Abstract
Free full text

Epidermal growth factor receptor binding is affected by structural determinants in the toxin domain of transforming growth factor-alpha-Pseudomonas exotoxin fusion proteins.
Abstract
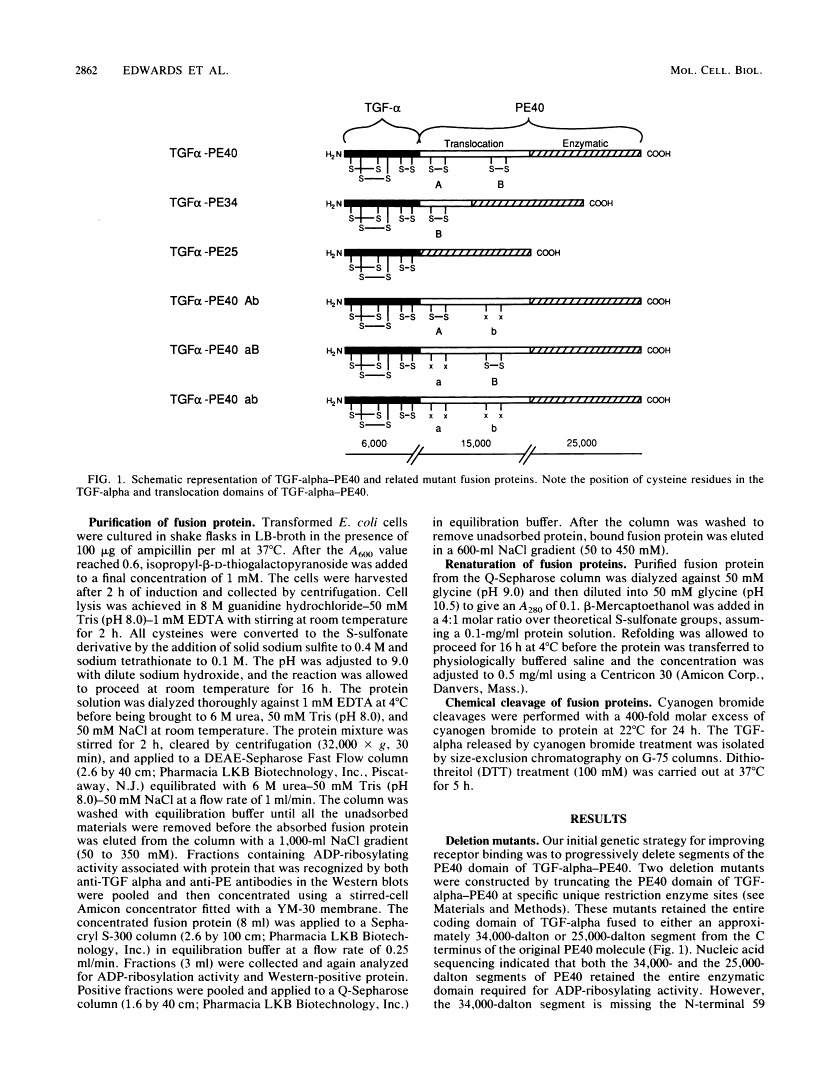
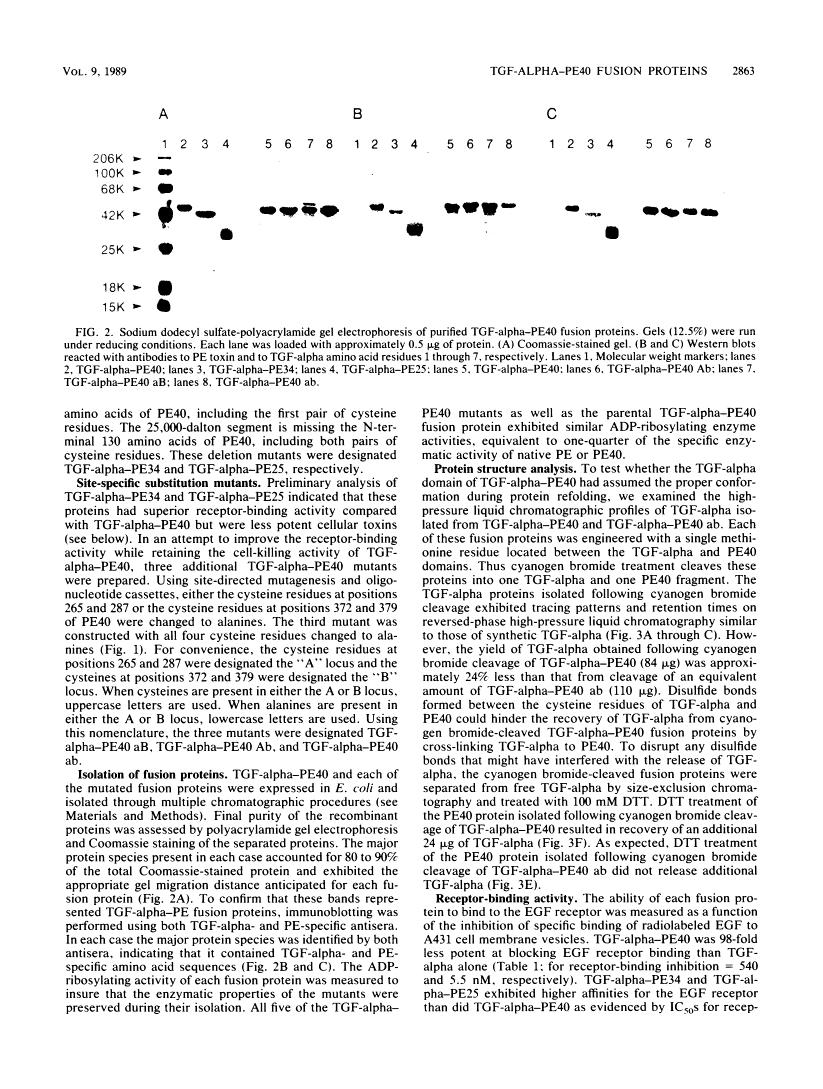
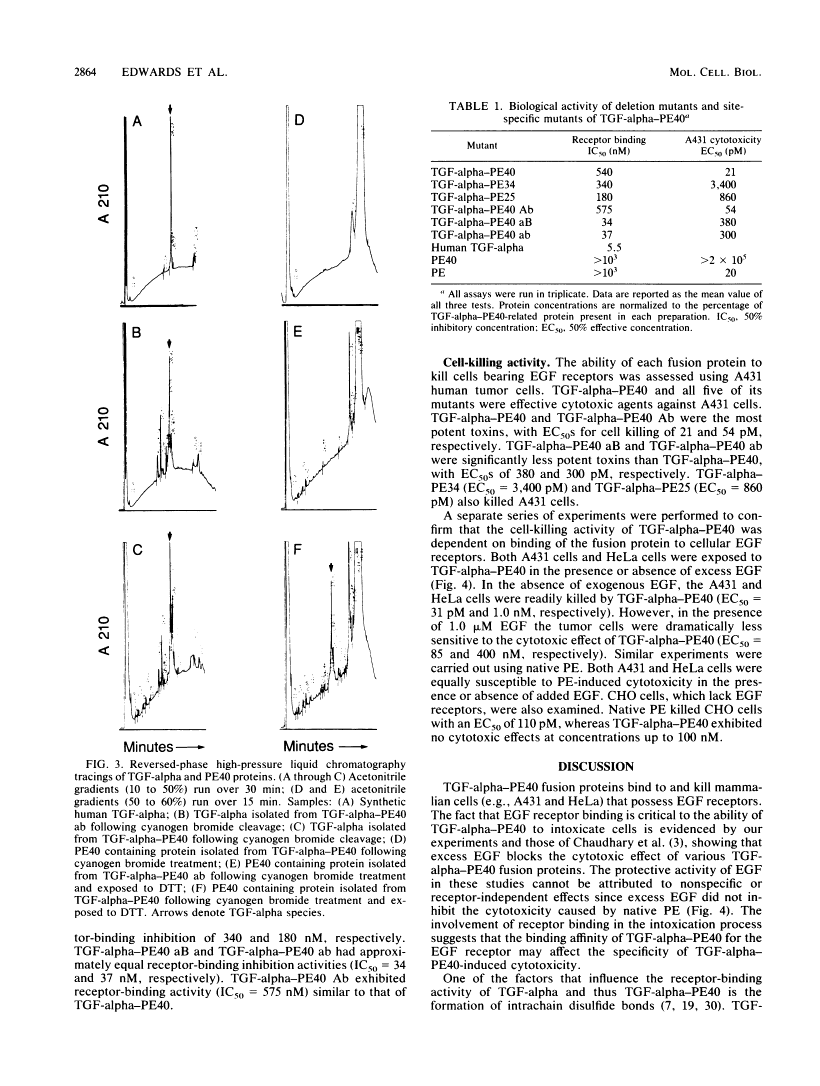
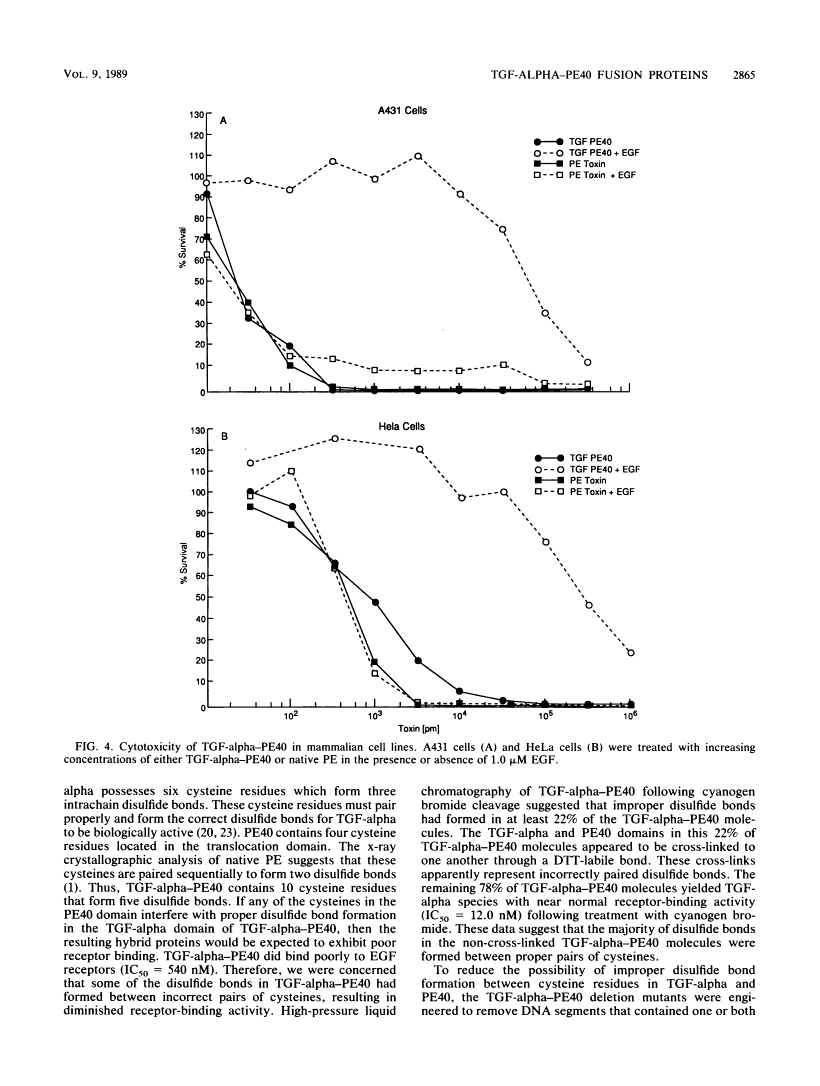
TGF-alpha-PE40 is a hybrid protein composed of transforming growth factor-alpha (TGF-alpha) fused to a 40,000-dalton segment of Pseudomonas exotoxin A (PE40). This hybrid protein possesses the receptor-binding activity of TGF-alpha and the cell-killing properties of PE40. These properties enable TGF-alpha-PE40 to bind to and kill tumor cells that possess epidermal growth factor (EGF) receptors. Unexpectedly, TGF-alpha-PE40 binds approximately 100-fold less effectively to EGF receptors than does native TGF-alpha (receptor-binding inhibition IC50 = 540 and 5.5 nM, respectively). To understand the factors governing receptor binding, deletions and site-specific substitutions were introduced into the PE40 domain of TGF-alpha-PE40. Removal of the N-terminal 59 or 130 amino acids from the PE40 domain of TGF-alpha-PE40 improved receptor binding (IC50 = 340 and 180 nM, respectively) but decreased cell-killing activity. Substitution of alanines for cysteines at positions 265 and 287 within the PE40 domain dramatically improved receptor binding (IC50 = 37 nM) but also decreased cell-killing activity. Similar substitutions of alanines for cysteines at positions 372 and 379 within the PE40 domain did not significantly affect receptor-binding or cell-killing activities. These studies indicate that the PE40 domain of TGF-alpha-PE40 interferes with EGF receptor binding. The cysteine residues at positions 265 and 287 of PE40 are responsible for a major part of this interference.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (1.4M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Click on the image to see a larger version.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured VS, Collier RJ, Carroll SF, McKay DB. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. [Europe PMC free article] [Abstract] [Google Scholar]
- Chaudhary VK, FitzGerald DJ, Adhya S, Pastan I. Activity of a recombinant fusion protein between transforming growth factor type alpha and Pseudomonas toxin. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4538–4542. [Europe PMC free article] [Abstract] [Google Scholar]
- Chaudhary VK, Xu YH, FitzGerald D, Adhya S, Pastan I. Role of domain II of Pseudomonas exotoxin in the secretion of proteins into the periplasm and medium by Escherichia coli. Proc Natl Acad Sci U S A. 1988 May;85(9):2939–2943. [Europe PMC free article] [Abstract] [Google Scholar]
- Chung DW, Collier RJ. Enzymatically active peptide from the adenosine diphosphate-ribosylating toxin of Pseudomonas aeruginosa. Infect Immun. 1977 Jun;16(3):832–841. [Europe PMC free article] [Abstract] [Google Scholar]
- Cohen S, Ushiro H, Stoscheck C, Chinkers M. A native 170,000 epidermal growth factor receptor-kinase complex from shed plasma membrane vesicles. J Biol Chem. 1982 Feb 10;257(3):1523–1531. [Abstract] [Google Scholar]
- Defeo-Jones D, Tai JY, Wegrzyn RJ, Vuocolo GA, Baker AE, Payne LS, Garsky VM, Oliff A, Riemen MW. Structure-function analysis of synthetic and recombinant derivatives of transforming growth factor alpha. Mol Cell Biol. 1988 Aug;8(8):2999–3007. [Europe PMC free article] [Abstract] [Google Scholar]
- Feinberg AP, Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. [Abstract] [Google Scholar]
- Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. [Abstract] [Google Scholar]
- Gray GL, Smith DH, Baldridge JS, Harkins RN, Vasil ML, Chen EY, Heyneker HL. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. [Europe PMC free article] [Abstract] [Google Scholar]
- Grunstein M, Hogness DS. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. [Europe PMC free article] [Abstract] [Google Scholar]
- Gusterson B, Cowley G, McIlhinney J, Ozanne B, Fisher C, Reeves B. Evidence for increased epidermal growth factor receptors in human sarcomas. Int J Cancer. 1985 Dec 15;36(6):689–693. [Abstract] [Google Scholar]
- Holmes DS, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. [Abstract] [Google Scholar]
- Hwang J, Fitzgerald DJ, Adhya S, Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. [Abstract] [Google Scholar]
- Iglewski BH, Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. [Europe PMC free article] [Abstract] [Google Scholar]
- Kelley VE, Bacha P, Pankewycz O, Nichols JC, Murphy JR, Strom TB. Interleukin 2-diphtheria toxin fusion protein can abolish cell-mediated immunity in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3980–3984. [Europe PMC free article] [Abstract] [Google Scholar]
- Kondo T, FitzGerald D, Chaudhary VK, Adhya S, Pastan I. Activity of immunotoxins constructed with modified Pseudomonas exotoxin A lacking the cell recognition domain. J Biol Chem. 1988 Jul 5;263(19):9470–9475. [Abstract] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. [Abstract] [Google Scholar]
- Lazar E, Vicenzi E, Van Obberghen-Schilling E, Wolff B, Dalton S, Watanabe S, Sporn MB. Transforming growth factor alpha: an aromatic side chain at position 38 is essential for biological activity. Mol Cell Biol. 1989 Feb;9(2):860–864. [Europe PMC free article] [Abstract] [Google Scholar]
- Lee DC, Rose TM, Webb NR, Todaro GJ. Cloning and sequence analysis of a cDNA for rat transforming growth factor-alpha. Nature. 1985 Feb 7;313(6002):489–491. [Abstract] [Google Scholar]
- Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985 Jan 10;313(5998):144–147. [Abstract] [Google Scholar]
- Marquardt H, Hunkapiller MW, Hood LE, Todaro GJ. Rat transforming growth factor type 1: structure and relation to epidermal growth factor. Science. 1984 Mar 9;223(4640):1079–1082. [Abstract] [Google Scholar]
- Maxam AM, Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. [Europe PMC free article] [Abstract] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. [Abstract] [Google Scholar]
- Murphy JR, Bishai W, Borowski M, Miyanohara A, Boyd J, Nagle S. Genetic construction, expression, and melanoma-selective cytotoxicity of a diphtheria toxin-related alpha-melanocyte-stimulating hormone fusion protein. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8258–8262. [Europe PMC free article] [Abstract] [Google Scholar]
- Riemen MW, Wegrzyn RJ, Baker AE, Hurni WM, Bennett CD, Oliff A, Stein RB. Isolation of multiple biologically and chemically diverse species of epidermal growth factor. Peptides. 1987 Sep-Oct;8(5):877–885. [Abstract] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. [Europe PMC free article] [Abstract] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. [Europe PMC free article] [Abstract] [Google Scholar]
- Winkler ME, Bringman T, Marks BJ. The purification of fully active recombinant transforming growth factor alpha produced in Escherichia coli. J Biol Chem. 1986 Oct 15;261(29):13838–13843. [Abstract] [Google Scholar]
- Winter G, Fersht AR, Wilkinson AJ, Zoller M, Smith M. Redesigning enzyme structure by site-directed mutagenesis: tyrosyl tRNA synthetase and ATP binding. Nature. 1982 Oct 21;299(5885):756–758. [Abstract] [Google Scholar]
- Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6899–6903. [Europe PMC free article] [Abstract] [Google Scholar]
Associated Data
Articles from Molecular and Cellular Biology are provided here courtesy of Taylor & Francis
Full text links
Read article at publisher's site: https://doi.org/10.1128/mcb.9.7.2860-2867.1989
Read article for free, from open access legal sources, via Unpaywall:
https://europepmc.org/articles/pmc362752?pdf=render
Free after 4 months at mcb.asm.org
http://mcb.asm.org/cgi/reprint/9/7/2860
Free to read at mcb.asm.org
http://mcb.asm.org/cgi/content/abstract/9/7/2860
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1128/mcb.9.7.2860-2867.1989
Article citations
EGFR-targeted Chimeras of Pseudomonas ToxA released into the extracellular milieu by attenuated Salmonella selectively kill tumor cells.
Biotechnol Bioeng, 113(12):2698-2711, 08 Jul 2016
Cited by: 8 articles | PMID: 27260220 | PMCID: PMC5083144
Optimization of expression and purification of two biologically active chimeric fusion proteins that consist of human interleukin-13 and Pseudomonas exotoxin in Escherichia coli.
Protein Expr Purif, 39(2):189-198, 01 Feb 2005
Cited by: 28 articles | PMID: 15642470
Recombinant toxins in haematologic malignancies and solid tumours.
Expert Opin Investig Drugs, 7(9):1405-1427, 01 Sep 1998
Cited by: 0 articles | PMID: 15992040
Immunotoxins for targeted cancer therapy.
Adv Drug Deliv Rev, 31(1-2):53-88, 01 Apr 1998
Cited by: 77 articles | PMID: 10837618
A circularly permuted recombinant interleukin 4 toxin with increased activity.
Proc Natl Acad Sci U S A, 91(15):6889-6893, 01 Jul 1994
Cited by: 52 articles | PMID: 8041715 | PMCID: PMC44303
Go to all (11) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Transforming growth factor alpha-Pseudomonas exotoxin fusion protein prolongs survival of nude mice bearing tumor xenografts.
Proc Natl Acad Sci U S A, 87(12):4697-4701, 01 Jun 1990
Cited by: 26 articles | PMID: 2352944 | PMCID: PMC54184
Cytotoxic activities of a fusion protein comprised of TGF alpha and Pseudomonas exotoxin.
FASEB J, 3(14):2647-2652, 01 Dec 1989
Cited by: 44 articles | PMID: 2556314
Biological activity of a transforming growth factor-alpha--Pseudomonas exotoxin fusion protein in vitro and in vivo.
J Ind Microbiol, 7(3):203-207, 01 Apr 1991
Cited by: 0 articles | PMID: 1367127
Pseudomonas exotoxin: chimeric toxins.
J Biol Chem, 264(26):15157-15160, 01 Sep 1989
Cited by: 54 articles | PMID: 2504717
Review