Abstract
Free full text

Primary ciliary dyskinesia and associated sensory ciliopathies
Abstract
Primary ciliary dyskinesia (PCD) is a genetic disease of motile cilia, which belongs to a group of disorders resulting from dysfunction of cilia, collectively known as ciliopathies. Insights into the genetics and phenotypes of PCD have grown over the last decade, in part propagated by the discovery of a number of novel cilia-related genes. These genes encode proteins that segregate into structural axonemal, regulatory, as well as cytoplasmic assembly proteins. Our understanding of primary (sensory) cilia has also expanded, and an ever-growing list of diverse conditions has been linked to defective function and signaling of the sensory cilium. Recent multicenter clinical and genetic studies have uncovered the heterogeneity of motile and sensory ciliopathies, and in some cases, the overlap between these conditions. In this review, we will describe the genetics and pathophysiology of ciliopathies in children, focusing on PCD, review emerging genotype-phenotype relationships, and diagnostic tools available for the clinician.
INTRODUCTION
Ciliopathies are a growing collection of disorders related to dysfunction of cilia, which are essential organelles that extend from the surface of most cells. Cilia are often segregated into primary (sensory) and motile (motor) cilia. The ciliary axoneme is evolutionarily conserved along the phylogenetic tree, and the structure of motile cilia is nearly identical to the flagella used for cell motility [1]. Thus Chlamydomonas reinhardtii, a biflagellated single cell organism, has been widely used to model ciliopathies, providing insights into the structure, function, and genetics of the human cilium [2,3]. Indeed, most genes implicated in motor cilia disease in humans have an algal counterpart (Table 1).
Table 1
Human genes known to be mutated in primary ciliary dyskinesia.
| Gene name | Locus | Protein location | TEM | Chlamydomonas orthologue |
|---|---|---|---|---|
| DNAH5 | Chr 5 | ODA | ODA truncation | ODA2/re11.g476050 |
| TXNDC3 | Chr 7 | ODA | ODA truncation | FAP67/Cre12.g558700 |
| DNAI1 | Chr 9 | ODA | ODA truncation | ODA9/DIC1/cre12.g536550 |
| DNAI2 | Chr 17 | ODA | ODA truncation | DIC2/Cre12.g506000 |
| DNAL1 | Chr 14 | ODA | ODA truncation | DIC1/Cre12.g536550 |
| CCDC114 | Chr 19 | ODA | ODA truncation | DCC2/Cre16.g666150 |
| ARMC4 | Chr 10 | ODA | ODA truncation | -- |
| CCDC151 | Chr 19 | ODA | ODA truncation | ODA10/Cre08.g361200 |
| CCDC103 | Chr 17 | ODA | ODA truncation | CCDC103/PR46/Cre06.g253404 |
| LRRC6 | Chr 8 | Cytoplasmic | ODA and IDA truncation | MOT48/Cre17.g739850 |
| HEATR2 | Chr 7 | Cytoplasmic | ODA and IDA truncation | HTR2/Cre09.g395500 |
| DYX1C1 | Chr 15 | Cytoplasmic | ODA and IDA truncation | Dyx1C1/Cre11.g467560 |
| DNAAF1 | Chr 16 | Cytoplasmic | ODA and IDA truncation | ODA7/DNAAF1/Cre01.g029150 |
| DNAAF3 | Chr 19 | Cytoplasmic | ODA and IDA truncation | PF22/DNAAF3/Cre01.g001657 |
| DNAAF2 | Chr 14 | Cytoplasmic | ODA and IDA truncation | PF13/DNAAF2/Cre09.g411400 |
| SPAG1 | Chr 8 | Cytoplasmic | ODA and IDA truncation | -- |
| C21orf59 | Chr 21 | Cytoplasmic | ODA and IDA truncation | FBB18/Cre16.g688450 |
| ZMYND10 | Chr 3 | Cytoplasmic | ODA and IDA truncation | ZMYND10/cre08.g358750 |
| HYDIN | Chr 16 | Central pair | Normal | Hydin/Cre01.g025400 |
| RSPH4A | Chr 6 | Radial spoke | Normal/central pair defect | RSP4/PF1/Cre05.g242500 |
| RSPH9 | Chr 6 | Radial spoke | Normal/central pair defect | RSP9/PF17/Cre07.g330200 |
| RSPH1 | Chr 21 | Radial spoke | Normal/central pair defect | RSP1/Cre03.g201900 |
| CCDC164 | Chr 2 | DRC | Normal/DRC defect | DRC1/Cre13.g607750 |
| CCDC39 | Chr 3 | DRC | Normal/DRC defect | PF8/Cre17.g701250 |
| CCDC40 | Chr 17 | DRC | Normal/DRC defect | PF7/Cre17.g698353 |
| CCNO | Chr 5 | Transcription | Reduced cilia number | -- |
| MCIDAS | Chr 5 | Transcription | Reduced cilia number | -- |
| DNAH11 | Chr 7 | ODA | Normal | ODA4/Cre09.g403800 |
| CCDC65 | Chr 12 | ODA | Normal | DRC2/Cre13.g607750 |
DRC: Dynein regulatory complex; Chr: chromosome; ODA: Outer dynein arm; IDA: Inner dynein arm Chlamydomonas genes are found at: http://phytozome.jgi.doe.gov/pz/portal.html#!info?alias=Org_Creinhardtii
Motor cilia line the surface of the upper and lower respiratory tract, and serve as the first line of airway defense against inhaled particulates and bacteria. A mature ciliated airway epithelial cell has approximately 200 uniform motor cilia that are oriented in the same direction. Their rhythmic beat is synchronous at a fairly constant frequency (8 to 12 Hz at room temperature), which results in a continuous wave critical to fluid movement along the conducting airways. Calcium signaling through gap junctions, and mechanical hydrodynamic interaction of neighboring cilia, synchronizes cilia motion along the respiratory epithelium [4].
Any disruption in the coordinated movement of motile cilia can lead to impaired mucociliary clearance and potentially disease. Cilia beat frequency can be affected by changes in the external environment, such as changes in redox conditions, mucus viscosity, bacterial toxins, and airborne pollutants (including cigarette smoke) [5–7]. Motor cilia express bitter taste receptors, identical to those in the tongue and nose [8] that allow the motile cilia to adjust their movement in response to changes in their immediate environment. These attributes suggest that motile cilia also possess sensory functions.
Motor cilia are complex structures composed of hundreds of proteins organized around a cylindrical scaffold of α- and β- tubulin, arranged as helical protofilaments in microtubular doublets (A and B tubules), that surround a central pair of microtubules and produce the characteristic “9 + 2” configuration seen on transmission electron microscopy (Figure 1). The central fibrillar structure, or axoneme, is covered by the cell membrane. The axoneme is anchored to the cytoplasm by a basal body (modified centriole), which is a specialized structure that is derived from centrosomes used by the cell during replication [9]. The cilium is separated from the cytoplasm by a functional diffusion barrier or gate [10], and recent evidence suggests that proteins localized to the cilia transition zone, a region between the basal body and ciliary axoneme, play a role in regulating the ciliary gate function [11,12]. Cilia have specialized transport mechanism that utilize intraflagellar transport (IFT), and continuously move essential proteins required for the formation and maintenance of cilia from the cytoplasm along the length of the axoneme (Figure 2).
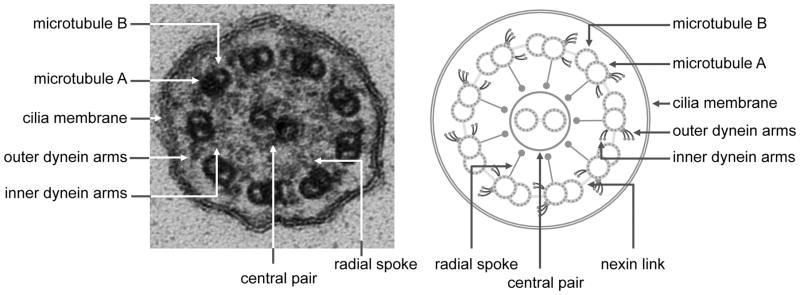
Electron photomicrograph and schematic diagram showing the ultrastructural features of the motor cilium.
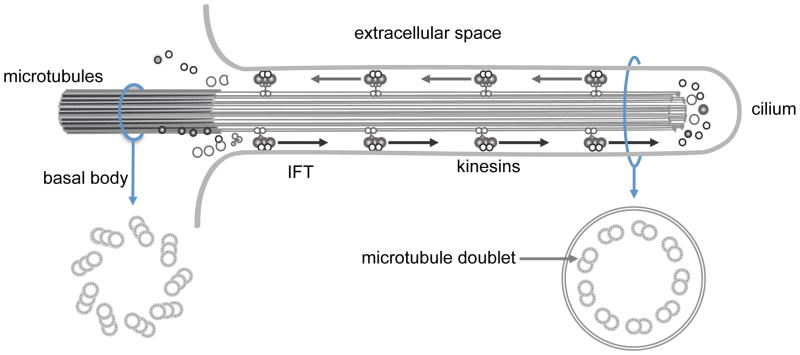
Schematic diagram showing the antegrade and retrograde transport of proteins along the length of ciliary axoneme via the intraflagellar transport proteins.
Motor cilia have a set of structural proteins collectively known as dynein proteins, which are lacking in primary (sensory) cilia. They form outer and inner dynein arms evident on electron microscopy (EM), that serve as ATPase motors to provide the cilium with the force required to bend. Dynein arms extend from the A tubule and interact with the B tubule of the neighboring outer pair. The force generated by the outer dynein arm proteins translates to a sliding motion of two neighboring tubules, while the inner dynein arms are central for controlling the rhythmic motion of cilia as part of a complex often referred to as the dynein regulatory complex (N-DRC) [13]. Other structures important for the function of the motor cilia include the nexin links and radial spokes. The nexin links are part of the DRC and extend between two adjacent microtubular doublets, thus limiting the sliding between microtubules. The radial spokes regulate dynein arm activity, sending signals from the central apparatus to the dynein arms. All these structures work in a coordinated fashion to produce a rhythmic and coordinated ciliary beat, and help maintain the alignment of the doublet microtubules [13,14]. Mutations in genes encoding for any of these structures may cause disease.
Primary (sensory) cilia are distinct from motile cilia, typically appearing as solitary structures extending from the surface of most mammalian cells during interphase. Sensory organs, including the eye retina, olfactory bulb in the nose, and cochlea in the ears are dependent on sensory cilia. In these organs, primary cilia evolved to form specialized sensory functions such as the retinal photoreceptors, cochlear stereocilia, and renal primary cilia [15,16]. Unlike motile cilia, most primary cilia are non-motile and lack key elements critical for motility, such as the central microtubules pair (“9 + 0” configuration), and dynein arms. An exception to this configuration is the kinocilia of the inner ear that retains a “9+2” configuration. Primary cilia detect changes in the cells external environment through mechanical stimulation, chemosensation, and in specialized cases, changes in light, temperature, and gravity [17–19]. Of note, while primary (sensory) cilia are present on undifferentiated airway epithelial cells, they have not been found on differentiated airway epithelium surface [20]. Primary cilia are essential for normal development and tissue differentiation, as evident by the many surface receptors present on these structures, including sonic hedgehog (SHH), epidermal growth factor receptor (EGFR), and platelet-derived growth factor receptor (PDGFR) [15,21,22]. Due to their ubiquitous nature, it is not surprising that genetic defects in primary cilia lead to syndromes and conditions that involve multiple organ systems that ostensibly appear unrelated [23,24] (Table 2).
Table 2
Clinical and respiratory manifestations associated with sensory ciliopathies.
| Primary ciliopathy | Typical clinical manifestations | Respiratory manifestations | Gene(s) |
|---|---|---|---|
| Autosomal recessive polycystic kidney disease | Cystic renal disease, hepatic fibrosis | Bronchiectasis | PKHD1 |
| Bardet-Biedl syndrome | Obesity, polydactyly, cognitive delays, retinitis pigmentosa., renal anomalies, anosmia, congenital heart disease | Motile ciliary tip vesicles (unclear clinical significance) | ARL6, BBS1-12, CEP290, MKKS, MKS1, MKS3, SDCCAG8, TRIM32, WDPCP |
| Retinitis pigmentosa | Photophobia, night blindness, progressive blindness, | Chronic sinusitis, serous otitis, recurrent bronchitis, bronchiectasis | RP1, RP2, RPGR, PRPH2, RP9, IMPDH1, PRPF31, CRB1, PRPF8, TULP1, CA4, HPRPF3, ABCA4, EYS, CERKL, FSCN2, TOPORS, SNRNP200, SEMA4A, PRCD, NR2E3, MERTK, USH2A, PROM1, KLHL7, BEST1, TTC8, C2ORF71, A RL6, ZNF513,BHDDS, PRPH2, LRAT, SPATA7, CRX |
| Usher syndrome | Congenital hearing loss, retinitis pigmentosa. | Bronchiectasis | MYO7A, GPR98, PDZD7, WHRN, HARS |
| Cranioectodermal dysplasia (Sensenbrenner syndrome) | Sagital craniosynostosis, facial anomalies, skeletal defects, hypodontia, nephronophthisis | Neonatal respiratory distress, recurrent bronchopneumonia, | WDR35, IFT43, WDR19 |
| Short-rib thoracic dysplasia (Ellis-van Creveld syndrome, Jeune syndrome, short rib-polydactyly syndrome, Mainzer-Saldino syndrome). | Small thoracic cage, short ribs, skeletal anomalies, polydactyly, cystic renal disease | atelectasis Pulmonary restriction, respiratory failure | WDR34, WDR35, DYNC2H1, NEK1, WDR60, TTC21B, WDR19, IFT140, IFT80, IFT139, IFT172, CEP120, KIAA0586 |
Nodal cilia are a third class of cilia that transiently appear in the ventral node of the gastrula during embryonic development. Nodal cilia consist of motile organelles with a “9+0” arrangement, surrounded by immotile sensory cilia. The lack of a central pair results in a rotatory motion of the motile nodal cilia, which produces a leftward flow of fluid across the surface of the embryonic node. This fluid flow is sensed by the sensory cilia and is responsible for determining body laterality [25–27]. Without this flow, left-right orientation becomes random, and results in laterality defects such as situs inversus totalis, situs ambiguous, and heterotaxy syndromes [28–31].
MOTOR CILIOPATHIES
Primary ciliary dyskinesia (CILD1: MIM 244400) is the first human disorder linked to motor ciliary dysfunction [32]. Primary ciliary dyskinesia is typically inherited in an autosomal recessive pattern, though rare cases of autosomal dominant and X-linked inheritance have been reported [33,34]. The pathogenesis of primary ciliary dyskinesia was uncovered 40 years ago, when the ultrastructural changes in the ciliary axoneme in affected individuals were first reported [32]. The frequency of primary ciliary dyskinesia was calculated as 1 in 10,000 to 20,000 live births, based on the prevalence of situs inversus totalis and bronchiectasis in population surveys from Norway and Japan, but these values likely underestimate its incidence in the general population. The prevalence of primary ciliary dyskinesia in children with repeated respiratory infections was approximated to be 5% [35].
Motor ciliary dysfunction leads to chronic airway infection and inflammation that result in progressive airway obstruction, atelectasis, and bronchiectasis, that can occur in young children. The upper respiratory tract is frequently involved in primary ciliary dyskinesia, clinically manifested as persistent rhinosinusitis that begins in infancy [36]. Middle ear involvement is common in children with primary ciliary dyskinesia, and can lead to conductive hearing loss. Approximately half of all primary ciliary dyskinesia subjects have situs inversus totalis or heterotaxy, since left-right laterality is a cilia-dependent mechanism, which can be associated with congenital heart disease, asplenia, or polysplenia [37]. It has been estimated that nearly 40% of patients with congenital heart disease associated with hetrotaxy have motile cilia dysfunction [37]. Other manifestations of primary ciliary dyskinesia include male and possibly female subinfertility and prenatal hydrocephalus [38].
PRIMARY CILIOPATHIES
Most cells of the body have a single non-motile, primary cilium, which contains specialized proteins and receptors to capture information from the local environment. These cilia are linked to various signaling pathways, and are associated with the regulation of planar cell polarity. Mutations in genes encoding for proteins associated with primary cilia lead to diverse syndromes and conditions, including retinitis pigmentosa, polycystic kidney disease, nephronophthisis, Bardet-Biedl syndrome, and various skeletal dysplasias. Although motile and sensory cilia share similar structures, motile cilia dysfunction is relatively rare in primary ciliopathies. In this section, we will discuss primary ciliopathies that may have a respiratory component, emphasizing the potential involvement of primary cilia in diseases of the lung and chest wall.
There are several conditions that encompass both motile and sensory cilia dysfunction caused by mutations in proteins that overlap both these organelles. Retinitis pigmentosa is a hereditary blindness caused by mutations in the retinitis pigmentosa GTPase regulator gene (RPGR). These patients can have symptoms identical to PCD [34,39].
One the earliest diseases to be associated with the primary cilium is autosomal dominant polycystic kidney disease, which occurs secondary to mutations in PKD1 and PKD2 genes that encode polycystin 1 and polycystin 2 respectively, both localized to the renal primary cilium [40]. Polycystin 1 and polycystin 2 are mechanoreceptors that detect urine flow in the renal tubules, and respond through calcium influx [41]. Loss or dysfunction of polycystins interferes with sensing mechanical cues that normally regulate renal morphogenesis and cause abnormal cyst formation. These receptors are also expressed on motile cilia, though their exact function in the context of the motor cilium is not known. Indeed, patients with autosomal dominant polycystic kidney disease have increased risk of airway disease and have an increased prevalence of radiographic bronchiectasis [42–44].
Bardet-Biedl syndrome is a rare, autosomal recessive disorder that is caused by mutations in BBS proteins that localize to the basal body of cilia and are important for intraflagellar transport [45,46]. Bardet-Biedl syndrome has varied clinical features, such as retinitis pigmentosa, polycystic kidneys, truncal obesity, polydactyly, intellectual disabilities, diabetes mellitus, hypogonadism, cardiovascular anomalies, and anosmia. BBS proteins also localize to the basal bodies of motile cilia in the airways. Animal models harboring mutations in BBS proteins have abnormal morphology in a fraction of motile cilia, including bulges filled with vesicles near the cilia tip [47]. The clinical importance of these findings is unclear, but encourages further research into the function of motile cilia in primary ciliopathies.
Other primary ciliopathies with evidence of motor cilia involvement include Usher syndrome, a rare genetic disease that affects the retinal photoreceptors and cochlear cilia, and is the leading genetic cause of combined hearing and sight loss. Proteins associated with Usher syndrome are thought to be unique to the retinal and cochlear cilia and mostly function in cell-cell adhesion, scaffold integrity and signaling [48]. While mutations that cause Usher syndrome are not associated with known motor cilia defects, there are individual case reports of patients with features similar to PCD [49,50].
Cranioectodermal dysplasia, Sensenbrenner syndrome, short-rib polydactyly, and Jeune asphyxiating thoracic dystrophy are another group of primary ciliopathies associated with skeletal dysplasia that affects the ribs cage leading to respiratory compromise [51]. These syndromes are collectively known as short-rib thoracic dysplasia, and are related to gene mutations that interfere with intraflagellar transport in primary cilia. Respiratory disease related to these disorders is due to the small, deformed thoracic cage that leads to pulmonary restriction, though a recent report hinted that motile cilia dysfunction contributed to respiratory insufficiency [52] in a child with cranioectodermal dysplasia due to biallelic WDR35 mutations. The actual link between WDR35 and motor cilia assembly or function is unknown.
DIAGNOSTIC APPROACH FOR PRIMARY CILIARY DYSKINESIA
Recent advances has allowed for improvements in the diagnosis of primary ciliary dyskinesia. Recognizing the clinical manifestations of motile cilia dysfunction continues to be the most important indication for diagnostic testing, and form the basis of criteria recommended by the PCD Foundation and the Genetic Disorders of Mucociliary Clearance consortium [53]. These manifestations are considered major criteria for the diagnosis of PCD, and include neonatal respiratory distress, laterality defects, persistent middle ear effusions, daily non-seasonal nasal congestion, and daily year-round wet cough that begins in infancy. The combination of persistent hypoxemia with situs abnormalities in a term infant without congenital cyanotic heart disease is consistent with primary ciliary dyskinesia and should prompt the clinician to pursue further evaluation. Children older than 1 month of age and older who presents with two or more of the aforementioned clinical manifestations, with at least one positive diagnostic test as discussed below, are likely to have PCD.
For years, clinicians have relied on transmission electron microscopy (Figure 1) to reveal ultrastructure changes in the cilia axoneme as means of confirming the disease, but this approach has significant limitations and can no longer be considered the sole “gold standard” for diagnosis, especially since up to 30% of patients with primary ciliary dyskinesia have normal ciliary ultrastructure [54–56]. Roughly 20% of patients with normal cilia ultrastructure as determined by electron microscopy will have mutations in the dynein axonemal heavy chain 11 (DNAH11; MIM603339) gene that encodes an outer dynein arm protein [55,57]. Other patients with mutations in the dynein regulatory complex can have subtle changes that are easily missed on electron microscopy [56,58]
The classic ultrastructural defects in primary ciliary dyskinesia typically involve absence or shortening of outer dynein arms with or without an inner dynein arm defect (15%). Isolated inner dynein arm abnormalities are rare and most have associated microtubular disorganization. Changes in cilia structure may also be secondary to airway infections and environmental pollutants exposures, and these changes have been erroneously attributed to primary ciliary dyskinesia. For instance, ciliary “disorientation” was once considered an ultrastuctural phenotype, but misalignment of the central pair is now thought to be an acquired defect and should not be used as a diagnostic criterion [59]. In contrast, ciliary aplasia, or reduction in the number of cilia, which were once believed to be due to secondary injury to the airway epithelium, have now been linked to genetic defects. Several patients were reported to have markedly reduced numbers of motor cilia on the surface of airway epithelia due to genetic defects of mother centriole generation and migration that are caused by rare gene mutations in CCNO and MCIDAS. Affected individuals have respiratory manifestations consistent with primary ciliary dyskinesia, with more rapid pulmonary function decline and early mortality [60].
Our current understanding of the genetic causes of PCD dictates that biallelic mutations of any gene encoding proteins involved in ciliary assembly, structure, or function can potentially cause primary ciliary dyskinesia. Massive parallel sequencing has been used to analyze regions of interest, and in the absence of candidates, whole exome sequencing has been used to successfully identify new candidate genes associated with primary ciliary dyskinesia [61–63]. The past three years have witnessed the discovery of a rapidly growing number of novel primary ciliary dyskinesia-associated genes. Advances in genetic testing have the potential to revolutionize diagnostics, and lead to earlier identification and treatment of primary ciliary dyskinesia. There are currently several commercially available gene panels that provide coverage of most known genes associated with primary ciliary dyskinesia. As more mutations are being identified, we expect genetic testing to ultimately become the preferred diagnostic option for primary ciliary dyskinesia.
Mutations in over 30 different genes have been implicated in primary ciliary dyskinesia, with clear relationship between genotype and ultrastructural phenotype. The genes currently implicated in primary ciliary dyskinesia encode proteins involved in axonemal structure, including the outer dynein arm; inner dynein arm and axonemal organization; and the central apparatus. Mutations in several genes that code cytoplasmic proteins, likely involved in ciliary assembly or protein transport, have been found in individuals with primary ciliary dyskinesia who lack both inner and outer dynein arms (Table 1). It is estimated that biallelic mutations in known primary ciliary dyskinesia-associated genes account for 70% of cases [64]. Many reported mutations causative of PCD are nonsense mutations or deletions, which result in loss of protein function. The association between rare sequence variants, such as those caused by missense mutations, and disease is more challenging. In most cases, a casual relationship often requires in vitro cell modeling. It should be emphasized that the clinical significance of common gene polymorphisms in PCD-associated genes is not always clear.
Relationships between genotype and clinical phenotypes in primary ciliary dyskinesia are emerging. Patients with inner dynein arm defects and axonemal disorganization represent approximately 12% of all primary ciliary dyskinesia cases. Mutations in CCDC39 (MIM613798)[65] and CCDC40 (MIM613799)[66], account for the majority of these patients, which produce inconsistent ultrastructual abnormalities characterized by disordered microtubules in only some cilia (5 to 20%). CCDC39 and CCDC40 are thought to function as a ruler dictating the precise repetition of structural proteins along the length of the axoneme [67]. Interestingly, CCDC39 and CCDC40 mutations are associated with more severe disease and poorer pulmonary function measures compared to other mutated primary ciliary dyskinesia-causing genes [64]. The reason for this phenomenon is not known.
Advances in imaging have allowed the use of high-speed video-microscopy as a diagnostic tool for primary ciliary dyskinesia mainly in Europe [68,69]. The normal beat frequency of human cilia ranges between 8–12 Hz, which can vary with testing temperature and tissue manipulation [70]. Slow or abnormal beating can be secondary to an acquired defect, and may lead to misdiagnoses. To circumvent some of these limitations, some centers have used in vitro airway epithelial cell cultures. Airway cells are obtained from patients and allowed to recover and differentiate in controlled cell cultures. This method can eliminate the non-specific changes caused by tissue manipulation or inflammation in the patient nasal passages, but it has limitations. This approache requires substantial experience and is best performed at centers that specialize in primary ciliary dyskinesia. Mutations characterized by subtle or variable changes in ciliary waveform can be missed. It should be emphasized that use of standard light microscopy is insufficient to screen or support the diagnosis of primary ciliary dyskinesia.
One of the most important diagnostic advances for primary ciliary dyskinesia is the use of nasal nitric oxide measurements, which has been adopted as the screening and diagnostic test of choice in North American centers, especially given the standardization of measurements [71]. The precise relationship between motile cilia and nitric oxide levels is unclear, though nitric oxide synthase localizes to the proximal ciliary axoneme [72] and several regulatory enzymes are localized to the basal bodies, suggesting some involvement in regulating cilia motility [73,74]. Nasal nitric oxide measurements are sensitive and specific for the diagnosis of primary ciliary dyskinesia in children five years and older [71,75], with sensitivity and specify approaching 98% and 99%, respectively [71], once cystic fibrosis is excluded, as nasal nitric oxide measurements can also be low in patients with cystic fibrosis. The accuracy of nasal nitric oxide measurement in younger children still needs to be established.
Recently, the PCD Foundation published a consensus statement with recommended criteria for the diagnosis of PCD. Standardization should clarify the process and improve diagnostic accuracy [53].
EXPERT COMMENTARY
Motor and sensory ciliopathies are a spectrum of diseases caused by dysfunction of the cilia. Primary ciliary dyskinesia is a rare, inherited disorder that is characterized by impaired ciliary function leading to diverse clinical manifestations, including chronic sinopulmonary disease, persistent middle ear effusions, laterality defects, and infertility. The growing number of cilia-related genes associated with primary ciliary dyskinesia has yielded new insights into the assembly, structure, and function of motor cilia and their involvement in disease. Emerging technologies hold promise for discovery of additional disease-associated genes and mutations. The availability of genetic testing has the potential to revolutionize diagnostic testing for primary ciliary dyskinesia and other ciliopathies, leading to earlier treatment of affected infants and children. Finally, recent discoveries of novel disease causing genes have lead to greater understanding of the basic cilia biology, and potentially could reveal targets to restore ciliary structure and function in diseases that do not have known cures.
FIVE-YEAR VIEW
We anticipate that our understanding of the genetics and pathophysiology of ciliopathies will continue to advance. With the discovery of novel disease-associated genes and use of model systems, the basic biology of cilia will be defined and functional networks elucidated, which will yield new therapeutic strategies to restore ciliary structure and function. Moreover, we expect that other genotype-phenotype relationships in primary ciliary dyskinesia and other ciliopathies will emerge, thus broadening the clinical spectrum of these diseases.
Acknowledgments
Financial support and conflicts of interest: The authors were supported in part by the National Institutes of Health (NIH) awards HL101465 (TWF), HL116211 (TWF), HL096458 (TWF), and the Children’s Discovery Institute (AH,TWF). The views expressed do not necessarily reflect the official policies of the Department of Health and Human Services; nor does mention by trade names, commercial practices, or organizations imply endorsement by the U.S government.
Footnotes
Financial disclosure
There are no previous publications or submissions with any overlapping information presented in this report. Moreover, this work is not and will not be submitted to any other journal while under consideration. Neither author has an actual or perceived conflict of interest concerning the information presented in the paper. Dr. Horani composed the first draft, and did not receive an honorarium or grant to write the manuscript. Both authors listed on the manuscript have reviewed and approved the content of the submission, and take full responsibility for the information provided.
References
Full text links
Read article at publisher's site: https://doi.org/10.1586/17476348.2016.1165612
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc4893162?pdf=render
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1586/17476348.2016.1165612
Article citations
Biallelic Variants in MNS1 Are Associated with Laterality Defects and Respiratory Involvement.
Cells, 13(12):1017, 11 Jun 2024
Cited by: 0 articles | PMID: 38920647 | PMCID: PMC11202006
Chlamydomonas dynein-preassembly-deficient mutants exhibit characteristic ciliary responses to viscous media.
MicroPubl Biol, 2024, 12 Mar 2024
Cited by: 0 articles | PMID: 38545438 | PMCID: PMC10966395
The morphological and functional diversity of apical microvilli.
J Anat, 242(3):327-353, 25 Oct 2022
Cited by: 9 articles | PMID: 36281951
Review
Biallelic DAW1 variants cause a motile ciliopathy characterized by laterality defects and subtle ciliary beating abnormalities.
Genet Med, 24(11):2249-2261, 08 Sep 2022
Cited by: 3 articles | PMID: 36074124 | PMCID: PMC10584193
PCD Genes-From Patients to Model Organisms and Back to Humans.
Int J Mol Sci, 23(3):1749, 03 Feb 2022
Cited by: 8 articles | PMID: 35163666 | PMCID: PMC8836003
Review Free full text in Europe PMC
Go to all (16) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Diseases
- (1 citation) OMIM - 244400
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Advances in the Genetics of Primary Ciliary Dyskinesia: Clinical Implications.
Chest, 154(3):645-652, 22 May 2018
Cited by: 59 articles | PMID: 29800551 | PMCID: PMC6130327
Review Free full text in Europe PMC
Picking up speed: advances in the genetics of primary ciliary dyskinesia.
Pediatr Res, 75(1-2):158-164, 05 Nov 2013
Cited by: 38 articles | PMID: 24192704 | PMCID: PMC3946436
Review Free full text in Europe PMC
Genetics and biology of primary ciliary dyskinesia.
Paediatr Respir Rev, 18:18-24, 11 Sep 2015
Cited by: 113 articles | PMID: 26476603 | PMCID: PMC4864047
Review Free full text in Europe PMC
Ciliary Dyneins and Dynein Related Ciliopathies.
Cells, 10(8):1885, 25 Jul 2021
Cited by: 12 articles | PMID: 34440654 | PMCID: PMC8391580
Review Free full text in Europe PMC
Funding
Funders who supported this work.
Children’s Discovery Institute
NHLBI NIH HHS (4)
Grant ID: U01 HL101465
Grant ID: U54 HL096458
Grant ID: R01 HL116211
Grant ID: U54 HL09640958




