Abstract
Free full text

Hypofractionated ablative radiotherapy for locally advanced pancreatic cancer
Abstract
The role of radiation in locally advanced unresectable pancreatic cancer (LAPC) is controversial. Randomized trials evaluating standard doses of chemoradiation have not shown a significant benefit from the use of consolidative radiation. Results from non-randomized studies of 3–5-fraction stereotactic body radiotherapy (SBRT) have been similar to standard chemoradiation, but with less toxicity and a shorter treatment time. Doses of SBRT have been reduced to subablative levels for the sake of tolerability. The benefit of both options is unclear. In contrast, ablative doses can be delivered using an SBRT technique in 15–28 fractions. The keys to the delivery of ablative doses are computed tomography (CT) image guidance and respiratory gating. Higher doses have resulted in encouraging long-term survival results. In this review, we present a comprehensive solution to achieving ablative doses for selected patients with pancreatic tumors by using a combination of classical, modern and novel concepts of radiotherapy: fractionation, CT image guidance, respiratory gating, intentional dose heterogeneity, and simultaneous integrated protection.
INTRODUCTION
The role of radiation in locally advanced unresectable pancreatic cancer (LAPC) is controversial. There have been five trials that have evaluated the role of standard chemoradiation after chemotherapy in the treatment of LAPC. The trial that has had the greatest impact on clinical practice thus far is the LAP 07 trial. Preliminary data from the LAP 07 trial revealed no clear benefit from consolidative chemoradiation following chemotherapy [1]. Results from the trial were presented at ASCO 2013 and showed no benefit to the use of consolidative chemoradiation after 4 months of gemcitabine-based chemotherapy compared with 6 months of chemotherapy alone. Issues regarding the off-protocol use of chemoradiation, compliance in the chemoradiation arm, and radiation therapy quality assurance require further clarity when the full manuscript is published. These results, coupled with the introduction of more active systemic regimens, have led to a shift at most academic centers to the much more selective use of consolidative chemoradiation. Four other randomized trials have compared chemotherapy with chemoradiation [2–5]. Results have been mixed: two trials modestly favored a chemotherapy approach [3, 4], whereas the other two trials modestly supported an initial chemoradiation strategy [2, 5]. The Fédération Francophone de Cancérologie Digestive and Société Française de Radiothérapie Oncologique (FFCD–SFRO) showed superior survival of gemcitabine alone to a poorly tolerated experimental chemoradiation regimen (60 Gy to large fields with cisplatin and 5FU) that had not been tested in a Phase I or II trial [3]. The only other recent trial to compare initial chemotherapy with chemoradiation was conducted by the Eastern Cooperative Oncology Group (ECOG 4201). This trial compared gemcitabine-based chemoradiation followed by weekly gemcitabine with gemcitabine alone. A median survival benefit was seen in the chemoradiation arm. This benefit came at the cost of increased gastrointestinal toxicity [5]. A number of US cooperative group trials evaluating gemcitabine-based chemotherapy in advanced pancreatic cancer have included patients with locally advanced disease without planned radiotherapy. Median survival durations of between 9.1 and 9.9 [6–8] months have been achieved in these subsets of patients, compared with between 12 and 14.3 months in the LAP07 and FFCD–SFRO trials. The reason for this difference is unclear. Collectively, what these randomized trials illustrate most clearly is the substantial degree to which standard therapies are limited in their effectiveness. Specifically, they offer no hope of long-term survival.
The rationale for the further study of the use of radiation therapy in LAPC is based on clinical and autopsy data indicating that 30% or more of patients die from complications related to local disease progression. As median survival durations improve in patients with locally advanced pancreatic cancer, local progression of disease will probably more commonly limit long-term survival. Better options for local tumor control than 50.4 Gy or low-dose SBRT (25–33 Gy in 5 fractions) are needed.
LOCAL TUMOR CONTROL IN LOCALLY ADVANCED PANCREATIC CANCER
Patients with pancreatic cancer want to live longer, and most of all they want some hope of cure. Although the natural history of pancreatic cancer is dominated by the development of metastatic disease, local tumor progression contributes significantly to morbidity and mortality. Locoregional progression is common, and it is clear that a subset of patients do not ever develop metastatic disease, and some patients with metastatic disease die from local tumor progression. A recent rapid autopsy series from Johns Hopkins reported that 28% of patients with locally advanced pancreatic cancer had no evidence of metastases at the time of death, and SMAD4 loss correlated with widespread distant metastatic disease [9]. We reported a similar rate of local progression–related death, as well as a correlation of SMAD4 expression with the pattern of disease related to death, in a Phase II trial of 69 patients with locally advanced pancreatic cancer that evaluated cetuximab-based chemotherapy followed by chemoradiation (50.4 Gy in 28 fractions). The median survival of 19.2 months was long enough to evaluate late local tumor progression (20% at 15 months, increased to 65% at 2 years). Local tumor progression was the dominant cause of death after 15 months [10]. The median survival in nearly all studies of locally advanced pancreatic cancer is too short to evaluate the highest-risk period for local tumor recurrence. The significance of 1-year local tumor control is often emphasized, but has little relevance. Local tumor progression is a barrier to long-term survival in locally advanced pancreatic cancer and is the reason there is almost never a tail to the survival curve. The best evidence for the impact of effective local tumor control on survival comes from resected patients: resection leads to a substantial median survival benefit, and apparent cure in 20% of patients. Similar results can be achieved in selected patients with the use of definitive doses of radiotherapy.
STEREOTACTIC BODY RADIOTHERAPY IN PANCREATIC CANCER
Stereotactic body radiotherapy (SBRT) is capable of precisely delivering high doses of radiation to small tumor volumes. SBRT is an attractive option for primary or metastatic tumors occurring in organs with parallel functional subunits, such as the lung or liver. Ablation of a small volume of surrounding normal liver or lung tissue around the tumor usually has no significant clinical consequence. In contrast, ablative doses near an organ with serial functional subunits, such as the gastrointestinal tract, are not possible without affecting organ function. If a radiosensitive structure such as the duodenum, small bowel, or stomach is in close proximity to a radioresistant target, such as a pancreatic tumor, ablative doses cannot be given without protracting the fractionation beyond the 3–5 fractions that are typically used for pancreatic cancer SBRT. To ensure safety, clinicians that use SBRT techniques for pancreatic cancer are presently giving palliative doses—e.g. 25–33 Gy in 3–5 fractions, which is roughly half the dose needed for ablation of small solid tumors (50 Gy in 5 fractions). The only prospective multi-institutional trial has defined a safe and convenient regimen (33 Gy in 5 fractions), but as with standard-fraction chemoradiation, there is no evidence of long-term survival benefit [11]. The rationale for using 3–5 fractions in a tumor that is surrounded in close proximity by bowel is inconsistent with the fundamental principle of fractionation. The dose has to be reduced, limiting the benefit. We have used an SBRT technique with the incorporation of the principle of fractionation.
HYPOFRACTIONATION USING AN SBRT TECHNIQUE
Accurate assumptions about the optimal choice of fraction size in the treatment of pancreatic cancer are problematic because there are generally only two datasets from which one can draw conclusions, 50.4–60 Gy given in conventional fractions and low-dose hypofractionated SBRT (25–33 Gy in 5 fractions). These treatments have led to little or no chance of long-term tumor control or median survival benefit. Changing the fraction size in these dose ranges has also not improved efficacy. As with many solid tumors, the primary limitation of local tumor control may be total dose. These total doses are low, certainly not definitive. Accurate targeting of the tumor, with diagnostic quality image guidance, leading to relative sparing of OARs, gives one the option of dose escalation. Our data suggest that giving a physical and bioequivalent dose that is twice as high as standard doses has led to unprecedented outcomes. The overriding consideration for the choice of fraction size for the simultaneous integrated boost (SIB) is a practical one. Based on our liver and pancreatic data, we think that a biological equivalent dose (BED10) of ~100 Gy is sufficient to achieve local tumor control. Eleven weeks of treatment to deliver 100 Gy at standard fractionation is not practical from a patient convenience or resource perspective, but 3–5 weeks is. Anything short of 3 weeks and the BED10 will probably have to be reduced to meet OAR constraints. For instance, we know that 3–5 fraction SBRT regimens do not lead to a significant chance of long-term tumor control. That is because the dose has to be reduced so much that the treatment becomes ineffective. We now have evidence that hypofractionation at 2.25–4.5 Gy per fraction to a high BED10 (70–100 GY) provides long-term tumor control. Whether this is due to the higher total dose or the fraction size is impossible to determine from our data. That determination would require an analysis of pooled data from future dose escalation studies.
Our treatment strategy incorporates fractionation, CT image guidance, respiratory gating, intentional dose heterogeneity, and simultaneous integrated protection. We started our novel treatment-planning approach with the assumption that allowing high and low dose inhomogeneity would help to control pancreatic cancers, which are almost always <1 cm from the gastrointestinal tract. We have tried to treat as much of the tumor as possible to an ablative dose, but areas abutting the GI tract have been restricted to a microscopic dose while the rest of the tumor has received an ablative dose. The hypoxic center of the tumor can safely receive very high doses (Fig. 1). We do not know yet whether the use of this central SIB improves tumor control. However, we have not seen significant toxicity from its use. In order to maximize the therapeutic index, we use the principle of fractionation to maximize BED10 delivered to the tumor. Using this SBRT technique, we deliver what would be definitive doses for most solid tumors in 15 or 28 fractions to the primary tumor. Feedback-assisted inspiration breath-hold respiratory gating with daily diagnostic-quality CT-on-rails, soft tissue image registration (Fig. 2), and intensity-modulated radiation therapy (IMRT) allows the safe delivery of up to a 2-fold increase in the BED10 compared with standard fractionation or low-dose SBRT. Our recently published series using definitive doses (IMRT to 63–70 Gy in 28 fractions or 67.5 Gy in 15 fractions; BED10, 77.2–97.9 Gy) [12] demonstrates a median time to local progression difference (20.1 vs 15 months, P = 0.03) as well as a survival benefit at 3 years (35%) and 5 years (18%) compared with the expected survival (<5%). These results compare favorably with surgical resection in patients with less advanced local disease and is proof of principle that definitive radiation doses can lead to a hope of long-term survival. We have typically excluded patients with tumors adjacent to the bowel. In doing so, we have achieved a median gross tumor volume (GTV) coverage of 96% of the prescription dose. Although high doses could be delivered with this technique to tumors abutting the bowel, the GTV coverage would be lower. However, the extent of GTV coverage does not appear to affect outcome in patients with GTV coverage as low as 70%, but more data are needed on this question.
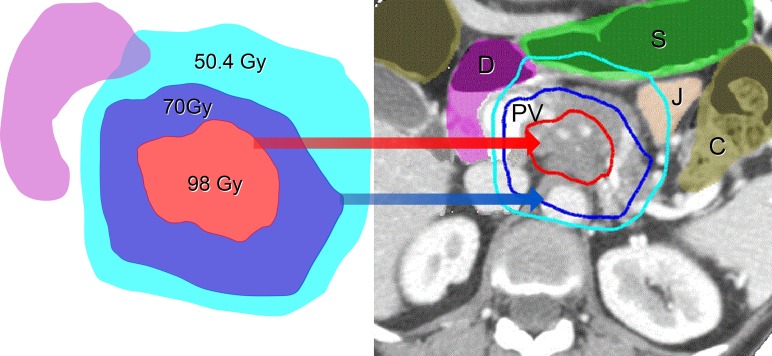
Simultaneous Integrated Boost (SIB) and Simultaneous Integrated Protection (SIP) in the Treatment Planning of Locally Advanced Pancreatic Cancer. This figure illustrates the proximity of gastrointestinal organs to a pancreatic tumor. This patient was treated with a dose of 70 Gy in 28 fractions to the GTV and 98 Gy to the hypoxic center, using feedback-assisted inspiration breath-hold gating and daily diagnostic-quality CT imaging to verify stomach position. GTV = gross tumor volume. S = stomach, J = jejunum, D = duodenum, C = colon, PV = collaterals from an occluded portal vein.
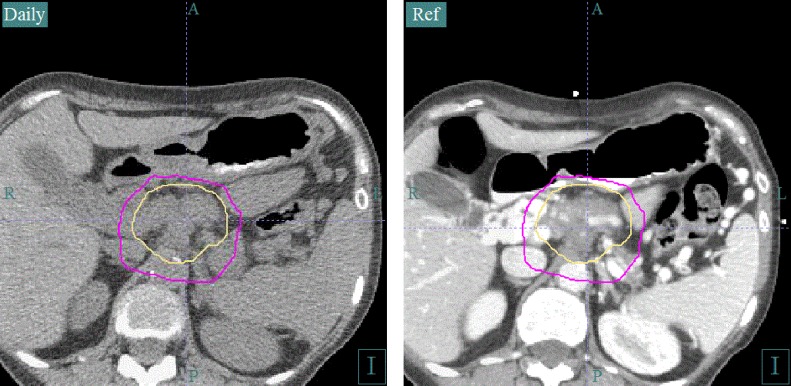
Daily CT image guidance allows monitoring of the stomach and small bowel. Daily CT image guidance is an essential component of the safe delivery of ablative doses of radiation to a tumor surrounded by gastrointestional luminal structures. An inspiration breath-hold technique is used to control respiratory motion. The intravenous contrast-enhanced simulation scan on the right is registered with the daily CT scan on the left for verification of luminal organ position. The daily CT can be used for adaptive planning if internal organ position is consistently different from that in the simulation CT.
CURRENT SIMULTANEOUS INTEGRATED BOOST WITH SIMULTANEOUS INTEGRATED PROTECTION TECHNIQUE
Our treatment-planning approach involves using IMRT with an SIB technique, typically with two or three different PTVs (a microscopic dose, an SIB to the GTV, and if possible an SIB to a higher dose to the hypoxic center). Areas of potential microscopic extension around the tumor, the celiac axis, and the superior mesenteric artery are treated with 37.5 Gy in 15 fractions or 50.4 Gy in 28 fractions using a 10-mm CTV + a 5-mm PTV expansion, for a total of 15 mm from GTV to PTV. The SIB to the GTV (67.5 Gy in 15 fractions or 70 Gy in 28 fractions) is treated with a 0–5-mm PTV. The decision to use 0–5 mm for the PTV of the high-dose SIB is based on the proximity of the bowel. The simultaneous integrated protection technique involves subtracting a planning-OAR-volume for all luminal structures (created by taking the 4D contour of the OAR and adding 5 mm) from this high-dose PTV. A contraction of the high-dose PTV volume of 5–10 mm is used to create the PTV within the hypoxic center, in selected patients. This volume receives 75 Gy in 15 fractions or 98 Gy in 28 fractions. Most of the time, the tumor is too small or the bowel is too close for this central high dose to be given. A representative plan is shown in Fig. 1.
The choice of fraction number is based on the proximity of the tumor to luminal GI structures. When the tumor is ≤1 cm of the GI tract, we always use 28 fractions to optimally spare those structures while achieving the highest minimum dose to the tumor. If the tumor is >1 cm from the bowel, we use 15 fractions. Our bowel dose constraints, with the inspiratory breath-hold gating and CT image guidance that we use, are based on a previous analysis [13]. We use a maximum point dose of 60 Gy in 28 fractions and 45 Gy in 15 fractions for the stomach and descending duodenum. For the transverse duodenum and jejunum, we use a 10% lower constraint because those structures are out of reach of an endoscopic argon plasma laser procedure, making the consequences of bleeding greater. Using these constraints, we have not had a significant bleeding event in 4 years. The only other OARs that may be of concern are the named arteries and the bile ducts. We have not seen pancreaticobiliary ductal strictures or vascular complications within the high-dose PTV (67.5 Gy/15 fx to 70 Gy/28 fx) in the treatment of liver or pancreatic cancers. Arterial rupture of the aorta has been reported with lung SBRT using similar bioequivalent doses in 3–5 fractions, but has not been reported elsewhere in smaller, lower-pressure arteries or at all in veins. Causing a ductal stricture within a pancreatic tumor is not a concern because the tumor has already caused irreversible occlusion of the involved ducts. Patients generally need metallic common bile duct stents for life and pancreatic ductal obstruction from the tumor is treated with enzyme supplementation. Since we have excluded named arteries from the extra-high dose volume (75 Gy/15 fx to 98 Gy/28 fx), we do not have data regarding the risk to arterial structures. Almost certainly, these higher doses would lead to a risk of arterial intimal proliferation or rupture.
SOLUTION FOR ORGAN MOTION
Since the target dose is up to twice the tolerance of the surrounding bowel, solutions for organ motion and image guidance are necessary in order to deliver safe treatment. Tracking, abdominal compression, and gating are all options for respiratory motion control that are used to reduce dose to normal tissues and escalate dose to tumors. We use a technique of feedback-guided inspiratory breath-hold gating using the Varian Real-Time Position Management system. This breath-hold technique is coupled with diagnostic-quality CT image guidance to verify the target position and the day-to-day variation of the position of the stomach, duodenum and jejunum with each fraction (Fig. 2). With a diagnostic non-contrast CT, we visualize and set up to pancreatic tumors while visualizing the interface of the tumor and GI structures. We also use these images for adaptive planning. Most adaptive planning is actually triggered by movement of OARs from simulation to treatment. CT-on-rails is not being marketed commercially at this time. Magnetic Resonance Imaging (MRI)-Linacs seem to be the way of the future for optimal image-guided radiotherapy in these challenging cases and have potential advantages over CT such as better visualization of tumors within the liver and improved image registration accuracy owing to better soft-tissue contrast than CT.
If a patient cannot voluntarily hold their breath for treatment, we use end expiratory gating during free breathing. Successful use of gating techniques requires a regular breathing pattern. We gate patients at end expiration is because there is less motion during that point in the respiratory cycle. Treatment with this technique requires the presence of fiducial markers, 4D simulation, and contouring the target and avoidance structures at end expiration. Positions 40 through 70 in the respiratory cycle move the least and are a good starting point to assess the gating window. A narrower gating window means a longer treatment time, but may be preferred to minimize motion. Fiducial position is verified with a gated kilovoltage image between IMRT beams to assess intrafraction variability of target position at end expiration. Image guidance is accomplished with fiducial alignment using KV images for target localization. Without soft tissue imaging, the day-to-day position of the luminal gastrointestinal structures cannot be verified.
The issue of intrafraction motion could be of clinical relevance. Most of our concern is with GTV coverage during treatment, but motion of the surrounding OARs could also be clinically relevant. Although we have not seen any radiation-related bleeding events since our constraints were modified four years ago, we have not formally studied this question, and to our knowledge it has not been addressed in the literature in this context. Our observation from the limited experience with clinically indicated repeat CT for positioning is that bowel and stomach motion does not happen rapidly enough for this to be a clinically relevant concern. In fact, it seems more likely that any motion would smear the higher doses within the bowel, which would be an advantage. The issue of intrafraction organ motion in general certainly requires further study. The only practical way to formally address these concerns is with MRI-Linacs, since real-time soft-tissue motion can be observed during treatment. Fortunately, they are now commercially available and these issues can be addressed more objectively.
TOG/NRG ONCOLOGY 1201
Radiation Therapy Oncology Group (RTOG)/NRG Oncology 1201[14] is a randomized Phase II trial that will evaluate the role of radiation dose escalation. It compares gemcitabine and abraxane alone with gemcitabine and abraxane followed by standard or 63 Gy using IMRT. It will also address the role of the use of SMAD4 expression to predict the pattern of disease progression. SMAD4 expression status is a stratification variable, and patients are randomly assigned to receive chemotherapy alone until progression in the standard arm, four cycles of chemotherapy followed by 50.4 Gy in 28 fractions, or four cycles of chemotherapy followed by 63 Gy in 28 fractions, with IMRT used in both chemoradiation arms. Concurrent capecitabine will be given with radiation. This trial will evaluate the role of SMAD4 as a predictive marker for chemoradiation decision-making and will provide further data on the role of chemoradiation in the context of more active chemotherapy.
REFERENCES
Articles from Journal of Radiation Research are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/jrr/rrw016
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/jrr/article-pdf/57/S1/i53/8038310/rrw016.pdf
Citations & impact
Impact metrics
Article citations
Effect of breathing phase number on the 4D robust optimization for pancreatic cancer intensity modulated proton therapy.
BMC Cancer, 24(1):1337, 30 Oct 2024
Cited by: 0 articles | PMID: 39478463 | PMCID: PMC11526620
Escalated-dose radiotherapy for unresected locally advanced pancreatic cancer: Patterns of care and survival in the United States.
Cancer Med, 13(12):e7434, 01 Jun 2024
Cited by: 0 articles | PMID: 38923407 | PMCID: PMC11200087
Radiation response assessment of organoids derived from patients with pancreatic cancer.
Clin Transl Radiat Oncol, 48:100829, 27 Jul 2024
Cited by: 0 articles | PMID: 39192878 | PMCID: PMC11347840
Prediction of Isolated Local Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Nationwide Study.
Ann Surg Oncol, 31(12):8264-8275, 27 Jun 2024
Cited by: 0 articles | PMID: 38937412 | PMCID: PMC11467030
A pilot study on interobserver variability in organ-at-risk contours in magnetic resonance imaging-guided online adaptive radiotherapy for pancreatic cancer.
Front Oncol, 14:1335623, 10 May 2024
Cited by: 0 articles | PMID: 38800394 | PMCID: PMC11116709
Go to all (44) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Ablative Radiotherapy Doses for Locally Advanced: Pancreatic Cancer (LAPC).
Cancer J, 23(6):350-354, 01 Nov 2017
Cited by: 12 articles | PMID: 29189331
Review
Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results.
Radiat Oncol, 14(1):95, 06 Jun 2019
Cited by: 89 articles | PMID: 31171025 | PMCID: PMC6555709
Review Free full text in Europe PMC
Linac-based stereotactic body radiation therapy for unresectable locally advanced pancreatic cancer: risk-adapted dose prescription and image-guided delivery.
Strahlenther Onkol, 194(9):835-842, 25 Apr 2018
Cited by: 21 articles | PMID: 29696321
Stereotactic body radiation therapy in pancreatic cancer: the new frontier.
Expert Rev Anticancer Ther, 14(12):1461-1475, 03 Sep 2014
Cited by: 18 articles | PMID: 25183386
Review





