Abstract
Free full text

Prevalence of Resistant Gram-Negative Bacilli in Bloodstream Infection in Febrile Neutropenia Patients Undergoing Hematopoietic Stem Cell Transplantation
Abstract
Bloodstream infection (BSI) is an important cause of morbidity and mortality in patients undergoing hematopoietic stem cell transplantation (HSCT). To evaluate the causative bacteria and identify risk factors for BSI associated mortality in febrile neutropenia patients undergoing HSCT, we collected the clinical and microbiological data from patients underwent HSCT between 2008 and 2014 and performed a retrospective analysis. Throughout the study period, among 348 episodes of neutropenic fever in patients underwent HSCT, 89 episodes in 85 patients had microbiological defined BSI with a total of 108 isolates. Gram-negative bacteria (GNB) were the most common isolates (76, 70.3%) followed by gram-positive bacteria (GPB, 29, 26.9%) and fungus (3, 2.8%). As to the drug resistance, 26 multiple drug resistance (MDR) isolates were identified. Resistant isolates (n =
= 23) were more common documented in GNB, mostly Escherichia coli (9/36, 25%) and Klebsiella pneumonia (6/24, 25%). A total of 12 isolated were resistant to carbapenem including 4 K pneumoniae (4/24, 16.7%), 3 Stenotrophomonas maltophilia, and 1 Pseudomonas aeruginosa and other 4 GNB isolates (Citrobacter freumdii, Pseudomonas stutzeri, Acinetobacter baumanii, and Chryseobacterium indologenes). As to the GPB, only 3 resistant isolates were documented including 2 methicillin-resistant isolates (Staphylococcus hominis and Arcanobacterium hemolysis) and 1 vancomycin-resistant Enterococcus faecium. Among these 85 patients with documented BSI, 11 patients died of BSI as primary or associated cause with a BSI-related mortality of 13.1
23) were more common documented in GNB, mostly Escherichia coli (9/36, 25%) and Klebsiella pneumonia (6/24, 25%). A total of 12 isolated were resistant to carbapenem including 4 K pneumoniae (4/24, 16.7%), 3 Stenotrophomonas maltophilia, and 1 Pseudomonas aeruginosa and other 4 GNB isolates (Citrobacter freumdii, Pseudomonas stutzeri, Acinetobacter baumanii, and Chryseobacterium indologenes). As to the GPB, only 3 resistant isolates were documented including 2 methicillin-resistant isolates (Staphylococcus hominis and Arcanobacterium hemolysis) and 1 vancomycin-resistant Enterococcus faecium. Among these 85 patients with documented BSI, 11 patients died of BSI as primary or associated cause with a BSI-related mortality of 13.1 ±
± 3.7% and 90-day overall survival after transplantation at 80.0
3.7% and 90-day overall survival after transplantation at 80.0 ±
± 4.3%. Patients with high-risk disease undergoing allo-HSCT, prolonged neutropenia (≥15 days) and infection with carbapenem-resistant GNB were associated with BSI associated mortality in univariate and multivariate analyses. Our report revealed a prevalence of GNB in BSI of neutropenic patients undergoing HSCT. Patients with high-risk diseases with prolonged neutropenia and carbapenem-resistant GNB were independent risk factors for BSI-related mortality.
4.3%. Patients with high-risk disease undergoing allo-HSCT, prolonged neutropenia (≥15 days) and infection with carbapenem-resistant GNB were associated with BSI associated mortality in univariate and multivariate analyses. Our report revealed a prevalence of GNB in BSI of neutropenic patients undergoing HSCT. Patients with high-risk diseases with prolonged neutropenia and carbapenem-resistant GNB were independent risk factors for BSI-related mortality.
INTRODUCTION
Neutropenic infection is a major adverse effect of cancer treatment particularly in patients receiving intensive chemotherapy or undergoing hematopoietic stem cell transplantation (HSCT).1,2 Microbiologically documented bloodstream infection (BSI) account for 10% to 30% of the febrile neutropenia (FN) episodes and remains an important cause of morbidity and mortality in HSCT recipients.3–5 The epidemiology data provided the basis for selection of empiric antibiotic therapy for FN. There has been a shift from the predominance of gram-negative bacteria (GNB) to predominance of gram-positive bacteria (GPB) in many centers over the last 2 decades.1,2 At most cancer centers, BSI infections are mainly caused by GPB (about 60%), GNB (about 25%), or fungi (about 10%).3–5
Recently, multiple drug resistance (MDR) pathogens became the most concern worldwide. Extended spectrum beta-lactamase (ESBL) producing Escherichia coli (E coli) is an increasing issue in the treatment of patients with hematological malignancies. Other MDR bacteria such as Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp. pathogens were also noticed.6 Although the prevalence and pattern of resistance varies among centers; the emergence of resistant bacteria became an even more important problem in China. In the latest released data from national surveillance CHINET study year 2013 based on a total of 84,572 clinical isolates, the average prevalence of methicillin-resistant strains in S aureus (MRSA) and coagulase-negative Staphylococcus was 45.2% and 73.5%, respectively, while the prevalence of ESBLs producing strains was 54.0% in E coli, 31.8% in Klebsiella spp. (K pneumoniae and K oxytoca) and 16.0% in Proteus mirabilis on average.7
Since neutropenic BSI and prevalence of drug resistance remained as important clinical issues for patients undergoing HSCT; therefore, we performed a retrospective study to evaluate the local epidemiology of causative bacteria, the prevalence of drug resistance in patients with BSI and the impact of BSI on the treatment outcome in the Blood and Marrow Transplantation Center, Rui Jin Hospital to further optimize the strategies of infection control and antibiotic prophylaxis and treatment in transplantation recipients.
PATIENTS AND METHODS
Study Design and Data Collection
We conducted a 7-year retrospective study of patients undergoing HSCT at the transplant unit of Department of Hematology at Rui Jin Hospital between January 1, 2008 and December 30, 2014. The aims of this study were: to describe epidemiology and outcome of BSI in neutropenic patients underwent HSCT; to understand the risk factors of BSI associated mortality. All patients undergoing HSCT with neutropenic (neutrophil count <
< 0.5
0.5 ×
× 109/L) fever with documented BSI were included for analyzed. All microbiology data were provided by the Department of Microbiology and clinical data were gathered from the patient's clinical records and prospectively kept transplantation database as appropriate. This study was a retrospective evaluation of epidemiology data and therefore did not require ethical approval based on advice of ethics committee, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine.
109/L) fever with documented BSI were included for analyzed. All microbiology data were provided by the Department of Microbiology and clinical data were gathered from the patient's clinical records and prospectively kept transplantation database as appropriate. This study was a retrospective evaluation of epidemiology data and therefore did not require ethical approval based on advice of ethics committee, Rui Jin Hospital, Shanghai Jiao Tong University School of Medicine.
Clinical Management
All patients undergoing transplantation were cared in rooms with laminar airflow until discharge. Before admission to blood and marrow transplantation unit, decontamination using chlorhexidine solution was performed. Routine surveillance cultures and active screening (stool, urine, nose, throat, vagina or penis, and anus) were obtained weekly. In case of colonization of multiple-drug resistance bacteria documented, further contact barrier precautions, environmental cleaning, and surface decontamination were carried out. All patients had an indwelling central venous catheter and received oral levofloxacin and fluconazole prophylaxis until engraftment and until day 100, respectively. Both types of prophylaxis were discontinued in case of need for antibacterial or antifungal therapy.
At the onset of fever (≥38.1°C) or in the presence of any clinical symptom compatible with an infection, 2 sets of blood cultures were drawn from peripheral vein and central catheter. An empirical antibiotic therapy was started, usually with imipenem or cefoperazone sulbactam alone. If patients developed severe mucositis or septic shock, vancomycin was added as combination therapy. Otherwise, vancomycin was added until 48 hr in case of persistent fever. For those patients who remained febrile for 5 days, an empirical antifungal therapy was also started after a chest computed tomography scan and blood test for (1–3)β-d-glucan (G) and/or galactomannan test. Additional blood cultures were then repeated at least every 2 to 3 days or clinically indicated throughout the febrile/infectious episode.
hr in case of persistent fever. For those patients who remained febrile for 5 days, an empirical antifungal therapy was also started after a chest computed tomography scan and blood test for (1–3)β-d-glucan (G) and/or galactomannan test. Additional blood cultures were then repeated at least every 2 to 3 days or clinically indicated throughout the febrile/infectious episode.
Definitions
According to the definitions of the Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation (EBMT), BSI was defined by the isolation of bacteria or fungi from any blood culture in the context of fever or other clinical signs consistent with infection.8,9 For coagulase-negative Staphylococci, at least 2 blood cultures were required to be positive. All episodes of BSI were then sub-classified into 4 categories: gram-positive, gram-negative, fungal, and polymicrobial. Polymicrobial BSI was defined as 2 or more pathogens were isolated in a single blood culture or in at least 2 separate blood cultures obtained 96 hr apart.8 For patients who had more than 1 BSI during the study period, the first episode was defined as the primary one, while subsequent episodes were numbered sequentially (2nd, 3rd, etc.). Fever was defined as temperature ≥38.1°C. Neutropenia and severe neutropenia were defined as an absolute granulocyte count <0.5 or <0.1
hr apart.8 For patients who had more than 1 BSI during the study period, the first episode was defined as the primary one, while subsequent episodes were numbered sequentially (2nd, 3rd, etc.). Fever was defined as temperature ≥38.1°C. Neutropenia and severe neutropenia were defined as an absolute granulocyte count <0.5 or <0.1 ×
× 109/L, respectively.
109/L, respectively.
BSI-related death was defined as following: BSI was considered as the primary cause of death if the patient died within 1 week after the last positive blood culture and no other cause (including the underlying disease, persistent neutropenia, other infections and hemorrhage) was identified. BSI was considered an associated cause of death when another cause was also present (uncontrolled underlying disease, persistent neutropenia, and/or graft versus host disease [GvHD]). Death not related or associated with BSI was defined as death considered due to other causes when BSI was cleared at time of death (as indicated by the lack of infection-related symptoms or positive cultures).8
Pathogen, Identification, and Antimicrobial Susceptibility Testing
Blood cultures were performed using the BACTEC 9240 (Becton Dickinson, Franklin Lakes, NJ) automated system. Collected isolates were checked by Vitek-2 system and/or phenotypic tests as previously described, and the isolates were recovered to conduct antimicrobial susceptibility testing with the disk diffusion method according to the guidelines of Clinical and Laboratory Standards Institute (CLSI).10,11 As to the drug resistance, the MRSA, vancomycin-resistant Enterococcus spp. (VRE), P aeruginosa, A baumannii, and Stenotrophomonas maltophilia resistant to at least 3 different groups of antibiotics including antipseudomonal penicillins, cephalosporins, carbapenems, aminoglycosides, and fluoro-quinolones were considered as MDR bacteria.12,13
Statistical Analysis
The SPSS 19 (Chicago, IL) package program was used for statistical analysis. The distribution of time-to-event endpoints such as overall survivor 90 days after transplantation was estimated using the Kaplan–Meier method. Probability of overall BSI-related mortality was calculated within 90 days after documentation of BSI using reciprocal cumulative incidence estimates. Log-rank test was used for univariate analysis to identify the potential risk factors associated with BSI-related mortality. Multivariate logistic regression analysis was performed to determine risk factors independently associated with BSI-related mortality. P values were reported as 2-sided and <0.05 were statistical significance.
RESULTS
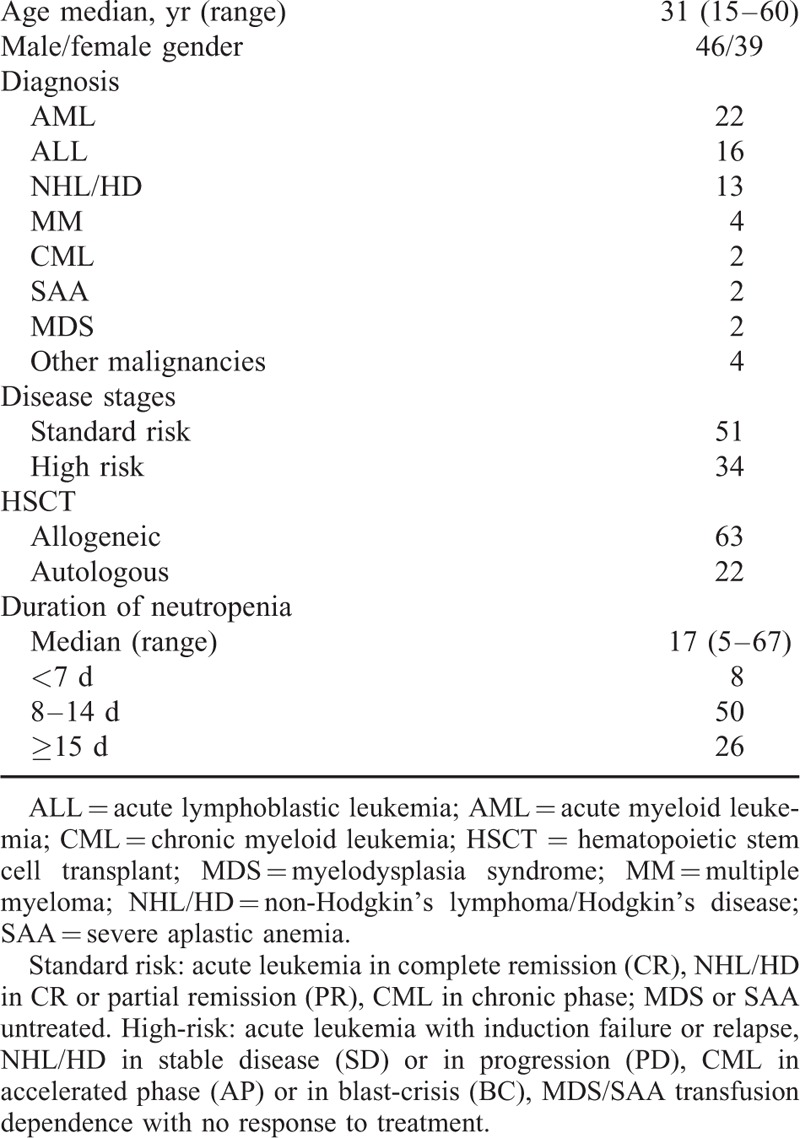
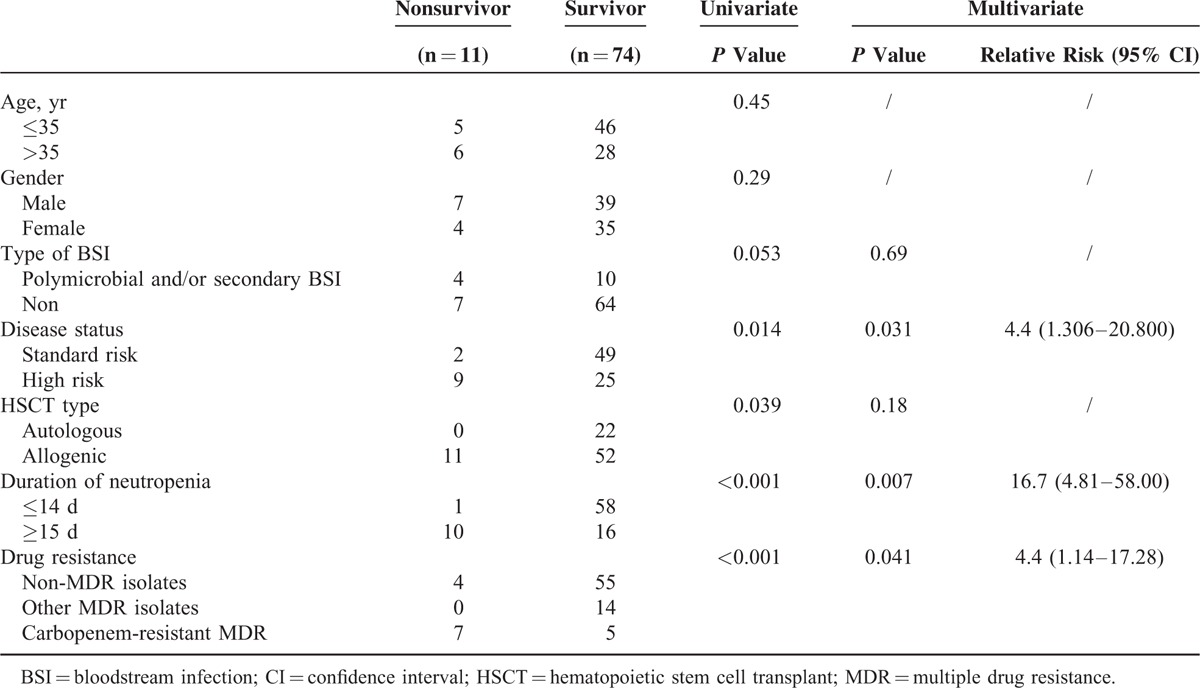
Throughout the study period, a total of 348 episodes of neutropenic fever in 273 patients were documented. Out of 348 episodes, only 89 (22.5%) episodes in 85 patients were found to have documented BSI (85 primary BSI and 4 secondary BSI). The basic characteristics were shown in Table Table1.1. A total of 51 patients with standard-risk disease received transplantation which was defined as acute leukemia in complete remission (CR), lymphoma in CR or partial remission (PR), chronic myeloid leukemia in chronic phase and untreated myelodysplasia syndrome (MDS) or severe aplastic anemia (SAA). A total of 34 patients had high-risk disease (acute leukemia with induction failure or relapse; lymphoma with stable disease or progression disease; chronic myeloid leukemia in accelerated phase or in blast-crisis and MDS/SAA remained transfusion dependence after previous treatment).
TABLE 1
Patients’ Characteristics
A total of 83 patients had documented BSI at median of 5 days (3–9) after transplantation. Among them 2 patients with primary engraft failure developed primary and secondary BSI during the prolonged neutropenia phase after transplantation. Other 2 patients with refractory disease undergoing allo-HSCT developed BSI during the conditioning phase with E coli and K pneumoniae, respectively, and then developed a secondary BSI on days 3 and 5 after allo-HSCT, respectively.
According to the definition of BSI, gram-positive and gram-negative BSI were 22 (25.9%) and 50 (58.8%), respectively. Only 1 fungal BSI (1/85, 1.1%) and 12 polymicrobial BSI were documented (14.1%). In these patients, a total of 108 isolates were recovered in which GNB were more frequently isolated (n =
= 76, 70.3%) than GPB (n
76, 70.3%) than GPB (n =
= 29, 26.9%), while only 3 isolates of Candidas (2.8%) were identified (Tables (Tables22 and and3).3). In the GNB, E coli was the most common bloodstream isolates (n
29, 26.9%), while only 3 isolates of Candidas (2.8%) were identified (Tables (Tables22 and and3).3). In the GNB, E coli was the most common bloodstream isolates (n =
= 36, 47.4%) followed by K pneumoniae (n
36, 47.4%) followed by K pneumoniae (n =
= 24, 31.6%) and then S maltophilia (n
24, 31.6%) and then S maltophilia (n =
= 3, 3.5%) and P aeruginosa (n
3, 3.5%) and P aeruginosa (n =
= 3, 3.5%), respectively. Acinetobacter baumannii and other GNB were much less as shown in Table Table2.2. For GPB, coagulase-negative Staphylococcus spp. (n
3, 3.5%), respectively. Acinetobacter baumannii and other GNB were much less as shown in Table Table2.2. For GPB, coagulase-negative Staphylococcus spp. (n =
= 20, 68.9%) were most commonly documented followed by Enterococcus (n
20, 68.9%) were most commonly documented followed by Enterococcus (n =
= 3, 10.3%) and S aureus (n
3, 10.3%) and S aureus (n =
= 2, 6.9%). For fungal isolates, 2 Candida krusei and 1 Candida tropicalis were documented. Of note, throughout the study period from year 2008 to 2014, GNB isolates were constantly predominant than GPB and fungal isolates (P
2, 6.9%). For fungal isolates, 2 Candida krusei and 1 Candida tropicalis were documented. Of note, throughout the study period from year 2008 to 2014, GNB isolates were constantly predominant than GPB and fungal isolates (P <
< 0.001) as shown in Table Table22.
0.001) as shown in Table Table22.
TABLE 2
Annual Distribute of Isolates From BSI Patients
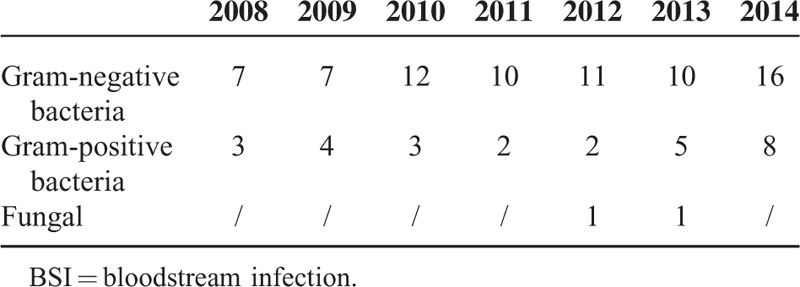
TABLE 3
Isolated Gram-Negative Pathogens and Drug Resistance Between 2008 and 2014
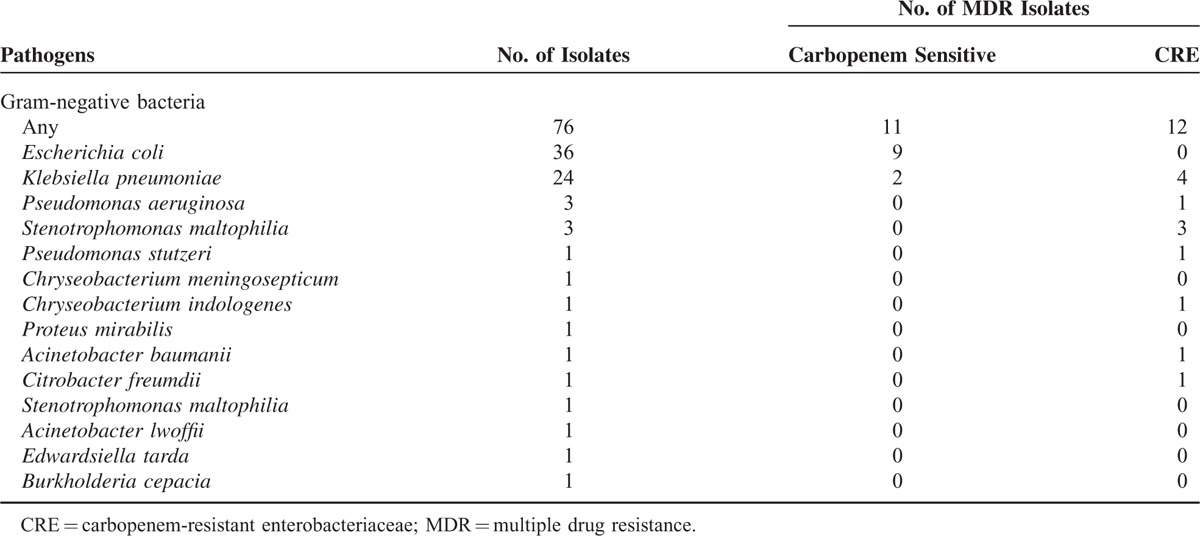
As to the drug resistance, a total of 25 MDR isolates were identified and predominantly documented in GNB such as E coli (9/36, 25.0%) and K pneumoniae (6/24, 25.0%) and S maltophilia (3 isolates) which is naturally resistant to broad-spectrum antibiotics including carbapenems as shown in Table Table3.3. Even when the S maltophilia were not included, the overall incidence of MDR isolates was as high as 30.1% (22/73). More importantly, besides S maltophilia isolates, a total of 9 isolated were resistant to carbapenem with an incidence of carbapenem-resistant enterobacteriaceae (CRE) as high as 12.3% (9/73) mostly documented in K pneumoniae (4/24, 16.7%) and other Enterobacteriaceae such as P aeruginosa (1/3, 33.3%), P stutzeri (n =
= 1), A baumanii (n
1), A baumanii (n =
= 1), C freumdii (n
1), C freumdii (n =
= 1), and Chryseobacterium indologenes (n
1), and Chryseobacterium indologenes (n =
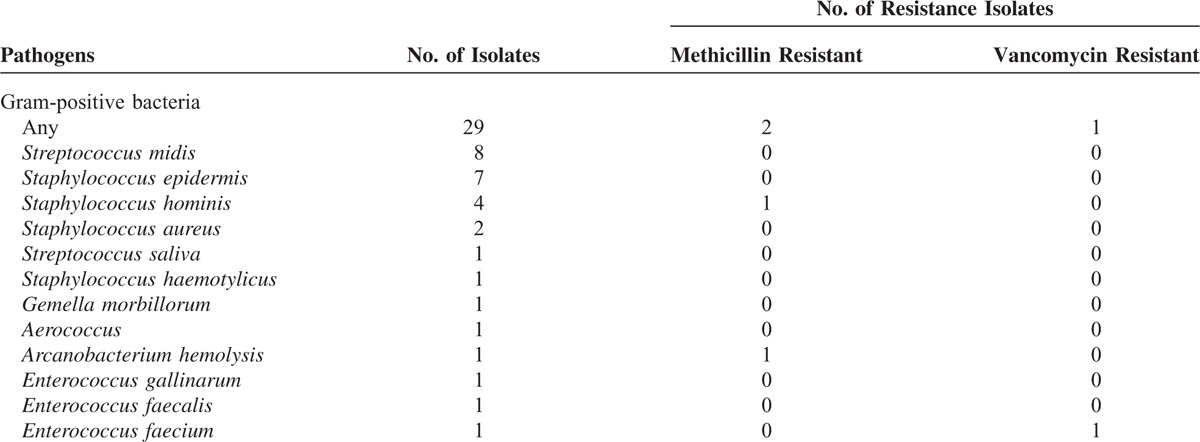
= 1) as shown in Table Table3.3. As contrary, few MDR isolates were identified in GPB isolates with only 1 vancomycin-resistant E faecium (3.4%) as shown in Table Table44.
1) as shown in Table Table3.3. As contrary, few MDR isolates were identified in GPB isolates with only 1 vancomycin-resistant E faecium (3.4%) as shown in Table Table44.
TABLE 4
Isolated Gram-Positive Pathogens and Drug Resistance Between 2008 and 2014
All alive patients were followed-up for at least 90 days after transplantation, a total of 17 patients died with a 90-day overall survival after transplantation at 80.0 ±
± 4.3% as shown in Figure Figure1.1. A total of 11 patients died with BSI considered as either primary (n
4.3% as shown in Figure Figure1.1. A total of 11 patients died with BSI considered as either primary (n =
= 4) or associated (n
4) or associated (n =
= 7) cause of death leading to a BSI-related mortality rate of 13.1
7) cause of death leading to a BSI-related mortality rate of 13.1 ±
± 3.7% (Fig. (Fig.1).1). Among them, 3 patients died within 7 days, 6 patients within 14 days, and 2 patients beyond 15 days after the documentation of BSI. The isolates identified in these patients were GNB in 8 patients including E coli (n
3.7% (Fig. (Fig.1).1). Among them, 3 patients died within 7 days, 6 patients within 14 days, and 2 patients beyond 15 days after the documentation of BSI. The isolates identified in these patients were GNB in 8 patients including E coli (n =
= 3, non-MDR isolates), K pneumoniae (n
3, non-MDR isolates), K pneumoniae (n =
= 2, both MDR/CRE), P aeruginosa (n
2, both MDR/CRE), P aeruginosa (n =
= 1, MDR/CRE), A baumanii (n
1, MDR/CRE), A baumanii (n =
= 1, MDR/CRE), and C freumdii (n
1, MDR/CRE), and C freumdii (n =
= 1, MDR/CRE). Other 3 patients died of polymicrobial BSI and all had at least 1 documented GNB: E coli (n
1, MDR/CRE). Other 3 patients died of polymicrobial BSI and all had at least 1 documented GNB: E coli (n =
= 1), P stutzeri (n
1), P stutzeri (n =
= 1, MDR/CRE), and S maltophilia (n
1, MDR/CRE), and S maltophilia (n =
= 1, MDR/CRE). A total of 6 patients died due to GvHD (n
1, MDR/CRE). A total of 6 patients died due to GvHD (n =
= 2), VOD (n
2), VOD (n =
= 1), cranial hemorrhage (n
1), cranial hemorrhage (n =
= 2), and relapse disease, respectively.
2), and relapse disease, respectively.

(A) The BSI-related mortality after documentation of BSI. (B) The 90-d overall survival of 85 patients who had BSI during the neutropenia. BSI = bloodstream infection.
For risk factors associated with BSI-related mortality, patients with high-risk disease (P =
= 0.014), undergoing allogeneic HSCT (P
0.014), undergoing allogeneic HSCT (P =
= 0.04), BSI with carbapenem-resistant GNB (P
0.04), BSI with carbapenem-resistant GNB (P <
< 0.001), and prolonged neutropenia (≥15 days, P
0.001), and prolonged neutropenia (≥15 days, P <
< 0.001) were associated with BSI-related mortality in univariate analysis while high-risk disease (P
0.001) were associated with BSI-related mortality in univariate analysis while high-risk disease (P =
= 0.031, RR 4.4), BSI with carbapenem-resistant GNB (P
0.031, RR 4.4), BSI with carbapenem-resistant GNB (P =
= 0.04, RR 4.4) and prolonged neutropenia (≥15 days, P
0.04, RR 4.4) and prolonged neutropenia (≥15 days, P =
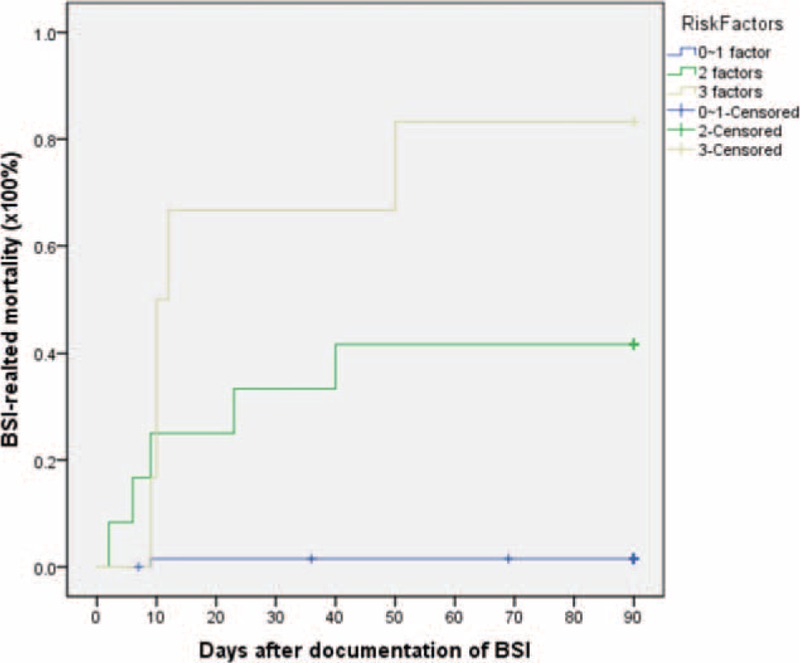
= 0.007, RR 16.7) remained significant with multivariate analysis (as shown in Table Table55 and Figure Figure2).2). When combined these 3 risk factors, it was possible to divide patients into 3 different risk groups for BSI-related mortality: low-risk with 0 to 1 risk factor, intermediate-risk with 2 risk factors, and high-risk with 3 risk factors. Patients in the low-risk group had a BSI-related mortality at 4.5
0.007, RR 16.7) remained significant with multivariate analysis (as shown in Table Table55 and Figure Figure2).2). When combined these 3 risk factors, it was possible to divide patients into 3 different risk groups for BSI-related mortality: low-risk with 0 to 1 risk factor, intermediate-risk with 2 risk factors, and high-risk with 3 risk factors. Patients in the low-risk group had a BSI-related mortality at 4.5 ±
± 1.5%, while intermediate- and high-risk patients had significantly increased BSI-related mortality to 41.7
1.5%, while intermediate- and high-risk patients had significantly increased BSI-related mortality to 41.7 ±
± 14.2% and 83.3
14.2% and 83.3 ±
± 15.2%, respectively, as shown in Fig. Fig.33.
15.2%, respectively, as shown in Fig. Fig.33.
TABLE 5
Analysis of Risk Factors for BSI Mortality

(A) Comparison of BSI-related mortality in patients receiving autologous HSCT (0) and allogeneic HSCT (17.8 ±
± 4.9%, P
4.9%, P =
= 0.04). (B) Comparison of BSI-related mortality in patients undergoing HSCT with standard-risk (22.6
0.04). (B) Comparison of BSI-related mortality in patients undergoing HSCT with standard-risk (22.6 ±
± 6.6%) and high-risk disease (4.5
6.6%) and high-risk disease (4.5 ±
± 3.1%, P
3.1%, P =
= 0.014). (C) Comparison of BSI-related mortality in patients with carbapenem-resistant gram-negative bacteria infection (70.0
0.014). (C) Comparison of BSI-related mortality in patients with carbapenem-resistant gram-negative bacteria infection (70.0 ±
± 14.5%) and non-CRE infection (5.4
14.5%) and non-CRE infection (5.4 ±
± 2.6%, P
2.6%, P <
< 0.001). (D) Comparison of BSI-related mortality in patients with neutropenia <15 d CRE (1.7
0.001). (D) Comparison of BSI-related mortality in patients with neutropenia <15 d CRE (1.7 ±
± 1.7%) and prolonged neutropenia (≥15 d, 38.5
1.7%) and prolonged neutropenia (≥15 d, 38.5 ±
± 9.5%, P
9.5%, P <
< 0.001). BSI = bloodstream infection; CRE
0.001). BSI = bloodstream infection; CRE =
= carbapenem-resistant enterobacteriaceae; HSCT = hematopoietic stem cell transplant.
carbapenem-resistant enterobacteriaceae; HSCT = hematopoietic stem cell transplant.
DISCUSSION
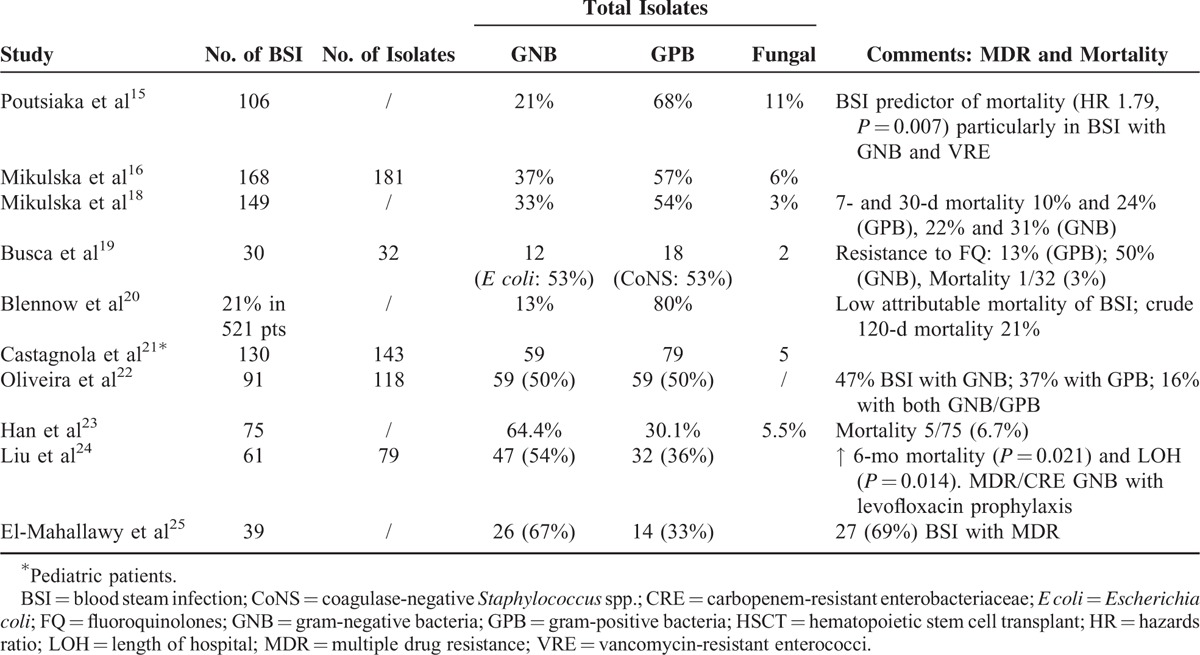
Up to 30% of neutropenic fever in cancer patients are associated with confirmed bacteremia.8,14–16 A large amount of prior clinical studies revealed that the etiological agents in neutropenic patients with BSI were commonly GPB followed by GNB and then fungi.1 In an analysis of bacteremia from 2 pooled European cohorts of 2142 patients with neutropenic fever between October 1994 and February 2005, gram-positive bacteremia had a frequency of 57% with 34% gram-negative bacteremias and 10% polymicrobial bacteremias. Mortality rates were 5% for gram-positive, 18% for gram-negative, and 13% for polymicrobial bacteremias.4 To the contrary, in a large-scale surveillance study including 7058 patients with hemato-oncology disease at National Taiwan University Hospital between 2002 and 2006, a total of 1307 nonduplicate bloodstream isolates were identified from neutropenic patients. GNB predominated (60%) with E coli (12%) followed by K pneumoniae (10%), Acinetobacter calcoaceticus–baumannii complex (6%), and S maltophilia (6%) as the most frequent causal bacteria while coagulase-negative Staphylococci and S aureus were the most common gram-positive pathogens.17
As to the BSIs in patients undergoing HSCT, there was also a significant variation of epidemiology as shown in Table Table6.6. Most European studies demonstrated a predominance of GPB (50–80%),15,16,18–21 while most studies from developing countries such as Asia-Pacific region demonstrated a predominance of GNB (54–67%).22–25 In our series, a constant dominance of gram-negative bacteremia over 7-year period was documented. These data were comparable to the surveillance study from Institute of Hematology, Peking University between January 2008 and October 2010. In their report of 75 BSI, the incidence of GNB, GPB, and fungal were 64.4%, 30.1%, and 5.5%, respectively. The mortality rate was 6.7% (5/75) while only 1 case of carbapenem-resistant GNB was identified.23
TABLE 6
Summary of BSI Study in Patients Undergoing HSCT
There are 2 possible explanations of the predominance of GNB in our study. First, though it is difficult to make confirmed conclusion due to limited data in HSCT patients, the 2 separate reports from China including ours (23) were comparable to the Chinese national surveillance studies of all clinical isolates (CHINET year 2013), which reported an overwhelming predominance of GNB (71.9%) over GPB (28.1%),7,26 thus reflects the local epidemiology in China which was significantly different from Europe and North American. Secondary, there were clinical reports from various studies show that epidemiological predominance of GNB causing severe infections in neutropenic patients was associated with antibacterial prophylaxis, while the levofloxacin was routinely used in our transplantation program as part of antibiotics prophylaxis.27–29
As to the drug resistance, in our series, the resistant isolates were mostly observed in GNB with more commonly in E coli (25%) and K pneumoniae (25%) which was compatible to the CHINET study.7 Of note, a relatively high percentage of carbapenem-resistant GNB was documented in our series. A total of 12 isolated out of 76 (15.7%) GNB isolates were MDR and carbapenem resistance. Even the natural resistant S maltophilia were excluded, the incidence of MDR and carbapenem resistant remained as high as 30.1% and 12.3%, respectively. This observation was significantly higher than the report from Peking University.23 More importantly, the BSI with carbapenem-resistant GNB was associated with a higher mortality rate (7/12) compared with all other patients (4/73) including BSI with other non-CRE MDR isolates. Since carbapenem-resistant Enterobacteriaceae were resistance to many classes of antibiotics, thus limiting the therapeutic options, those patients who underwent stem cell transplantation and were infected with carbapenem-resistant GNB were very high-risk patients and should be carefully monitored.30,31
To our surprise, our data not only revealed a lower incidence of GPB BSI, but also a relative low incidence of drug resistance (no MRSA and only 1 VRE) while the prevalence of MRSA were reported as high as 47.9% in the national surveillance CHINET study and VRE was also more commonly documented in recent years.7,26 There was no clear explanation for these findings because gram-positive MDR is also reported being associated with fluoroquinolones prophylaxis.27,29
As to the mortality of BSI, the type of pathogen, underlying disease and status played a pivotal role.18 Prior studies revealed that bacteremia due to GNB is usually associated with higher mortality, and the risk is further increased due to antimicrobial resistance.27,28 In the present analysis, all 11 patients died of BSI were associated with GNB infection. The particular high mortality of BSI with CRE (70.0 ±
± 14.5%, Fig. Fig.3)3) infection might have contributed to the significantly high BSI-related mortality associated with GNB. To the contrary, no patients died of gram-positive BSI in our series, which is compatible to the low mortality as previously reported.18 Besides, underlying disease and prolonged neutropenia also played a pivotal role in BSI-related mortality in our study. BSI mortality was high in high-risk patients usually with uncontrolled disease compared to those with standard-risk disease at HSCT. Such a difference might be due to the fact that patients with high-risk disease usually have received multiple cycles of intensive chemotherapy, resulting in more severe immunosuppression, longer hospitalization, higher exposure to antimicrobial treatment and sometimes, the presence of severe infections before HSCT or prolonged pretransplantation neutropenia as previously reported.18,32
14.5%, Fig. Fig.3)3) infection might have contributed to the significantly high BSI-related mortality associated with GNB. To the contrary, no patients died of gram-positive BSI in our series, which is compatible to the low mortality as previously reported.18 Besides, underlying disease and prolonged neutropenia also played a pivotal role in BSI-related mortality in our study. BSI mortality was high in high-risk patients usually with uncontrolled disease compared to those with standard-risk disease at HSCT. Such a difference might be due to the fact that patients with high-risk disease usually have received multiple cycles of intensive chemotherapy, resulting in more severe immunosuppression, longer hospitalization, higher exposure to antimicrobial treatment and sometimes, the presence of severe infections before HSCT or prolonged pretransplantation neutropenia as previously reported.18,32
Limitations of the study include its retrospective nature, limited number of patients with BSI, diagnosis, and treatment procedure based on single hospital protocol. Although the data reflect real-life practice in our hospital, these limitations precluded any confirmed conclusion particularly concerning the analysis of risk factors for BSI-related mortality.
Overall, our report revealed a unique epidemiology feature of BSIs in patients undergoing HSCT, which was characterized by predominance of GNB with emerging of MDR/CRE isolates. This may highlight the importance to raise awareness of the local epidemiology data and the drug resistance features to guide the infection control, optimization of empirical antibiotic therapy, and antimicrobial therapy stewardship particularly for those patients undergoing with high-risk diseases, when encountered with prolonged neutropenia.1,12,33
Acknowledgments
The authors thank Dr Ming Wan and Dr Jun-Cai Xu from Shanghai Clinical Research Center for assistance in the statistics analysis.
Footnotes
Abbreviations: ALL = acute lymphoblastic leukemia, AML = acute myeloid leukemia, BSI = bloodstream infection, CML = chronic myeloid leukemia, CoNS = coagulase-negative Staphylococcus spp., CR = complete remission, CRE = carbapenem-resistant enterobacteriaceae, E coli = Escherichia coli , EBMT = European Society for Blood and Marrow Transplantation, ESBL = extended spectrum beta-lactamase, FN = febrile neutropenia, FQ = fluoroquinolones, G = (1–3)β-d-glucanG, GM = galactomannan, GNB = gram-negative bacteria, GPB = gram-positive bacteria, GvHD = graft versus host disease, HD = Hodgkin's disease, HSCT = hematopoietic stem cell transplantation, LOH = length of hospital, MDR = multiple drug resistance = MDS, myelodysplasia syndrome = MM, multiple myeloma = MRCNS, methicillin-resistant coagulase-negative Staphylococcus = MRSA, methicillin-resistant Staphylococcus aureus = NHL, non-Hodgkin's lymphoma = OS, overall survivor = PR, partial remission = SAA, severe aplastic anemia = VOD, veno-occlusion disease = VRE, vancomycin-resistant Enterococcus spp.
LW and YW have contributed equally to the manuscript.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
Articles from Medicine are provided here courtesy of Wolters Kluwer Health
Full text links
Read article at publisher's site: https://doi.org/10.1097/md.0000000000001931
Read article for free, from open access legal sources, via Unpaywall:
https://pdfs.journals.lww.com/md-journal/2015/11110/Prevalence_of_Resistant_Gram_Negative_Bacilli_in.15.pdf?token=method|ExpireAbsolute;source|Journals;ttl|1542345750082;payload|mY8D3u1TCCsNvP5E421JYK6N6XICDamxByyYpaNzk7FKjTaa1Yz22MivkHZqjGP4kdS2v0J76WGAnHACH69s21Csk0OpQi3YbjEMdSoz2UhVybFqQxA7lKwSUlA502zQZr96TQRwhVlocEp/sJ586aVbcBFlltKNKo+tbuMfL73hiPqJliudqs17cHeLcLbV/CqjlP3IO0jGHlHQtJWcICDdAyGJMnpi6RlbEJaRheGeh5z5uvqz3FLHgPKVXJzddFRrD2hcIwdDP9eSnSkfs2mPEvU99Dm4n7+Bkg7weOE=;hash|6VI+BG760ha3PQJi6mVYdg==
Citations & impact
Impact metrics
Article citations
Systematic review on epidemiology of Escherichia coli in bloodstream infection of patients undergoing hematopoietic stem cell transplantation.
Germs, 14(1):85-94, 31 Mar 2024
Cited by: 0 articles | PMID: 39169974 | PMCID: PMC11333837
Review Free full text in Europe PMC
An Emergent Change in Epidemiologic and Microbiological Characteristics of Bloodstream Infections in Adults With Febrile Neutropenia Resulting From Chemotherapy for Acute Leukemia and Lymphoma at Reference Centers in Chile, Ecuador, and Peru.
Open Forum Infect Dis, 11(3):ofae052, 01 Feb 2024
Cited by: 0 articles | PMID: 38444817 | PMCID: PMC10913838
Global impact of antibacterial resistance in patients with hematologic malignancies and hematopoietic cell transplant recipients.
Transpl Infect Dis, 25 Suppl 1:e14169, 20 Oct 2023
Cited by: 1 article | PMID: 37864309 | PMCID: PMC10844985
Review Free full text in Europe PMC
Clinical Characteristics and Prognosis of Bloodstream Infection with Carbapenem-Resistant Pseudomonas aeruginosa in Patients with Hematologic Malignancies.
Infect Drug Resist, 16:4943-4952, 31 Jul 2023
Cited by: 0 articles | PMID: 37546370 | PMCID: PMC10402715
Bloodstream infections due to Carbapenem-Resistant Enterobacteriaceae in hematological patients: assessment of risk factors for mortality and treatment options.
Ann Clin Microbiol Antimicrob, 22(1):41, 18 May 2023
Cited by: 3 articles | PMID: 37202758 | PMCID: PMC10197250
Go to all (37) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The place of ceftazidime/avibactam and ceftolozane/tazobactam for therapy of haematological patients with febrile neutropenia.
Int J Antimicrob Agents, 57(6):106335, 07 Apr 2021
Cited by: 8 articles | PMID: 33838223
Incidence, Risk Factors and Outcome of Pre-engraftment Gram-Negative Bacteremia After Allogeneic and Autologous Hematopoietic Stem Cell Transplantation: An Italian Prospective Multicenter Survey.
Clin Infect Dis, 65(11):1884-1896, 01 Nov 2017
Cited by: 58 articles | PMID: 29020286
Characteristics of gram-negative bacteremia during febrile neutropenia among allogeneic hematopoietic stem cell transplant recipients on levofloxacin prophylaxis.
Eur J Clin Microbiol Infect Dis, 40(5):941-948, 13 Nov 2020
Cited by: 3 articles | PMID: 33185742
Global prevalence of carbapenem resistance in neutropenic patients and association with mortality and carbapenem use: systematic review and meta-analysis.
J Antimicrob Chemother, 72(3):668-677, 01 Mar 2017
Cited by: 44 articles | PMID: 27999023
Review