Abstract
Free full text

The Growing Threat of Multidrug-Resistant Gram-Negative Infections in Patients with Hematologic Malignancies
Abstract
Prolonged neutropenia and chemotherapy-induced mucositis render patients with hematologic malignancies highly vulnerable to Gram-negative bacteremia. Unfortunately, multidrug-resistant (MDR) Gram-negative bacteria are increasingly encountered globally, and current guidelines for empirical antibiotic coverage in these patients may not adequately treat these bacteria. This expansion of resistance, coupled with traditional culturing techniques requiring 2-4 days for bacterial identification and antimicrobial susceptibility results, have grave implications for these immunocompromised hosts. This review characterizes the epidemiology, risk factors, resistance mechanisms, recommended treatments, and outcomes of the MDR Gram-negative bacteria that commonly cause infections in patients with hematologic malignancies. We also examine infection prevention strategies in hematology patients, such as infection control practices, antimicrobial stewardship, and targeted decolonization. Finally, we assess strategies to improve outcomes of infected patients, including gastrointestinal screening to guide empirical antibiotic therapy, new rapid diagnostic tools for expeditious identification of MDR pathogens, and use of two new antimicrobial agents, ceftolozane/tazobactam and ceftazidime/avibactam.
Introduction
Patients with hematologic malignancies are at high risk of Gram-negative bacteremia because of chemotherapy-induced gastrointestinal mucositis and prolonged periods of neutropenia. Furthermore, they rely on immediate active antibacterial therapy to prevent severe morbidity and mortality when infected with Gram-negative bacteria. Unfortunately, multidrug-resistant (MDR) Gram-negative bacteria are becoming increasingly common pathogens in this vulnerable population [1-5]. Current guidelines and algorithms for empirical treatment of febrile neutropenia may not adequately cover MDR Gram-negative bacteria and identification of these pathogens by culture typically takes 2-4 days [6]. Thus, neutropenic patients with an MDR Gram-negative infection may have long delays until they receive appropriate antimicrobial therapy, which in turn could lead to poor outcomes.
Particularly problematic MDR Gram-negative bacteria that are often resistant to first-line empirical antibacterial therapies include extended-spectrum β-lactamase (ESBL)-, AmpC β-lactamase-, and carbapenemase-producing Enterobacteriaceae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia. The objectives of this review are to summarize what is known about the epidemiology and outcomes of infections due to these MDR Gram-negative bacteria in patients with hematologic malignancies, provide recommendations for treatment, and outline potential strategies to mitigate the threats posed by these pathogens.
Extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E)
The Enterobacteriaceae are a family of bacteria that inhabit the gastrointestinal tract and are the most common causes of Gram-negative bacteremia in patients with hematologic malignancies [7,8]. Prominent pathogens in this family include Escherichia coli, Klebsiella pneumoniae, and Enterobacter species. β-lactamases are enzymes that hydrolyze and inactivate β-lactam antibiotics and are the most common causes of β-lactam resistance among Enterobacteriaceae. ESBLs are specific β-lactamases (Table 1) that are capable of hydrolyzing penicillins, extended-spectrum cephalosporins (e.g., ceftriaxone, ceftazidime, and occasionally cefepime), and aztreonam, but not carbapenems. ESBLs are most commonly identified in E. coli and Klebsiella species.
Table 1
Multidrug-Resistant (MDR) Organism-Specific Resistance Mechanisms and Treatment Recommendations.
| Organism(s) | Resistance Profiles | β-lactam Resistance Mechanisms | Preferred Treatments | Treatment Considerations |
|---|---|---|---|---|
| ESBL-E (most common in E.coli and K. pneumoniae) |
|
|
|
|
| AmpC-E (most common in SPICE organisms) |
|
|
|
|
| CRE (most common in K. pneumoniae) |
|
|
| •High-dose, prolonged infusion carbapenem may be added if MIC ≤8 μg/mL |
| MDR Pseudomonas aeruginosa |
|
|
|
|
| Acinetobacter baumannii |
|
|
|
|
| Stenotrophomonas maltophilia |
|
|
|
|
Abbreviations: ESBL-E , extended-spectrum β-lactamase-producing Enterobacteriaceae; 3rd-gen. ceph., 3rd-generation cephalosporin (e.g., ceftriaxone); AmpC-E, AmpC β-lactamase-producing Enterobacteriaceae; SPICE: Serratia marcescens, Providencia species, indole-positive Proteus species, Citrobacter species, and Enterobacter species; CRE, carbapenem-resistant Enterobacteriaceae; KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; MIC, minimum inhibitory concentration; TMP-SMX, trimethoprim/sulfamethoxazole.
There are numerous reports documenting the emergence of bacteremia due to ESBL-E in patients with hematologic malignancies (Table 2). These reports suggest that in many areas of the world, ESBL-E comprise 17-37% of all bacteremias due to Enterobacteriaceae in this population, and that this incidence is increasing [2,4,9]. Risk factors for ESBL-E bacteremia in patients with hematologic malignancies include recent hospitalizations or antibiotic exposure, intensive care unit (ICU) admissions, and prolonged durations of hospitalization and neutropenia [2,10-14].
Table 2
Studies Characterizing the Prevalence and Associated Mortality Rates of Bloodstream Infections (BSIs) due to Extended-Spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) in Patients with Hematologic Malignancies.
| Reference | Country | Years | Organism(s) | % of Isolates that were ESBL-E | Mortality after ESBL-E BSI vs. non-ESBL-E BSI | Mortality Type |
|---|---|---|---|---|---|---|
| [Guidol 2010] | Spain | 2006-2009 | Escherichia coli | 17% (13/76) | 38% vs. 7%1 | 30-day |
| [Cornejo-Jaurez 2012] | Mexico | 2004-2009 | Escherichia coli | 31% (205/760)2 | 34% vs. 19%1 | Overall |
| [Kang 2012] | Korea | 2008-2009 | Escherichia. coli Klebisella pneumoniae | 24% (37/156) | 45% vs. 14%1 | 30-day |
| Metan [2013] | Turkey | 2006-2011 | Enterobacteriaceae | 33% (40/120) | 13% vs. 16% | 7-day |
| Ha [2013] | Korea | 2010-2012 | Escherichia coli | 32% (42/130) | 14% vs. 10% | 30-day |
| Kim [2013] | Korea | 2007-2008 | Escherichia coli Klebisella pneumoniae | 26% (26/101) | 15% vs. 5% | 30-day |
| Trecarichi [2015] | Italy | 2009-2012 | Enterobacteriaceae | 37% (98/265)3 | 26% vs. 5%1 | 21-day |
Reported mortality rates after ESBL-E bacteremia in patients with hematologic malignancies range from 13%-45% (Table 2) [2,7,10-12,14-16]. The majority of studies note an increased mortality rate after ESBL-E bacteremia compared to non-ESBL-E bacteremias in this population [2,7,10,11]. Importantly, inappropriate initial antibiotic therapy in ESBL infection has repeatedly been shown to be a risk factor for increased mortality [2,10,11,17].
Current guidelines for the management of initial fever and neutropenia in patients with hematologic malignancies recommend empirical therapy with cefepime, piperacillin-tazobactam (PTZ), meropenem, or imipenem [6]. Many centers also use ceftazidime for primary empirical therapy. Although ESBL-E are typically susceptible to carbapenems, the majority are resistant to ceftazidime, 30-40% are resistant to cefepime, and 5-30% are resistant to PTZ [18,19]. Even when ESBL-E test susceptible to cefepime or PTZ, clinical data in non-neutropenic patients suggest that infections due to ESBL-E may not respond as well to these agents as compared to carbapenems. A propensity score-matched, observational study of ESBL-E bacteremias revealed that patients treated with cefepime were more likely to have a clinical or microbiological failure and 30-day mortality than those who received carbapenem therapy [20]. With regards to PTZ, a recent observational study of patients with ESBL-E bacteremia demonstrated a nearly two-fold increase in the risk of death when PTZ was used empirically instead of a carbapenem, despite the fact all isolates were susceptible to both PTZ and carbapenems [21].
One potential explanation for the increase in mortality seen with cefepime and PTZ is that the minimum concentrations of these antibiotics required to inhibit growth of ESBL-E increase when the number of organisms inoculated is increased [22]. This inoculum effect is not seen when carbapenems are tested against ESBL-E. Based on current data, carbapenems remain the preferred agents for the treatment of ESBL-E bacteremias in neutropenic patients, regardless of reported cefepime or PTZ susceptibility results. Carbapenems should also be considered as empirical therapies in neutropenic patients known to be colonized or previously infected with ESBL-E or at institutions where rates of ESBL-E bacteremias are particularly high.
AmpC β-lactamase-producing Enterobacteriaceae (AmpC-E)
Like ESBLs, AmpC β-lactamases are capable of inactivating penicillins and most cephalosporins, but not carbapenems. Unlike ESBLs, they are not effectively inhibited by β-lactamase inhibitors, such as clavulanate and tazobactam [23]. Enterobacteriaceae that most commonly harbor AmpC β-lactamases are often referred to as the SPICE organisms (Serratia marcescens, Providencia, indole-positive Proteus, Citrobacter, and Enterobacter species). These organisms, particularly Enterobacter species, frequently possess chromosomal AmpC β-lactamases genes that are often expressed at only a low level. However, the expression of these enzymes can be markedly upregulated upon exposure to β-lactam antibiotics. Thus, SPICE organisms may initially test susceptible to third-generation cephalosporins, such as ceftriaxone or ceftazidime, but subsequently develop resistance to these antibiotics during therapy due to inducible expression of these enzymes [24,25].
Data regarding the incidence of AmpC-E infections in patients with hematologic malignancies are limited because most clinical microbiology laboratories do not perform phenotypic or genotypic testing to detect AmpC. However, recent studies demonstrate that Enterobacter spp. (which typically have AmpC β-lactamases) cause 5-8% of Gram-negative bacteremias in patients with hematologic malignancies, making them the 4th most common cause of Gram-negative bacteremia in this population [1,7,15,26].
As with ESBL-E infections, carbapenems are generally considered the first-line treatment option for serious infections due to AmpC-E because they are stable to hydrolysis by most AmpC enzymes and do not exhibit an inoculum effect [27]. However, no randomized trials have been conducted to definitively determine the optimal therapy [23]. Penicillins and 3rd-generation cephalosporins, such as ceftriaxone and ceftazidime, should not be used as targeted therapy to treat these infections, because 8-19% of patients who receive these therapies for AmpC-E infections will up-regulate AmpC expression and develop resistance on therapy [24,25]. Cefepime, a 4th-generation cephalosporin, may have a role in the treatment of AmpC-E infections, as it has relative stability against AmpC β-lactamases compared to other cephalosporins. Two observational studies of AmpC-E infections showed no differences in outcomes between patients treated with either carbapenems or cefepime [28,29]. The role of PTZ in the treatment of serious AmpC-E infections is not well established. Tazobactam does not efficiently inhibit the AmpC enzyme, but limited observational data suggest that PTZ has similar effectiveness to carbapenems when AmpC-E test susceptible to PTZ [30,31]. Fluoroquinolones, which are not affected by AmpC enzymes, are another option in treating AmpC-E infections. A recent meta-analysis demonstrated favorable outcomes with fluoroquinolones for this indication [31].
Carbapenem-resistant Enterobacteriaceae (CRE)
Over the last decade, there has been a worldwide emergence of Enterobacteriaceae that are not only resistant to cephalosporins, but are also resistant to carbapenems. In areas with high rates of CRE, the most common resistance mechanism is the presence of a carbapenemase, an enzyme capable of hydrolyzing and inactivating carbapenems and all other β-lactam agents (Table 1) [32-34]. These enzymes are also stable against commonly used β-lactamase inhibitors, such as tazobactam, and are most commonly found in Klebsiella pneumoniae. The genes that encode for carbapenemases are typically located on plasmids, and these genes can be transferred both within bacterial species and across different species and genera. These plasmids also frequently carry genes conferring resistance to other antibiotic classes, such as fluoroquinolones and aminoglycosides, leaving few treatment options.
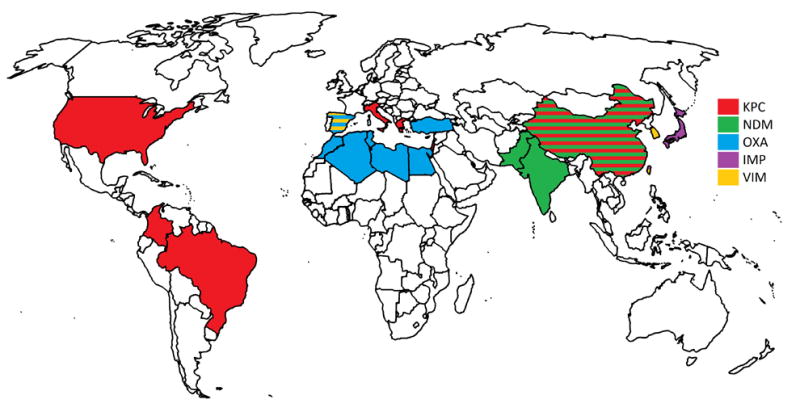
Different carbapenemases predominate in different geographical areas (Figure 1). K. pneumoniae carbapenemase (KPC) is common in the United States, South America, Italy, Greece, Israel, and China, whereas New Delhi metallo-β-lactamases (NDM) predominate in India and Pakistan, and OXA-48-type carbapenemases predominate in Mediterranean Europe, North Africa, and Turkey [32,35].
The epidemiology of CRE infections in patients with hematologic malignancies has only recently been investigated. In the general population, prior exposures to many classes of antibiotics, not just carbapenems, are risk factors for CRE infection [36-38]. In a study of neutropenic patients with hematologic malignancies, exposures to β-lactam/β-lactamase inhibitors, trimethoprim-sulfamethoxazole, glucocorticoids, and having a prior culture that grew CRE were independent risk factors for CRE bacteremia [39].
The overall reported mortality rates after CRE infections in patients with hematologic malignancies have ranged from 44-72% [32,33,40,41]. Furthermore, the majority of deaths in these studies were related to the CRE infections. Two factors likely contribute to these exceptionally high mortality rates. First, it typically takes 2-3 days to detect CRE from blood cultures using traditional microbiologic methods, and most patients do not receive CRE-active therapy during this time. Second, the treatment options for CRE infections are extremely limited because of their extensive resistance profiles.
Antibiotics that have retained activity against CRE include polymyxins (colistin and polymyxin B), tigecycline, fosfomycin, and occasionally aminoglycosides. Unfortunately, each of these options has major limitations (Table 3). The polymyxins have considerable nephrotoxicity and neurotoxicity, are less effective than β-lactam agents for the treatment of Gram-negative bacteremia in oncology patients, and resistance to polymyxins is increasingly identified among CRE [42,43,44]. Tigecycline has low bloodstream and urinary concentrations and its use has been associated with increased mortality in randomized trials [45]. Fosfomycin is not available as an intravenous formulation in the U.S., and resistance can develop quickly on therapy [46]. Similar to the polymyxins, aminoglycosides are limited by nephrotoxicity and historical data suggest that aminoglycoside monotherapy is associated with comparatively poor outcomes after Gram-negative bacteremia in neutropenic patients [47]. Aminoglycosides also carry a risk of otovestibular toxicity. Finally, aminoglycosides are not reliably active against CRE, as almost all are resistant to tobramycin and approximately one-half are resistant to gentamicin and amikacin [48,49,50,51].
Table 3
Characteristics of agents with activity against MDR Gram-negative bacteria
| Antimicrobial Agent | Mechanism of Action | Dosage1 | Targeted Organisms | Limitations |
|---|---|---|---|---|
| Carbapenems | ||||
 Meropenem, Imipenem, and Doripenem (IV) Meropenem, Imipenem, and Doripenem (IV) | Inhibits bacterial cell wall synthesis by binding to PBPs |
|
|
|
| Ceftazidime-avibactam (IV) |
| 2.5 gm every 8 h |
|
|
| Ceftolozane-tazobactam (IV) |
|
| MDR P. aeruginosa |
|
| Polymyxins | ||||
 Colistin (IV) Colistin (IV) | Binds to LPS and phospholipids in the outer cell membrane, leading to leakage of intracellular contents |
|
|
|
 Polymyxin B (IV) Polymyxin B (IV) | Same as colistin | 1.5–2.5 mg/kg (15 000–25 000 units) per day | Same as colistin | Same as colistin, except also achieves low concentrations in the urinary tract |
| Aminoglycosides | ||||
 Gentamicin and Tobramycin (IV) Gentamicin and Tobramycin (IV) | Bind to 16S rRNA portion of the 30S ribosomal subunit, blocking mRNA translation. |
|
|
|
 Amikacin (IV) Amikacin (IV) | Same as gentamicin |
| Same as gentamicin, but less active vs. CRE | Same as gentamicin, but less nephrotoxicity and ototoxicity [50] |
| Tigecycline (IV) | Binds to the 30S ribosomal subunit, blocking the binding of tRNA | 100 mg loading dose, followed by 50 mg every 12 h |
|
|
| Minocycline (IV and oral) | Similar to tigecycline | 200 mg loading dose, followed by 100 mg every 12 h |
|
|
| Fosfomycin | Inhibits peptidoglycan (and thus cell wall) synthesis |
|
|
|
| Trimethoprim-sulfamethoxazole (IV and oral) | Inhibits synthesis of folic acid at 2 different steps | 10-15 mg/kg trimethoprim component per day, in divided doses, every 8-12 h | Stenotrophomonas maltophilia |
|
Abbreviations: IV, intravenous; LPS, lipopolysaccharide; CBA, colistin base activity; IU, international units; CRE, carbapenem-resistant Enterobacteriaceae; MDR, multidrug-resistant; ESBL-E, ESBL-producing Enterobacteriaceae; AmpC-E; AmpC β-lactamase-producing Enterobacteriaceae; UTIs, urinary tract infections; PBP, Penicillin-binding protein; Klebsiella pneumoniae carbapenemase.
The optimal therapeutic regimen for CRE infections has yet to be identified, as no large randomized clinical trials comparing treatment options have been completed. Observational studies of CRE bacteremia in the general population suggest that combination therapy with at least two antibiotics to which the infecting organism tests susceptible is more effective than monotherapy [52-55]. These studies also demonstrate that adjunctive therapy with a carbapenem, in combination with active agents, may be associated with decreased mortality despite the presence of carbapenemases. These improved outcomes were generally observed when high doses and prolonged infusions of carbapenems were used (e.g., 2 gm. of meropenem infused over 3 hours, every 8 hours) and when the minimum inhibitory concentration (MIC) of the carbapenem was ≤8 mg/L [52,55].
Ceftazidime/avibactam is a new agent with potent in vitro activity against KPC-producing, but not NDM-producing, Enterobacteriaceae. This compound was recently approved in the U.S. for complicated intra-abdominal and urinary tract infections and represents the first approved β-lactam/β-lactamase inhibitor with activity against KPC-producing CRE [56]. However, clinical trials that led to approval of this agent enrolled very few patients with CRE infection or patients with hematologic malignancies [57]. Data are urgently needed to assess the effectiveness of this promising compound for the treatment of CRE infections, particularly in immunocompromised hosts, such as patients with hematologic malignancies.
MDR Pseudomonas aeruginosa
In a previous era where empirical antibacterial therapy was withheld until positive culture results in neutropenic patients with hematologic malignancies, mortality rates after Pseudomonas aeruginosa bacteremia was 50% after 3 days and 70% after 7 days [58]. Thus, recommendations for empirical antibacterial therapy in this population have largely focused on ensuring immediate coverage against P. aeruginosa to improve outcomes in infected patients [6]. P. aeruginosa remains the second or third most common cause of bacteremia in patients with hematologic malignancies [1,7,15,26,59]. Unfortunately, many P. aeruginosa have now developed resistance to recommended anti-pseudomonal β-lactam agents for fever and neutropenia. This resistance is mediated by a number of mechanisms (Table 1), including the production of β-lactamases, changes to porins that permit passage of β-lactams through the bacterial outer membrane, and efflux pumps that remove β-lactams and agents of other antibiotic classes [60]. Although resistance rates vary geographically, a recent multicenter study of bloodstream infections (BSIs) in patients with hematologic malignancies in Italy found that only 45% of P. aeruginosa were susceptible to ceftazidime, 58% to piperacillin-tazobactam, and 29% to meropenem [7]. Not surprisingly, outcomes of patients infected with these MDR strains of P. aeruginosa were poor (the 21-day mortality was 42%, compared to 13% for non-MDR strains) and inappropriate empirical therapy was associated with increased mortality.
Given these resistance patterns and outcomes, empirical therapy for P. aeruginosa infections in patients with hematologic malignancies should probably include both an antipseudomonal β-lactam and an aminoglycoside (or a fluoroquinolone if this agent is not being used prophylactically) prior to availability of susceptibility data. Once susceptibilities are known, therapy can often be tailored to a single active β-lactam agent, as most observational studies have not identified a benefit to combination therapy for P. aeruginosa bacteremia in patients with cancer [47,61]. However, it should be noted that P. aeruginosa develops resistance on therapy in approximately 10% of cases [62]. This most commonly occurs during treatment of pneumonia, where the organism burden is highest, and when carbapenems are used as monotherapy [63,64]. Although in vitro data suggest that combination therapy can prevent the emergence of resistance, there is currently no clinical data to support this practice [65,66].
Prolonged infusion of β-lactam agents (dosing the antibiotic over hours instead of over 30 minutes) has also been shown to decrease the emergence of resistance on therapy in vitro [67]. Additionally, two single-center observational studies demonstrated decreased mortality in critically ill patients with Pseudomonas aeruginosa infections after switching from a standard infusion of PTZ and cefepime to prolonged infusions of these agents [68,69]. Although no randomized clinical trials have been conducted to definitively support this practice, it is reasonable to use prolonged infusion β-lactam regimens for P. aeruginosa infections in patients with hematologic malignancies, provided that venous access is sufficient.
P. aeruginosa that are resistant to cephalosporins, PTZ, and carbapenems have historically been treated with polymyxins or aminoglycosides as agents of last resort. However, these compounds have high toxicity rates and are associated with poor outcomes in oncology patients with Pseudomonas aeruginosa bacteremia compared to β-lactam agents [43,47]. Two new cephalosporin/β-lactamase inhibitor combinations were recently approved in the U.S. that offer a potential advance in therapy for patients infected by these MDR P. aeruginosa strains: ceftolozane/tazobactam and ceftazidime/avibactam. Of P. aeruginosa that are resistant to ceftazidime, PTZ, and meropenem, approximately 70% are susceptible to ceftolozane/tazobactam and ceftazidime/avibactam [70,71]. However, these antibiotics have only been approved in the U.S. for complicated intra-abdominal and urinary tract infections. Both agents currently lack clinical data for treatment of BSIs, infections due to carbapenem-resistant organisms, or infections in patients with hematologic malignancies. However, given the major limitations of polymyxin and aminoglycoside monotherapy for the treatment of MDR P. aeruginosa infections, their use should be considered when treatment options are limited.
Acinetobacter baumannii
Acinetobacter baumannii is a Gram-negative coccobacillus that primarily causes infection in hospitalized and immunocompromised patients [72]. It is classically associated with hospital-acquired pneumonia, but can also be found in bloodstream, skin, urinary tract, and intraabdominal infections. The prevalence of A. baumannii infection in patients with hematologic malignancies is highly dependent on geographical location. Studies from Western Europe report that A. baumannii causes <2% of Gram-negative bacteremias in this population [1,3]. In contrast, studies from New York City and Turkey report that 9% and 14%, respectively, of Gram-negative bacteremias in patients with hematologic malignancies are caused by Acinetobacter species [26,73].
A. baumannii is frequently an extensively drug-resistant pathogen. Like P. aeruginosa, it has both intrinsic and acquired mechanisms of resistance, including β-lactamases, outer membrane protein changes, and efflux pumps (Table 1) [74]. An international surveillance study of over 5,000 A. baumannii clinical isolates demonstrated that only 22% were susceptible to cefepime, 18% to PTZ, and 36% to meropenem [75]. The most frequently active agents were minocycline (79% susceptible) and colistin (99% susceptible) for MDR A. baumannii infections [76].
Given its extensive resistance to first-line therapies for fever and neutropenia, it is not surprising that A. baumannii bacteremia in patients with hematologic malignances is associated with exceptionally high mortality rates [77,78]. Carbapenem resistance, pneumonia as the source of bacteremia, and inappropriate antimicrobial therapy have been shown to be independent risk factors for mortality in MDR A. baumannii infections [78]. Polymyxin-based combination therapies and minocycline are frequently used to treat these infections, and ampicillin-sulbactam (only the sulbactam portion has activity) and tigecycline are also potential therapies [79,80]. However, the optimal therapies for MDR A. baumannii have not been clearly elucidated.
Stenotrophomonas maltophilia
Stenotrophomonas maltophilia is a globally emerging MDR Gram-negative bacteria that is frequently found in the environment, such as water, soil, and plants [81]. It is an increasingly common cause of respiratory tract and other invasive infections in hospitalized patients, particularly in immunocompromised hosts. It is intrinsically resistant to carbapenems and thus should be considered as a potential pathogen in patients with hematologic malignancies who develop sepsis while receiving carbapenem therapy (Table 1). It also frequently carries other genetic determinants that lead to resistance to other β-lactams, fluoroquinolones, aminoglycosides, and tetracyclines, and has a propensity to form biofilms on vascular catheters and prosthetic material [82].
S. maltophilia causes 2-7% of Gram-negative bacteremias in patients with hematologic malignancies [7,15,83-85]. Neutropenia and having a hematologic malignancy are both risk factors for S. maltophilia infections [86]. Other notable risk factors include the presence of an indwelling catheter, broad-spectrum antibiotic exposure, and prolonged hospitalization, all common occurrences in patients with hematologic malignancies [15,87,88]. Two observational studies of patients with hematologic malignancies who developed S. maltophilia bacteremia demonstrated attributable mortality rates of 24% and 38%, respectively, rates comparable to those of P. aeruginosa bacteremia [89,90]. Severe neutropenia, pneumonia, and severe sepsis were all independent risk factors for mortality.
Trimethoprim/sulfamethoxazole (TMP/SMX) is the preferred therapy for S. maltophilia infections and approximately 90% of isolates are susceptible [81,90,91]. Fluoroquinolones, most commonly levofloxacin, are considered alternatives to TMP/SMX therapy. Observational studies have shown similar outcomes between levofloxacin and TMP-SMX for S. maltophilia infections [92,93]. However, resistance to levofloxacin appears to be increasing, particularly in the setting of fluoroquinolone prophylaxis, which limits its use as empirical therapy [93,94]. Other potential treatment options include tigecycline and minocycline [85,95].
Preventing MDR Gram-negative Bacterial Infections
Infection Control Practices
The limited therapeutic options and poor outcomes associated with MDR Gram-negative bacterial infections in patients with hematologic malignancies underscore the importance of preventing these infections. The primary goal of infection prevention efforts is to decrease the risk of acquiring MDR Gram-negative pathogens among patients who are located on an oncology ward. Recommended infection prevention strategies that are most pertinent to preventing acquisition of MDR Gram-negatives are ensuring strict adherence to hand hygiene, environmental cleaning and decontamination practices, use of contact precautions for patients known to be colonized or infected with MDR Gram-negative bacteria, and placing particularly high-risk patients, such as HSCT recipients, in private rooms [96,97]. Although the effectiveness of each of these strategies in preventing MDR Gram-negative infections on hematologic oncology wards are unclear, they appear to be effective when implemented together as a bundled intervention [98]. Active surveillance, where patients are screened for colonization with MDR Gram-negative bacteria and subsequently placed on contact precautions if found to be colonized, is another tool to decrease transmission. This approach has been successfully implemented in non-hematology settings to decrease the incidence of carbapenem-resistant Gram-negative bacteria [99,100]. However, few studies have attempted to assess the role of active surveillance in preventing transmission of MDR Gram-negative bacteria on hematology wards.
Antimicrobial stewardship also plays an important role in preventing MDR Gram-negative bacterial infections in this population. The use of broad-spectrum antimicrobial agents, particularly β-lactams, is consistently identified as an independent risk factor for MDR Gram-negative infections in oncology patients [10,32,101]. Effective antimicrobial stewardship in oncology programs requires close collaborations between oncologists, infectious diseases physicians, microbiologists, clinical pharmacists, and infection preventionists [102]. Local epidemiology and multidisciplinary expertise should be utilized to develop and implement protocols and treatment algorithms for common scenarios, such as a febrile neutropenia or sepsis. Other recommended practices are de-escalation of broad-spectrum antibacterial treatment once a non-MDR pathogen is identified, optimization of antibacterial dosages, and daily assessments for the need for continued antibacterial therapy [96].
Screening for Targeted Decolonization
In addition to using active surveillance to place colonized patients on contact precautions, another potential use of identifying colonized patients is that they may be candidates for targeted decolonization strategies. The role for decolonization as a means to prevent future infection in patients screening positive for MDR Gram-negative bacteria is unclear. Given their predominance in the gastrointestinal tract, selective digestive decontamination (SDD) using oral aminoglycosides and/or colistin has been best evaluated as means to prevent CRE infections in colonized patients. The largest cohort study investigating SDD in patients with CRE colonization, however, only achieved a 44% eradication rate [103]. Importantly, 16% of isolates treated with monotherapy developed resistance to the oral antibiotic that was used. This development of resistance following SDD has been documented in numerous others studies [104-106]. SDD has also not been shown to prevent infections in colonized oncology patients. In a study of 15 patients undergoing HSCT who were colonized with carbapenem-resistant Klebsiella pneumoniae (CRKP), eight patients developed post-transplant CRKP bacteremia despite receiving SDD with oral gentamicin [107]. Given the already narrow arsenal available to treat MDR Gram-negative infections, the risk of increasing resistance to the few available treatment options and unclear clinical benefit has limited the adoption of SDD in hematology units.
Improving Outcomes Once Infected
Screening for Targeted Empiric Antibiotic Therapy
The emergence of MDR Gram-negative bacteria that are often resistant to first-line empirical therapies warrants consideration of strategies to identify patients with hematologic malignancies who are at high risk of developing infections due to these organisms. These patients could potentially have their empirical therapy modified to ensure coverage of the MDR bacteria that they are at high risk of being infected with.
This strategy appears to offer the most promise for identifying hematologic oncology patients at high risk of infection with MDR Enterobacteriaceae. In a multicenter prospective study examining stool samples or rectal swabs from patients with hematologic malignancies in Germany, 4 (7%) of the 55 patients colonized with ESBL-E developed ESBL-E BSI, compared to only 1 (0.2%) of 442 patients who were not colonized with ESBL-E [108]. A multivariate analysis showed that ESBL-E colonization was the most important risk factor for ESBL-E BSI. Other investigators found that 22% of patients with hematologic malignancies who were colonized with ESBL-producing E. coli (ESBL-EC) developed subsequent ESBL-EC bacteremia [109]. A multicenter study of HSCT recipients in Italy also identified high rates of colonization to infection for CRKP. CRKP colonization was identified in 1% of autologous HSCT recipients and 2.4% of allogeneic HSCT recipients [33]. CRKP infection occurred after transplantation in 26% and 39% of colonized autologous and allogeneic HSCT recipients, respectively.
These data suggest that patients with hematologic malignancies who are colonized with MDR Enterobacteriaceae have a high risk of developing subsequent infection due to these organisms. Thus, hematologic oncology centers with high rates of infections due to MDR Enterobacteriaceae may consider implementing a program of screening for gastrointestinal colonization with these organisms and modifying the empirical therapy of colonized patients. However, more data are needed on the benefits and risks of initiating such a surveillance program before this strategy can be strongly recommended. Another caveat is that assessing for gastrointestinal colonization may not be an effective method to identify patients at high risk of P. aeruginosa infection. In one study of allogeneic HSCT recipients, the majority of patients who developed P. aeruginosa infection were not found to be previously colonized by assessing fecal samples, suggesting that many of these infections originated outside of the gastrointestinal tract [110].
Rapid Identification
Another important strategy to improve outcomes of patients with hematologic malignancies who are infected with MDR Gram-negative bacteria is to implement new technologies in the clinical microbiology laboratory to more rapidly identify these pathogens. This in turn could lead to shorter delays in administration of effective antimicrobial therapy. Two real-time multiplex PCR systems were recently approved in the U.S. that detect a variety of bacteria and yeast, plus important resistance determinants, directly from positive blood culture bottles. Both systems, the Verigene® Gram-Negative Blood Culture Test (Nanosphere, Northbrook IL, USA) and the FilmArray Blood Culture Identification Panel (Biofire Diagnostics, bioMérieux, Salt Lake City, UT, USA), detect the most common Gram-negative pathogens, including E. coli, K. pneumoniae, Enterobacter spp., P. aeruginosa, and A. baumannii. Both systems also detect the KPC gene that confers carbapenem resistance among Enterobacteriaceae in the U.S. and many other countries. Additionally, the Verigene platform detects other carbapenemase genes and the most common ESBL gene, CTX-M.
Use of these systems should decrease the time to identification of most Gram-negative bloodstream pathogens from 24-72 hours after culture positivity to 2 hours. They would also permit rapid detection of CRE bacteremia, which should lead to more timely therapy for these lethal infections. However, these assays are unlikely to provide susceptibility information for P. aeruginosa or A. baumannii that are detected and they do not detect Stenotrophomonas maltophilia. Clinical data to assess the impact of the use of these systems on clinical outcomes are also limited. One randomized study that was conducted on all positive blood cultures in a clinical microbiology laboratory showed that use of the FilmArray system decreased the use of broad-spectrum antimicrobials, but had no impact on mortality or length of stay [111]. More data are needed to better understand the potential benefits of these powerful tools in providing more timely appropriate therapy to patients with hematologic malignancies and ultimately improving outcomes in these patients.
Conclusions
This review summarizes the current understanding of the epidemiology, recommended treatments, outcomes, and preventative strategies for infections due to MDR Gram-negative bacteria in patients with hematologic malignancies. Given the expanding nature of this threat and this particularly vulnerable patient population, there is a critical need to identify the optimal strategies to prevent these infections and improve the outcomes of patients with these infections.
Acknowledgments
This review was supported in part by the National Institute of Allergy and Infectious Diseases (T32 AI007613; K23 AI114994) and the National Center for Advancing Translational Sciences (UL1 TR000457) of the National Institutes of Health.
Footnotes
Potential conflicts of interest: Disclosure forms provided by the authors are available with the full text of this article at ---
References
Full text links
Read article at publisher's site: https://doi.org/10.1080/10428194.2016.1193859
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc5027842?pdf=render
Citations & impact
Impact metrics
Article citations
Multidrug-Resistant Bacteria in Immunocompromised Patients.
Pharmaceuticals (Basel), 17(9):1151, 30 Aug 2024
Cited by: 0 articles | PMID: 39338313 | PMCID: PMC11434862
Review Free full text in Europe PMC
An Emergent Change in Epidemiologic and Microbiological Characteristics of Bloodstream Infections in Adults With Febrile Neutropenia Resulting From Chemotherapy for Acute Leukemia and Lymphoma at Reference Centers in Chile, Ecuador, and Peru.
Open Forum Infect Dis, 11(3):ofae052, 01 Feb 2024
Cited by: 0 articles | PMID: 38444817 | PMCID: PMC10913838
Increasing trends of colistin resistance in patients at high-risk of carbapenem-resistant Enterobacteriaceae.
Ann Med, 54(1):1-9, 01 Dec 2022
Cited by: 9 articles | PMID: 36373970 | PMCID: PMC9668277
Comparison between hospital- and community-acquired septic shock in children: a single-center retrospective cohort study.
World J Pediatr, 18(11):734-745, 23 Jun 2022
Cited by: 0 articles | PMID: 35737181 | PMCID: PMC9556399
Factors Associated with Gram-Negative Bacteremia and Mortality in Neutropenic Patients with Hematologic Malignancies in a High-Resistance Setting.
Infect Dis Clin Microbiol, 4(2):87-98, 13 Jun 2022
Cited by: 0 articles | PMID: 38633337 | PMCID: PMC10985816
Go to all (27) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Third generation cephalosporin resistant Enterobacteriaceae and multidrug resistant gram-negative bacteria causing bacteremia in febrile neutropenia adult cancer patients in Lebanon, broad spectrum antibiotics use as a major risk factor, and correlation with poor prognosis.
Front Cell Infect Microbiol, 5:11, 12 Feb 2015
Cited by: 44 articles | PMID: 25729741 | PMCID: PMC4325930
Multidrug-resistant bacteria in hematology patients: emerging threats.
Future Microbiol, 11:767-780, 19 May 2016
Cited by: 11 articles | PMID: 27196948
Review
The place of ceftazidime/avibactam and ceftolozane/tazobactam for therapy of haematological patients with febrile neutropenia.
Int J Antimicrob Agents, 57(6):106335, 07 Apr 2021
Cited by: 8 articles | PMID: 33838223
Treatment of infections caused by multidrug-resistant Gram-negative bacteria: report of the British Society for Antimicrobial Chemotherapy/Healthcare Infection Society/British Infection Association Joint Working Party.
J Antimicrob Chemother, 73(suppl_3):iii2-iii78, 01 Mar 2018
Cited by: 127 articles | PMID: 29514274
Review
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR000457
NIAID NIH HHS (2)
Grant ID: K23 AI114994
Grant ID: T32 AI007613
National Center for Advancing Translational Sciences (1)
Grant ID: UL1 TR000457
National Institute of Allergy and Infectious Diseases (1)
Grant ID: K23 AI114994,T32 AI007613