Abstract
Free full text

Characteristics and Outcomes of Complicated Intra-abdominal Infections Involving Pseudomonas aeruginosa from a Randomized, Double-Blind, Phase 3 Ceftolozane-Tazobactam Study
Abstract
Ceftolozane-tazobactam is active against Gram-negative pathogens, including multidrug-resistant Pseudomonas aeruginosa. In a subgroup analysis of patients with complicated intra-abdominal infections (cIAIs) involving P. aeruginosa from a phase 3 program, ceftolozane-tazobactam demonstrated potent in vitro activity against P. aeruginosa. Clinical cure in the microbiologically evaluable population was 100% (26/26) for ceftolozane-tazobactam plus metronidazole and 93.1% (27/29) for meropenem. These findings support the use of ceftolozane-tazobactam in the management of cIAI when P. aeruginosa is suspected or confirmed. (This study has been registered at ClinicalTrials.gov under registration no. NCT01445665 and NCT01445678.)
TEXT
Complicated intra-abdominal infections (cIAIs) are caused by Gram-negative bacteria, with Enterobacteriaceae being the most common pathogen. Pseudomonas aeruginosa is the third-most-common Gram-negative bacteria in cIAI (1), and increasing rates of P. aeruginosa resistance are a global concern (2, 3).
Ceftolozane-tazobactam, in combination with metronidazole, is approved for the treatment of cIAI (4). Ceftolozane-tazobactam has potent activity against many drug-resistant Gram-negative pathogens, including most extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae (5, 6), and is minimally affected by common P. aeruginosa resistance mechanisms (7). Compared with approved β-lactam antibiotics, including meropenem and piperacillin-tazobactam, ceftolozane-tazobactam displays more potent in vitro activity against P. aeruginosa (8).
The Assessment of the Safety Profile and Efficacy of Ceftolozane/Tazobactam in Complicated Intra-Abdominal Infections (ASPECT-cIAI) study was a global phase 3 program that demonstrated the efficacy of ceftolozane-tazobactam plus metronidazole to be similar to that of meropenem in patients with cIAI (NCT01445665 and NCT01445678) (9). This analysis was conducted to determine the characteristics and clinical outcomes of the subgroup of patients with P. aeruginosa infection.
(Part of this research was presented as poster 251 at IDWeek, the annual meeting of the Infectious Diseases Society of America [IDSA], the Society for Healthcare Epidemiology of America [SHEA], the HIV Medicine Association [HIVMA], and the Pediatric Infectious Diseases Society [PIDS], 8 to 12 October 2014, Philadelphia, PA.)
In ASPECT-cIAI, patients (age, ≥18 years) with cIAI were randomly assigned 1:1 to receive intravenous ceftolozane-tazobactam (1.5 g containing 1,000 mg ceftolozane and 500 mg tazobactam) plus metronidazole (500 mg) every 8 h or intravenous meropenem (1 g every 8 h) plus placebo for 4 to 14 days. Efficacy was assessed at the test-of-cure visit 24 to 32 days after initiation of the study drug. Clinical cure was defined as the resolution of or significant improvement in signs and symptoms of the index infection, such that no additional antibacterial therapy or intervention was necessary. Descriptive statistics were used to compare baseline characteristics (microbiological intent-to-treat [MITT] population) and clinical outcomes (microbiologically evaluable [ME] population) of patients with and without P. aeruginosa infection. Descriptions of inclusion/exclusion criteria and study design were published previously (9).
MIC cutoffs for susceptibility to ceftolozane-tazobactam and meropenem were based on Clinical and Laboratory Standards Institute (CLSI) definitions (10). Multidrug resistance (MDR) in P. aeruginosa was based on CLSI breakpoints and defined as nonsusceptibility to ≥3 drug classes known to be active against P. aeruginosa. P. aeruginosa isolates were screened for AmpC overexpression.
In the MITT population, 8.9% (72/806) of patients had P. aeruginosa infection at baseline; 4 patients had P. aeruginosa as the only infecting pathogen. Baseline demographic characteristics were similar between patients with and those without P. aeruginosa infection (Table 1). P. aeruginosa infection was more frequent in North America (17.6% [9/51]) than in Europe (7.9% [50/635]) and more commonly isolated in patients with colonic (14.4% [7/118]) or appendiceal (11.2% [43/384]) infections. In patients with P. aeruginosa infection, 65.3% (47/72) received previous antibacterial therapy, compared with 56.8% (417/734) of patients without P. aeruginosa infection. Previous therapies included metronidazole (41.7%), ceftriaxone (12.5%), and cefotaxime (8.3%); mean duration of therapy (7.8 days) was the same for patients with and those without P. aeruginosa infection. In total, 8.9% (4/5) of patients for whom previous antibacterial therapy was ineffective (amoxicillin-clavulanic acid and ertapenem; cefotaxime, metronidazole, and piperacillin-tazobactam; metronidazole and cefuroxime axetil; and metronidazole, ceftriaxone sodium, and cefuroxime axetil) had P. aeruginosa infection.
TABLE 1
Baseline demographics of all patients in the ASPECT-cIAI trial (microbiological intent-to-treat population)
| Characteristic | P. aeruginosa at baseline (n = 72) | No P. aeruginosa at baseline (n = 734) | Total (n = 806) |
|---|---|---|---|
| Sex, male (n [%]) | 48 (66.7) | 418 (56.9) | 446 (57.8) |
| Race, white (n [%]) | 63 (87.5) | 692 (94.3) | 755 (93.7) |
| Mean age (SD) (yr) | 49.5 (19.3) | 50.7 (17.4) | 50.6 (17.5) |
    ≥75 yr (n [%]) ≥75 yr (n [%]) | 8 (11.1) | 75 (10.2) | 83 (10.3) |
| Mean body mass index (SD) (kg/m2) | 27.1 (6.3) | 26.9 (5.3) | 26.9 (5.4) |
| Baseline APACHE II score category (n [%])a | |||
    <10 <10 | 61 (84.7) | 596 (81.2) | 657 (81.5) |
    ≥10 ≥10 | 11 (15.3) | 137 (18.7) | 148 (18.4) |
| Creatinine clearance (ml/min) (n [%]) | |||
    Normal (≥80) Normal (≥80) | 47 (65.3) | 516 (70.3) | 563 (69.9) |
    Mild renal impairment (>50 to <80) Mild renal impairment (>50 to <80) | 24 (33.3) | 183 (24.9) | 207 (25.7) |
    Moderate renal impairment (≥30 to ≤50) Moderate renal impairment (≥30 to ≤50) | 1 (1.4) | 35 (4.8) | 36 (4.5) |
| Geographic origin (n [%]) | |||
    Europe Europe | 50 (69.4) | 585 (79.7) | 635 (78.8) |
    North America North America | 9 (12.5) | 42 (5.7) | 51 (6.3) |
    South America South America | 6 (8.3) | 75 (10.2) | 81 (10.0) |
    Rest of world Rest of world | 7 (9.7) | 32 (4.4) | 39 (4.8) |
| Anatomic site of infection (n [%]) | |||
    Appendix Appendix | 43 (59.7) | 341 (46.5) | 384 (47.6) |
    Biliary cholecystitis/cholangitis Biliary cholecystitis/cholangitis | 5 (6.9) | 138 (18.8) | 143 (17.7) |
    Stomach/duodenum Stomach/duodenum | 4 (5.6) | 75 (10.2) | 79 (9.8) |
    Colon Colon | 17 (23.6) | 101 (13.8) | 118 (14.6) |
    Small bowel Small bowel | 1 (1.4) | 41 (5.6) | 42 (5.2) |
    Parenchymal (liver) Parenchymal (liver) | 1 (1.4) | 32 (4.4) | 33 (4.1) |
    Parenchymal (spleen) Parenchymal (spleen) | 0 | 4 (0.5) | 4 (0.5) |
    Other Other | 1 (1.4) | 15 (2.0) | 16 (2.0) |
Most P. aeruginosa (97.2% [70/72]) and non-P. aeruginosa (92.9% [682/734]) infections were community acquired, and P. aeruginosa was more likely to be isolated as part of a polymicrobial infection (94.4% [68/72]). All three cases of concurrent bacteremia in patients with P. aeruginosa occurred with polymicrobial infections; bacteremia was a result of Propionibacterium acnes, Eggerthella lenta, and Enterococcus faecalis infection, and all patients were deemed to be clinically cured.
Both ceftolozane-tazobactam and meropenem were highly active in vitro against P. aeruginosa, with an MIC required to inhibit the growth of 90% of isolates (MIC90) of 2 μg/ml for ceftolozane-tazobactam and 4 μg/ml for meropenem. Ceftolozane-tazobactam was the most potent agent tested; 97.1% of isolates were inhibited at an MIC of ≤4 μg/ml, whereas susceptibility to meropenem was 89.9% (Fig. 1A). Based on MIC90 values, ceftolozane-tazobactam (MIC90, 2 μg/ml) was 32-fold more active than piperacillin-tazobactam (MIC90, 64 μg/ml) and 8-fold more active than ceftazidime, cefepime, aztreonam, or gentamicin (MIC90, 16 μg/ml for each).
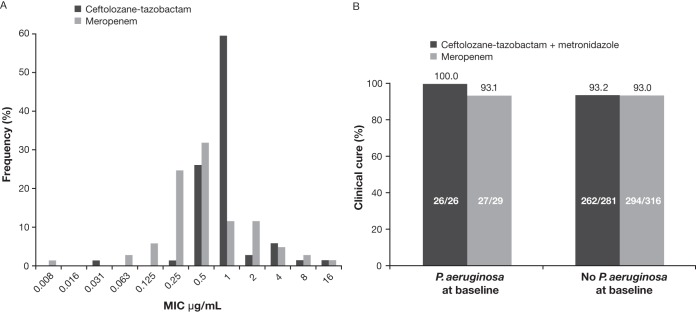
MIC distribution and clinical outcomes with ceftolozane-tazobactam and meropenem. (A) Distribution of ceftolozane-tazobactam and meropenem MICs for 69 Pseudomonas aeruginosa isolates identified at the screening visit (microbiological intent-to-treat population). (B) Clinical cure rate at the test-of-cure visit for patients with and without baseline P. aeruginosa infection, by treatment group (microbiologically evaluable population, which includes patients with pathogens at baseline who were susceptible or resistant to study drug).
In the MITT population, 15.7% (11/70) of molecularly characterized P. aeruginosa isolates overexpressed AmpC; the MIC range was 0.5 to 16 μg/ml for ceftolozane-tazobactam and 0.25 to 8 μg/ml for meropenem. Three patients in the meropenem group had MDR P. aeruginosa; the MIC range was 4 to 16 μg/ml for ceftolozane-tazobactam and 2 to 4 μg/ml for meropenem. In the ME population, 10 patients had P. aeruginosa infection that overexpressed AmpC, and 3 patients had MDR P. aeruginosa (Table 2).
TABLE 2
In vitro activity of ceftolozane-tazobactam and comparator antibacterials against AmpC-producing and MDR P. aeruginosa isolates identified at screening visit (microbiologically evaluable population)
| Treatment group | Clinical outcome | MIC (μg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|
| Ceftolozane-tazobactam | Meropenem | Aztreonam | Cefepime | Ceftazidime | Gentamicin | Piperacillin-tazobactam | ||
| AmpC producers (n = 10) | ||||||||
    Ceftolozane-tazobactam Ceftolozane-tazobactam | Cure | 0.5 | 1 | 4 | 2 | 2 | 1 | 8 |
    Ceftolozane-tazobactam Ceftolozane-tazobactam | Cure | 1 | 0.5 | 8 | 2 | 4 | 2 | 16 |
    Ceftolozane-tazobactam Ceftolozane-tazobactam | Cure | 1 | 1 | 8 | 4 | 4 | 2 | 8 |
    Ceftolozane-tazobactam Ceftolozane-tazobactam | Cure | 2 | 2 | 8 | 16 | 16 | >16 | 64 |
    Ceftolozane-tazobactam Ceftolozane-tazobactam | Cure | 1 | 4 | 4 | 4 | 4 | 2 | 8 |
    Meropenema Meropenema | Cure | 4 | 2 | 16 | 16 | 16 | >16 | 128 |
    Meropenem Meropenem | Cure | 1 | 0.5 | 4 | 4 | 4 | 1 | 8 |
    Meropenem Meropenem | Cure | 1 | 0.25 | 0.5 | 2 | 1 | 2 | ≤0.25 |
    Meropenem Meropenem | Cure | 16 | 2 | 32 | 32 | >32 | >16 | >128 |
    Meropenem Meropenem | Cure | 4 | 4 | >32 | 32 | >32 | 16 | >128 |
| MDRb (n = 3) | ||||||||
    Meropenema Meropenema | Cure | 4 | 2 | 16 | 16 | 8 | >16 | 128 |
    Meropenem Meropenem | Cure | 16 | 2 | 32 | 32 | >32 | >16 | >128 |
    Meropenem Meropenem | Cure | 4 | 4 | >32 | 32 | >32 | 16 | >128 |
Clinical cure rates in the ME population for patients with and without P. aeruginosa infection at baseline (regardless of pathogen susceptibility to study treatment) are summarized in Fig. 1B. For two patients with P. aeruginosa in the meropenem group, treatment was ineffective because of persistent/recurrent abdominal infection that necessitated additional intervention. Both treatments were 100% effective against overexpressed AmpC and MDR P. aeruginosa isolates (Table 2).
Understanding the risk factors associated with P. aeruginosa involvement in cIAI is important for making empirical treatment decisions (11, 12). In this study, nearly 10% of patients had P. aeruginosa infection, consistent with the findings in previous studies (13,–16), and previous antibacterial exposure was more frequent among those with P. aeruginosa. Prophylactic metronidazole and third-generation cephalosporins were common previous treatments, which potentially predisposed patients to P. aeruginosa infection.
All patients in the ME population with P. aeruginosa infection had a 100% clinical cure rate with ceftolozane-tazobactam plus metronidazole. In this study of primarily community-acquired cIAIs, the prevalence of MDR P. aeruginosa was low; nevertheless, ceftolozane-tazobactam had potent in vitro activity against P. aeruginosa (MIC90, 2 μg/ml). Because of the small number of patients in this nonrandomized subgroup analysis, the summary of data might have been subject to bias.
Ceftolozane-tazobactam plus metronidazole was effective in AmpC-overexpressing strains of P. aeruginosa, consistent with in vitro studies that have shown ceftolozane's stability against P. aeruginosa resistance mechanisms, including hydrolysis by AmpC enzymes, upregulation of efflux pumps, and decreases in porin expression (7, 17, 18).
Ceftolozane-tazobactam has been shown to be active against strains of P. aeruginosa that are resistant to carbapenems, piperacillin-tazobactam, cephalosporins, fluoroquinolones, and aminoglycosides, including the majority of MDR isolates (17, 19, 20), with the exception of metallo-β-lactamases. The ASPECT-cIAI findings suggest that ceftolozane-tazobactam will be an important addition to the available antibacterials used in the treatment of cIAIs, especially when P. aeruginosa is implicated.
ACKNOWLEDGMENTS
This study was funded by Merck & Co., Inc., Kenilworth, NJ, USA.
Medical writing and editorial assistance was provided by Tracy T. Cao and Meryl Mandle from ApotheCom, Yardley, PA, USA. This assistance was funded by Merck & Co., Inc.
M.W.P. is an employee and B.M., E.H., and J.N.S. are former employees of Merck & Co., Inc., Kenilworth, NJ, USA. J.A. has participated in advisory boards for Cubist Pharmaceuticals.
We and employees of the study sponsor were involved in the study design, data collection, and interpretation and in the decision to submit the work for publication.
REFERENCES
Articles from Antimicrobial Agents and Chemotherapy are provided here courtesy of American Society for Microbiology (ASM)
Full text links
Read article at publisher's site: https://doi.org/10.1128/aac.03074-15
Read article for free, from open access legal sources, via Unpaywall:
https://aac.asm.org/content/aac/60/7/4387.full.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/aac.03074-15
Article citations
Extensively drug-resistant Pseudomonas aeruginosa: clinical features and treatment with ceftazidime/avibactam and ceftolozane/tazobactam in a tertiary care university hospital center in Portugal - A cross-sectional and retrospective observational study.
Front Microbiol, 15:1347521, 13 Feb 2024
Cited by: 4 articles | PMID: 38414772 | PMCID: PMC10896734
Perspectives on the use of ceftolozane/tazobactam: a review of clinical trial data and real-world evidence.
Future Microbiol, 19(6):465-480, 22 Jan 2024
Cited by: 0 articles | PMID: 38252038 | PMCID: PMC11216532
Review Free full text in Europe PMC
What to Do with the New Antibiotics?
Antibiotics (Basel), 12(4):654, 27 Mar 2023
Cited by: 3 articles | PMID: 37107016 | PMCID: PMC10135159
Review Free full text in Europe PMC
An Update on Eight "New" Antibiotics against Multidrug-Resistant Gram-Negative Bacteria.
J Clin Med, 10(5):1068, 04 Mar 2021
Cited by: 42 articles | PMID: 33806604 | PMCID: PMC7962006
Review Free full text in Europe PMC
The safety of ceftolozane-tazobactam for the treatment of acute bacterial infections: a systemic review and meta-analysis.
Ther Adv Drug Saf, 12:20420986211027096, 15 Jul 2021
Cited by: 1 article | PMID: 34349976 | PMCID: PMC8290504
Go to all (17) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT01445678
- (1 citation) ClinicalTrials.gov - NCT01445665
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Ceftolozane/Tazobactam Plus Metronidazole for Complicated Intra-abdominal Infections in an Era of Multidrug Resistance: Results From a Randomized, Double-Blind, Phase 3 Trial (ASPECT-cIAI).
Clin Infect Dis, 60(10):1462-1471, 10 Feb 2015
Cited by: 199 articles | PMID: 25670823 | PMCID: PMC4412191
Analysis of patients with diabetes and complicated intra-abdominal infection or complicated urinary tract infection in phase 3 trials of ceftolozane/tazobactam.
BMC Infect Dis, 17(1):316, 02 May 2017
Cited by: 9 articles | PMID: 28464828 | PMCID: PMC5414364
Does moderate renal impairment affect clinical outcomes in complicated intra-abdominal and complicated urinary tract infections? Analysis of two randomized controlled trials with ceftolozane/tazobactam.
J Antimicrob Chemother, 72(3):900-905, 01 Mar 2017
Cited by: 12 articles | PMID: 27999024
Ceftolozane/tazobactam for the treatment of complicated intra-abdominal infections.
Expert Opin Pharmacother, 16(2):271-280, 22 Dec 2014
Cited by: 12 articles | PMID: 25529765
Review
Funding
Funders who supported this work.
 b
b 




