC in Tris-HCl buffer (50 mM, pH 7.4). The conversion of 5-FC to 5-FU was evaluated by UV-visible spectrophotometry.
3.3 Microencapsulation of engineered E. coli in alginate microcapsules and AMF induced heating
Most enzyme-prodrug strategies involve either localization of exogenously administered enzymes to the tumor cells (ADEPT) or transformation of the tumor cells so as to yield autologous expression of prodrug converting biocatalysts (GDEPT). However, the therapeutic enzymes’ immunogenicity and susceptibility to proteolytic decay can limit the efficacy of these treatment strategies. Microencapsulation of cytosine deaminase in immunoisolative matrices like alginate can provide immunoprotection without affecting its catalytic activity and cytotoxicity in presence of 5-FC (Funaro et al., 2012), but even encapsulated enzymes suffer from long term in vivo stability issues. Additionally, the activity of many enzymes is dependent upon various co-factors, which are often available only within the intracellular environment. Thus, encapsulated engineered cells able to express cytosine deaminase in vivo and on demand might constitute an improved means for in situ synthesis of 5-FU within the tumor microenvironment (Nemani et al., 2013).
Sodium alginate is a naturally occurring hydrogel matrix used in the encapsulation of a wide variety of therapeutic macromolecules. It is a marine polysaccharide, which forms a microporous matrix through ionotropic gelation, usually with calcium chloride, resulting in an immunoprotective matrix based on size-exclusion of large molecules of the immune system. Alginate microcapsules possess long term in vivo mechanical stability and can be tailored to minimize immunogenicity ensuring long term graft survival. Clinical applications of alginate microcapsules has included the transplantation of pancreatic islets, from both autologous and non-autologous sources, for the treatment of type 1 diabetes mellitus (Zimmermann et al., 2007). Alginate microcapsules have also been used in the delivery of live therapeutic bacteria (Chang and Prakash, 1998). While encapsulation of cytosine deaminase producing E. coli in alginate microcapsules addresses the issues of immunogenicity and long term enzyme stability, the therapeutic utility of encapsulated cells is dependent upon controlled induction of cytosine deaminase expression. We envisioned that thermoregulated cytosine deaminase expression could be triggered remotely by AMF activation of magnetic nanoparticles that might be co-encapsulated with the E. coli expression hosts.
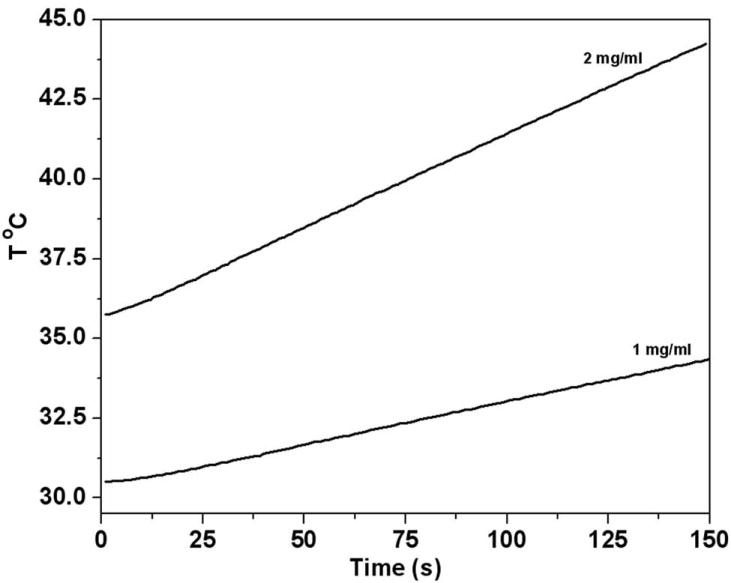
The engineered E. coli were co-encapsulated with MNP in alginate microcapsules and were imaged using SEM and TEM (Fig. 4). It is observed from the SEM images that MNP aggregate in clusters of ~1.5–2 μm in diameter (Fig. 4a). From the TEM images it is evident that the clusters comprise iron oxide particles which are approximately ~30–60 nm in diameter (Fig. 4b). For AMF hyperthermia, the rate of heating and the temperature achieved is dependent on both the field strength and the concentration of magnetic nanoparticles within the microcapsule. The optimum concentration of MNP required to raise the temperature of the alginate microcapsules to 42–43 °C was determined using alginate microspheres containing four different nanoparticle concentrations (0.25, 0.5, 1.0 and 2.0 mg /ml) and evaluated at a field strength of 400 Oe (Supplementary Fig. S3). At an MNP concentration of 2 mg/ml, a temperature of 42–43 °C could be achieved within 3 minutes using a field strength of 450 Oe (Fig. 5), which was used for all subsequent experiments.
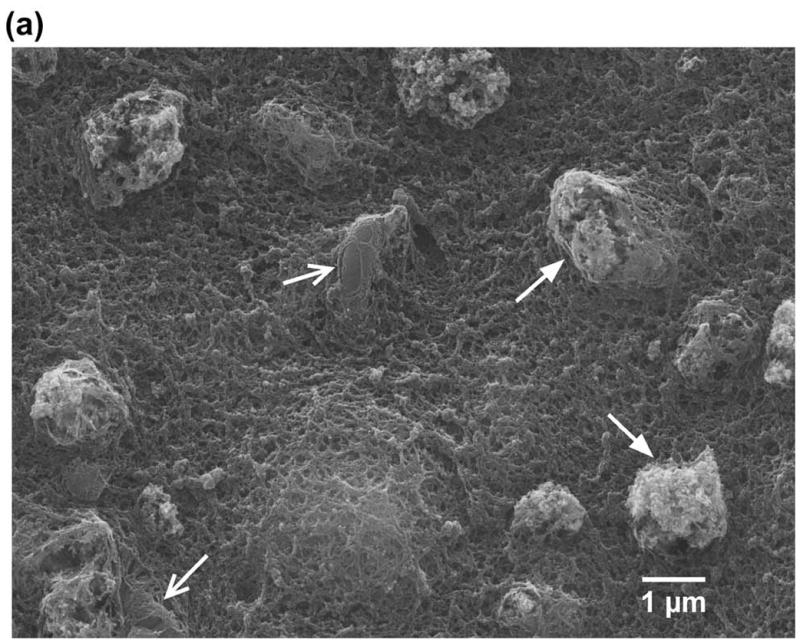
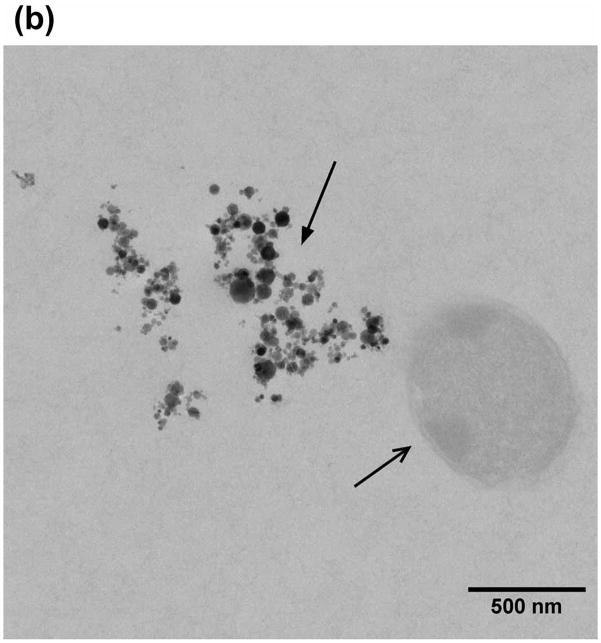
Morphology of the alginate microcapsules containing the engineered E. coli (open arrows) and the iron oxide nanoparticles (MNP, closed arrows) characterized by scanning electron microscopy (a) and Tunneling electron microscopy (b). The MNP aggregate in clusters of ~1.5–2 μm (a) and individual particles are ~30–60 nm in diameter (b).
3.4 Tumor cell toxicity of AMF induced cytosine deaminase/5-FC treatment using Alg-IO-EC microcapsules
Magnetic nanoparticles have been used in a variety of biomedical applications such as drug delivery, radionuclide and gene delivery, imaging, biochemical assays and in hyperthermia based cancer therapy (Frimpong and Hilt, 2010). Brule et al (Brule et al., 2011) have shown that AMF induced hyperthermia enhances doxorubicin release when co-encapsulated with MNP in alginate microcapsules resulting in efficient tumor cell kill. Ciofani et al (Ciofani et al., 2009) proposed a bimodal therapeutic approach involving MNPHT and heat induced drug release. They hypothesized that by co-encapsulating a therapeutic drug with magnetic nanoparticles in polymeric matrices such as alginate, AMF induced heating of the nanoparticles would not only lead to heating of tumors but would also trigger drug release. Ortner et al (Ortner et al., 2012) engineered human embryonic kidney cells (HEK293-C5) that could express green fluorescent protein (GFP) under the transcriptional control of a heat shock promoter. The engineered cells were co-encapsulated with magnetic nanoparticles in microcapsules and GFP expression was triggered by AMF induced heating. No GFP expression was observed in the absence of the nanoparticles.
We have demonstrated the cytotoxic effects of MNPHT-induced cytosine deaminase expression from encapsulated bacterial cells. Engineered E. coli were co-encapsulated with MNP in alginate microcapsules (Alg-IO-EC) through a process of ionic gelation. The resulting microcapsules are spherical in nature with an average diameter of 800–900 μm (Fig. 6a). As described in section 3.3, the microcapsules were subjected to AMF at 450 Oe to attain a temperature of 42–43 °C, and the desired target temperature could be maintained continuously for ~30 min by subsequently reducing the power of the field (Fig. 6b). In 5-FC cell kill experiments with three common cancer lines, the efficacy of Alg-IO-EC microcapsules subjected to AMF was comparable to that of microcapsules heated at 42–43 °C in a water bath (Fig. 7). This result is consistent with the earlier spectroscopic analysis of 5-FC to 5-FU conversion (Fig. 3), and it provides a functional validation of MNPHT-induced cytosine deaminase expression in the alginate microcapsules. Importantly, the cytotoxicity observed with MNPHT-induced Alg-IO-EC treatments was comparable to direct treatment with 5-FU. Interestingly, we observed basal cytotoxicity from Alg-IO-EC microcapsule treatment in the absence of 5-FC prodrug. This unexpected toxicity may be due to shedding of bacterial toxins (e.g. lipopolysaccharide) or competition between bacterial and mammalian cells for media nutrients. Regardless, the results clearly show that efficient 5-FC mediated cell kill requires the presence of cytosine deaminase expressing E. coli.

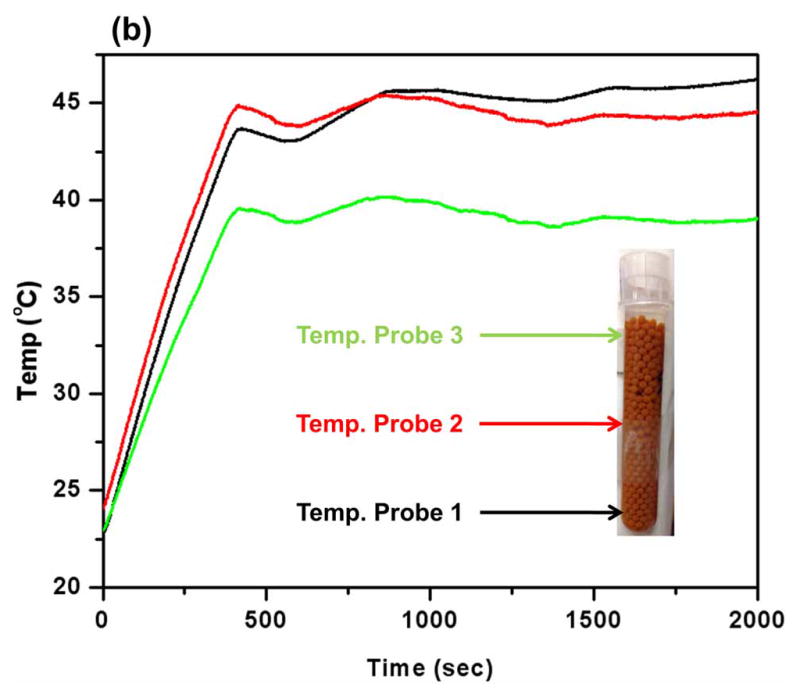
AMF induced magnetic nanoparticle hyperthermia in alginate microcapsules containing magnetic iron (III) oxide. Engineered E. coli were co-encapsulated with iron oxide nanoparticles in alginate microcapsules (a). The tube containing the microcapsules was placed in a brass coil, and three optical fiber probes were placed in the tube to record the temperature. The microcapsule suspension was exposed to a magnetic field of 450 Oe until the temperature reached 43 °C, at which point the field strength was reduced so as to maintain the desired temperature (b).
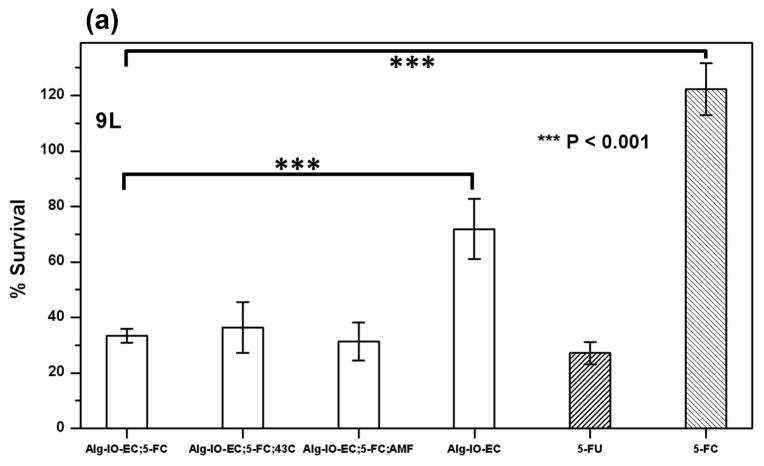
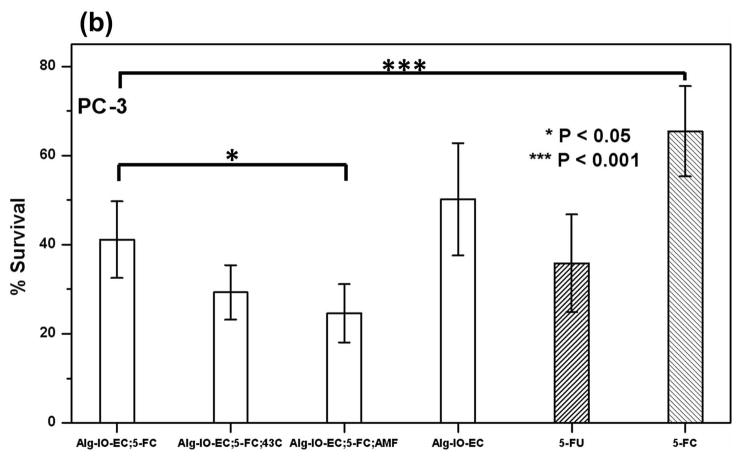
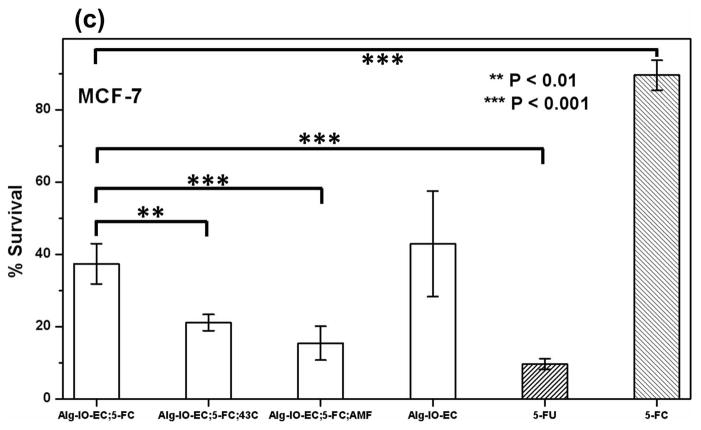
Tumor cell cytotoxicity of the engineered thermosensitive NM522 cells co-encapsulated with magnetic iron (III) oxide nanoparticles in alginate microcapsules (Alg-IO-EC). Three tumor cell lines; 9L rat glioma cells (a), PC-3, human prostate cancer cells (b) and MCF-7, human breast cancer cells (c) were cultured in media supplemented with Alg-IO-EC microcapsules and 5-FC (0.5 mM), subjected to cytosine deaminase/5-FC enzyme prodrug therapy, and cytotoxicity was compared against 5-FC (0.5 mM) and 5-FU (0.5 mM) (shaded bars indicating absence of E. coli). Cytosine deaminase expression was induced thermally by either heating in a 43 °C water bath or exposure to AMF. Cells cultured in media containing the microcapsules but no 5-FC served as the control. Cytotoxicity was evaluated by standard MTT assay, and the percentage survival relative to untreated cells is reported. Asterisk indicates significant difference from basal cytotoxicity levels observed in case of Alg-IO-EC microcapsule/5-FC treatment at 37 °C (One way ANOVA with Dunnett’s post-hoc test, n=6).
In contrast to studies with the two human cancer cell lines, experiments with rat 9L glioma cells revealed that thermally-induced cytosine deaminase expression did not significantly enhance cytotoxicity relative to cultures treated with 5-FC and Alg-IO-EC at 37 °C. This could be due to the higher 5-FU sensitivity of 9L cells and/or tumor cell starvation resulting from rapid proliferation; it bears noting that a single concentration of therapeutic agent may not be appropriate for all cancers, given that different tumor cell lines display varying sensitivity to 5-FU (Miller et al., 2002). Therefore, follow-on studies will tailor therapeutic regimens to tumor cell lines by varying E. coli cell density within the microcapsules to minimize toxicity from bacterial toxins and modulating the 5-FC dosage. In this initial proof-of-concept, however, we have demonstrated the feasibility of remotely activating enzyme-prodrug therapy using MNPHT. Here, we have focused on MNPHT as a remote trigger for enzyme prodrug therapy, but MNPHT can itself induce tumor cell kill (Giustini et al., 2010; Kobayashi, 2011). Therapeutic application of MNPHT is based on the rationale that tumor cells are more susceptible to heat than normal cells (van der Zee, 2002). Magnetic nanoparticles are first delivered to sites of malignancy and then activated using a radio frequency AMF. The resulting energy deposition induces local hyperthermia and leads to tumor cell kill (Kobayashi, 2011; Tietze et al., 2012). The temperatures usually achieved are in the range of 42–45 °C (Kobayashi, 2011), which is sufficient to both kill cancer cells and activate recombinant protein expression from heat inducible promoters. Thus, our engineered treatment platform could manifest multiple modes of action.
We have chosen a strategy of thermally induced expression over constitutive expression to maximize productivity of cytosine deaminase – protein expression is higher in high copy number plasmids under the transcriptional control of an inducible promoter (Balbas, 2001). This inducible expression should facilitate efficient, de novo synthesis of 5-FU from a fewer number of E. coli cells and reduce the dosage of 5-FC. Moreover, the overexpression of recombinant proteins represents a severe metabolic burden on bacterial hosts which often initiate various stress responses (Hoffmann and Rinas, 2004). To minimize any confounding complications associated with such stress responses, we will control cytosine deaminase expression, such that it is produced “on demand” and only when needed. Our envisioned strategy for advancing the proposed therapy to the clinic is briefly outlined in Chart 1. We will enhance the mechanical stability of the alginate microcapsules by using BaCl2 for ionic gelation and coating the microcapsules with additional layers of polyelectrolytes to prevent degradation of the microcapsules and leakage of E. coli. These methods are well known to enhance mechanical stability and prevent microcapsule rupture. Furthermore, we will incorporate suicide genes into our engineered bacteria that can be triggered upon release from the alginate microcapsules. Our proposed platform can be easily adapted for the expression of other therapeutic biomolecules from recombinant bacterial and autologous cells. Externally triggered expression provides a mechanism to achieve multiple activations in therapies requiring repeated dosing.
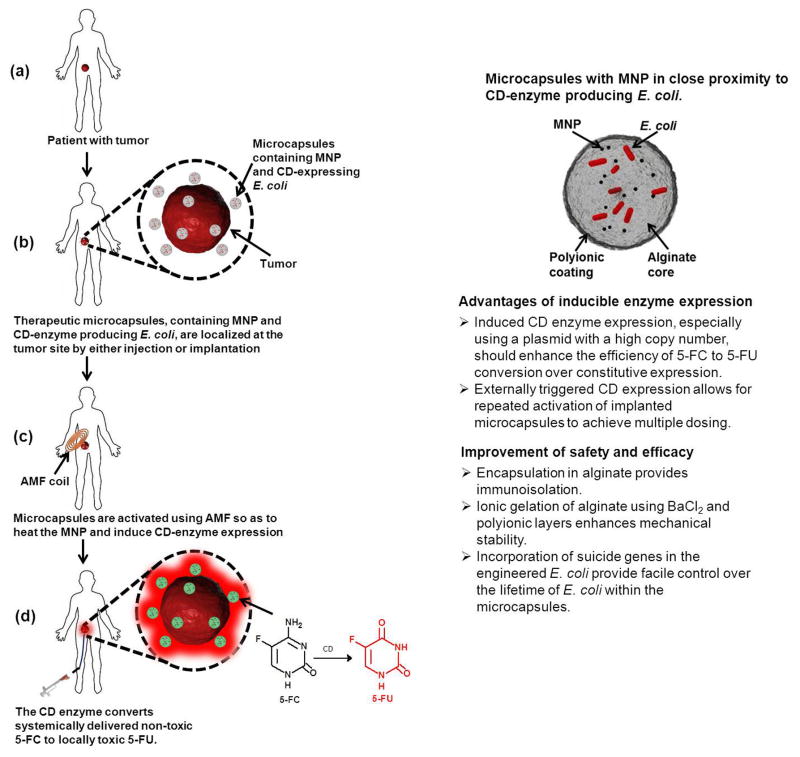
Experimental strategy for future in vivo experiments for evaluating the therapeutic efficacy of the proposed enzyme-prodrug therapy in patients with a localized tumor (a). Alginate microcapsules containing the engineered E. coli and magnetic nanoparticles (MNP) are surgically implanted into the tumor or angiographically delivered to the vicinity of the tumor (b). The patient is then subjected to an external alternating magnetic field (AMF) to heat the MNP, thereby activating the E. coli (c). The heat activated E. coli convert systemically administered non-toxic prodrug 5-FC to its potent chemotherapeutic form 5-FU locally in the vicinity of the tumor thereby localizing the dose and reducing systemic toxicity (d). The advantages of inducible enzyme expression, as well as the improvements in safety and efficacy by encapsulating engineered bacteria are detailed here (right panel).
Enzyme-prodrug therapy has been shown previously to enhance tumor cell sensitivity to radiation therapy (Huang et al., 2009; Robson et al., 2005). In a mouse model of human glioma, Kaliberov et al. (Kaliberov et al., 2007) observed higher survival rates with a combination therapy as opposed to treatment with either cytosine deaminase/5-FC or radiation alone. Enzyme-prodrug therapy, temporally triggered by the tumor environment or other treatment modalities, sensitizes tumor cells and enhances overall therapeutic efficacy (Brade et al., 2003; Sun et al., 2012). Brade et al. (Brade et al., 2003) placed two suicide genes, cytosine deaminase and TK, under transcriptional control of the hsp70b promoter, and they demonstrated killing of breast cancer cells via hyperthermia-induced protein expression. In the clinic, hyperthermia has been combined with chemotherapy in the treatment of inoperable and metastatic tumors (Issels, 2008). Thus, the literature provides substantial evidence supporting the hypothesis that MNPHT-triggered enzyme prodrug therapy may yield a therapeutic advantage over its individual components, and moreover that it might combine synergistically with radiation and other treatment modalities. Combinatorial therapy is often more effective than monotherapy in the treatment of cancers, and we speculate that MNPHT-triggered enzyme prodrug therapy could benefit from inherent therapeutic synergy as well as synergy with other treatment modalities.
4.0 Conclusion
We have engineered a bacterial cell line designed to express cytosine deaminase under heat stress, and we have co-encapsulated these bacteria with MNP in immunoprotective alginate microcapsules. The thermoregulatory promoter system could be activated by MNPHT resulting in cytosine deaminase expression that efficiently converted 5-FC to 5-FU. Cell kill studies showed that this prodrug conversion led to tumor cell toxicity for three different cancer cell lines. We speculate that, once optimized for particular cancers, our highly engineered treatment platform can be adapted to provide combinatorial therapy that will exhibit improved therapeutic efficacy relative to chemotherapy or MNPHT monotherapies. To our knowledge, this system is the first example of an on demand enzyme-prodrug therapy utilizing encapsulated, engineered bacteria and triggered by magnetic nanoparticle hyperthermia.
Acknowledgments
pbCD540FT is a kind gift from Prof. J. M. Brown, Stanford University School of Medicine. We wish to thank Rendall Strawbridge, Prof. Jack Hoopes for help with the AMF treatment and Dr. Charles Daghlian and Christopher Ogomo for help with SEM and TEM image acquisition. This work was supported by a pilot grant from the Dartmouth Center for Cancer Nanotechnology Excellence (NIH U54 CA151662; BG, NVK).
Abbreviation list
| 5-FC | 5-fluorocytosine |
| 5-FU | 5-fluorouracil |
| AMF | alternating magnetic field |
| MNP | magnetic nanoparticles |
| MNPHT | magnetic nanoparticle hyperthermia |
| PCR | polymerase chain reaction |
| DNA | Deoxyribonucleic acid |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| PBS | phosphate buffered saline |
| Alg-IO-EC | Alginate-iron (III) oxide-E. coli |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altaner C. Prodrug cancer gene therapy. Cancer Lett. 2008;270:191–201. [Abstract] [Google Scholar]
- Balbas P. Understanding the art of producing protein and nonprotein molecules in Escherichia coli. Mol Biotechnol. 2001;19:251–267. [Abstract] [Google Scholar]
- Baneyx F. Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol. 1999;10:411–421. [Abstract] [Google Scholar]
- Brade AM, Szmitko P, Ngo D, Liu FF, Klamut HJ. Heat-directed suicide gene therapy for breast cancer. Cancer Gene Ther. 2003;10:294–301. [Abstract] [Google Scholar]
- Brule S, Levy M, Wilhelm C, Letourneur D, Gazeau F, Menager C, Le Visage C. Doxorubicin release triggered by alginate embedded magnetic nanoheaters: A combined therapy. Adv Mater. 2011;23:787–790. [Abstract] [Google Scholar]
- Chang TM, Prakash S. Therapeutic uses of microencapsulated genetically engineered cells. Mol Med Today. 1998;4:221–227. [Abstract] [Google Scholar]
- Chang TM, Prakash S. Procedures for microencapsulation of enzymes, cells and genetically engineered microorganisms. Mol Biotechnol. 2001;17:249–260. [Abstract] [Google Scholar]
- Chen X, Liu Z, Zhang J, Zhang W, Kowal P, Wang PG. Reassembled biosynthetic pathway for large-scale carbohydrate synthesis: α-gal epitope producing “superbug” ChemBioChem. 2002;3:47–53. [Abstract] [Google Scholar]
- Ciofani G, Riggio C, Raffa V, Menciassi A, Cuschieri A. A bi-modal approach against cancer: Magnetic alginate nanoparticles for combined chemotherapy and hyperthermia. Med Hypotheses. 2009;73:80–82. [Abstract] [Google Scholar]
- Cirone P, Potter M, Hirte H, Chang P. Immuno-isolation in cancer gene therapy. Curr Gene Ther. 2006;6:181–191. [Abstract] [Google Scholar]
- Cunningham C, Nemunaitis J. A phase I trial of genetically modified Salmonella typhimurium expressing cytosine deaminase (TAPET-CD, VNP20029) administered by intratumoral injection in combination with 5-fluorocytosine for patients with advanced or metastatic cancer. Protocol No: Cl-017 Version: April 9, 2001. Hum Gene Ther. 2001;12:1594–1596. [Abstract] [Google Scholar]
- Denny WA, Wilson WR. The design of selectively-activated anti-cancer prodrugs for use in antibody-directed and gene-directed enzyme-prodrug therapies. J Pharm Pharmacol. 1998;50:387–394. [Abstract] [Google Scholar]
- Encell LP, Landis DM, Loeb LA. Improving enzymes for cancer gene therapy. Nat Biotechnol. 1999;17:143–147. [Abstract] [Google Scholar]
- Frimpong RA, Hilt JZ. Magnetic nanoparticles in biomedicine: Synthesis, functionalization and applications. Nanomedicine (Lond) 2010;5:1401–1414. [Abstract] [Google Scholar]
- Fuchita M, Ardiani A, Zhao L, Serve K, Stoddard BL, Black ME. Bacterial cytosine deaminase mutants created by molecular engineering show improved 5-fluorocytosine-mediated cell killing in vitro and in vivo. Cancer Res. 2009;69:4791–4799. [Europe PMC free article] [Abstract] [Google Scholar]
- Funaro M, Nemani VK, Chen Z, Bhujwalla ZM, Griswold K, Gimi B. Immunoprotected, microencapsulated bacterial cytosine deaminase mediated conversion of 5-fluorocytosine to 5-fluorouracil. Cancer Res; Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31–Apr 4; Chicago, IL Philadelphia (PA). AACR; 2012. p. Abstract # 5632. [Google Scholar]
- Giustini AJ, Petryk AA, Cassim SM, Tate JA, Baker I, Hoopes PJ. Magnetic nanoparticle hyperthermia in cancer treatment. Nano Life. 2010;1:17–32. [Europe PMC free article] [Abstract] [Google Scholar]
- Hamstra DA, Rice DJ, Fahmy S, Ross BD, Rehemtulla A. Enzyme/prodrug therapy for head and neck cancer using a catalytically superior cytosine deaminase. Hum Gene Ther. 1999;10:1993–2003. [Abstract] [Google Scholar]
- Hay MP, Sykes BM, Denny WA, Wilson WR. A 2-nitroimidazole carbamate prodrug of 5-amimo-1-(chloromethyl)-3-[(5,6,7-trimethoxyindol-2-yl)carbony l]-1,2-dihydro-3h--benz[e]indole (amino-seco-cbi-tmi) for use with ADEPT and GDEPT. Bioorg Med Chem Lett. 1999;9:2237–2242. [Abstract] [Google Scholar]
- Hoffmann F, Rinas U. Stress induced by recombinant protein production in Escherichia coli. Adv Biochem Eng Biotechnol. 2004;89:73–92. [Abstract] [Google Scholar]
- Huang SY, Zhang DS, Han JQ, Zhang N, Zhang SZ, Mu WL, Wei FC. Radiosensitization and anti-tumour effects of cytosine deaminase and thymidine kinase fusion suicide gene in human adenoid cystic carcinoma cells. J Int Med Res. 2009;37:479–490. [Abstract] [Google Scholar]
- Ichikawa T, Tamiya T, Adachi Y, Ono Y, Matsumoto K, Furuta T, Yoshida Y, Hamada H, Ohmoto T. In vivo efficacy and toxicity of 5-fluorocytosine/cytosine deaminase gene therapy for malignant gliomas mediated by adenovirus. Cancer Gene Ther. 2000;7:74–82. [Abstract] [Google Scholar]
- Issels RD. Hyperthermia adds to chemotherapy. Eur J Cancer. 2008;44:2546–2554. [Abstract] [Google Scholar]
- Jana S, Deb JK. Strategies for efficient production of heterologous proteins in Escherichia coli. Appl Microbiol Biotechnol. 2005;67:289–298. [Abstract] [Google Scholar]
- Kaliberov SA, Market JM, Gillespie GY, Krendelchtchikova V, Della Manna D, Sellers JC, Kaliberova LN, Black ME, Buchsbaum DJ. Mutation of Escherichia coli cytosine deaminase significantly enhances molecular chemotherapy of human glioma. Gene Ther. 2007;14:1111–1119. [Abstract] [Google Scholar]
- Kobayashi T. Cancer hyperthermia using magnetic nanoparticles. Biotechnol J. 2011;6:1342–1347. [Abstract] [Google Scholar]
- Lohr M, Hummel F, Faulmann G, Ringel J, Saller R, Hain J, Gunzburg WH, Salmons B. Microencapsulated, CYP2B1-transfected cells activating ifosfamide at the site of the tumor: The magic bullets of the 21st century. Cancer Chemother Pharmacol. 2002;49(Suppl 1):S21–24. [Abstract] [Google Scholar]
- Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. [Abstract] [Google Scholar]
- Makrides SC. Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev. 1996;60:512–538. [Europe PMC free article] [Abstract] [Google Scholar]
- Mieschendahl M, Muller-Hill B. F′-coded, temperature-sensitive lambda cI857 repressor gene for easy construction and regulation of lambda promoter-dependent expression systems. J Bacteriol. 1985;164:1366–1369. [Europe PMC free article] [Abstract] [Google Scholar]
- Miller CR, Williams CR, Buchsbaum DJ, Gillespie GY. Intratumoral 5-fluorouracil produced by cytosine deaminase/5-fluorocytosine gene therapy is effective for experimental human glioblastomas. Cancer Res. 2002;62:773–780. [Abstract] [Google Scholar]
- Nemani VK, Ennis RE, Griswold KE, Gimi B. Heat-induced expression of cytosine deaminase for enzyme-prodrug therapy. Cancer Res; Proceedings of the 104th Annual Meeting of the American Association for Cancer Research; 2013 Apr 6–10; Washington, DC Philadelphia (PA). AACR; 2013. p. Abstract # 3300. [Google Scholar]
- Ortner V, Kaspar C, Halter C, Tollner L, Mykhaylyk O, Walzer J, Gunzburg WH, Dangerfield JA, Hohenadl C, Czerny T. Magnetic field-controlled gene expression in encapsulated cells. J Control Release. 2012;158:424–432. [Europe PMC free article] [Abstract] [Google Scholar]
- Palomares LA, Estrada-Mondaca S, Ramirez OT. Production of recombinant proteins: Challenges and solutions. Methods Mol Biol. 2004;267:15–52. [Abstract] [Google Scholar]
- Prakash S, Jones ML. Artificial cell therapy: New strategies for the therapeutic delivery of live bacteria. J Biomed Biotechnol. 2005;2005:44–56. [Europe PMC free article] [Abstract] [Google Scholar]
- Robson T, Worthington J, McKeown SR, Hirst DG. Radiogenic therapy: Novel approaches for enhancing tumor radiosensitivity. Technol Cancer Res Treat. 2005;4:343–361. [Abstract] [Google Scholar]
- Rooseboom M, Commandeur JN, Vermeulen NP. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol Rev. 2004;56:53–102. [Abstract] [Google Scholar]
- Salmons B, Lohr M, Gunzburg WH. Treatment of inoperable pancreatic carcinoma using a cell-based local chemotherapy: Results of a phase I/II clinical trial. J Gastroenterol. 2003;38(Suppl 15):78–84. [Abstract] [Google Scholar]
- Sangro B, Mazzolini G, Ruiz M, Ruiz J, Quiroga J, Herrero I, Qian C, Benito A, Larrache J, Olague C, Boan J, Penuelas I, Sadaba B, Prieto J. A phase I clinical trial of thymidine kinase-based gene therapy in advanced hepatocellular carcinoma. Cancer Gene Ther. 2010;17:837–843. [Abstract] [Google Scholar]
- Sanoff HK, Carpenter WR, Freburger J, Li L, Chen K, Zullig LL, Goldberg RM, Schymura MJ, Schrag D. Comparison of adverse events during 5-fluorouracil versus 5-fluorouracil/oxaliplatin adjuvant chemotherapy for stage III colon cancer: A population-based analysis. Cancer. 2012;118:4309–4320. [Europe PMC free article] [Abstract] [Google Scholar]
- Sun X, Xing L, Deng X, Hsiao HT, Manami A, Koutcher JA, Clifton Ling C, Li GC. Hypoxia targeted bifunctional suicide gene expression enhances radiotherapy in vitro and in vivo. Radiother Oncol. 2012;105:57–63. [Europe PMC free article] [Abstract] [Google Scholar]
- Tietze LF, Schmuck K. Prodrugs for targeted tumor therapies: Recent developments in adept, gdept and pmt. Curr Pharm Des. 2011;17:3527–3547. [Abstract] [Google Scholar]
- Tietze R, Lyer S, Durr S, Alexiou C. Nanoparticles for cancer therapy using magnetic forces. Nanomedicine (Lond) 2012;7:447–457. [Abstract] [Google Scholar]
- Vajda A, Marignol L, Foley R, Lynch TH, Lawler M, Hollywood D. Clinical potential of gene-directed enzyme prodrug therapy to improve radiation therapy in prostate cancer patients. Cancer Treat Rev. 2011;37:643–654. [Abstract] [Google Scholar]
- Valdez-Cruz NA, Caspeta L, Perez NO, Ramirez OT, Trujillo-Roldan MA. Production of recombinant proteins in e. Coli by the heat inducible expression system based on the phage lambda pL and/or pR promoters. Microb Cell Fact. 2010;9:18. [Europe PMC free article] [Abstract] [Google Scholar]
- van der Zee J. Heating the patient: A promising approach? Ann Oncol. 2002;13:1173–1184. [Abstract] [Google Scholar]
- Villaverde A, Benito A, Viaplana E, Cubarsi R. Fine regulation of cI857-controlled gene expression in continuous culture of recombinant Escherichia coli by temperature. Appl Environ Microbiol. 1993;59:3485–3487. [Europe PMC free article] [Abstract] [Google Scholar]
- Xu G, McLeod HL. Strategies for enzyme/prodrug cancer therapy. Clin Cancer Res. 2001;7:3314–3324. [Abstract] [Google Scholar]
- Zhang J, Kowal P, Chen X, Wang PG. Large-scale synthesis of globotriose derivatives through recombinant E. coli. Org Biomol Chem. 2003;1:3048–3053. [Abstract] [Google Scholar]
- Zimmermann H, Shirley SG, Zimmermann U. Alginate-based encapsulation of cells: Past, present, and future. Curr Diab Rep. 2007;7:314–320. [Abstract] [Google Scholar]
Full text links
Read article at publisher's site: https://doi.org/10.1016/j.jbiotec.2015.03.008
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4417421?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Article citations
Novel Directed Enzyme Prodrug Therapy for Cancer Treatment Based on 2'-Deoxyribosyltransferase-Conjugated Magnetic Nanoparticles.
Biomolecules, 14(8):894, 24 Jul 2024
Cited by: 0 articles | PMID: 39199282 | PMCID: PMC11352528
Remote Activation of Enzyme Nanohybrids for Cancer Prodrug Therapy Controlled by Magnetic Heating.
ACS Nano, 17(13):12358-12373, 26 Jun 2023
Cited by: 7 articles | PMID: 37358244 | PMCID: PMC10339790
An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery.
J Nanobiotechnology, 19(1):399, 29 Nov 2021
Cited by: 22 articles | PMID: 34844632 | PMCID: PMC8630862
Review Free full text in Europe PMC
Innovative Approaches of Engineering Tumor-Targeting Bacteria with Different Therapeutic Payloads to Fight Cancer: A Smart Strategy of Disease Management.
Int J Nanomedicine, 16:8159-8184, 16 Dec 2021
Cited by: 9 articles | PMID: 34938075 | PMCID: PMC8687692
Review Free full text in Europe PMC
Molecular Basis of NDT-Mediated Activation of Nucleoside-Based Prodrugs and Application in Suicide Gene Therapy.
Biomolecules, 11(1):120, 18 Jan 2021
Cited by: 6 articles | PMID: 33477716 | PMCID: PMC7831932
Go to all (9) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Effect of alginate microencapsulation on the catalytic efficiency and in vitro enzyme-prodrug therapeutic efficacy of cytosine deaminase and of recombinant E. coli expressing cytosine deaminase.
J Microencapsul, 33(1):64-70, 07 Dec 2015
Cited by: 2 articles | PMID: 26642874
Human amniotic fluid-derived stem cells expressing cytosine deaminase and thymidine kinase inhibits the growth of breast cancer cells in cellular and xenograft mouse models.
Cancer Gene Ther, 19(6):412-419, 13 Apr 2012
Cited by: 29 articles | PMID: 22498724
Stabilizing Alginate Confinement and Polymer Coating of CO-Releasing Molecules Supported on Iron Oxide Nanoparticles To Trigger the CO Release by Magnetic Heating.
Inorg Chem, 54(23):11236-11246, 23 Nov 2015
Cited by: 9 articles | PMID: 26595858
Escherichia coli cytosine deaminase: Structural and biotechnological aspects.
Biotechnol Appl Biochem, 71(1):5-16, 24 Sep 2023
Cited by: 0 articles | PMID: 37743549
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: U54 CA151662
Grant ID: P30 CA023108
the Dartmouth Center for Cancer Nanotechnology Excellence (1)
Grant ID: CA151662