Abstract
Background
Chikungunya virus (CHIKV) is a reemerging pathogen transmitted by Aedes aegypti and Aedes albopictus mosquitoes. The ongoing Caribbean outbreak is of concern due to the potential for infected travelers to spread the virus to countries where vectors are present and the population is susceptible. Although there has been no autochthonous transmission of CHIKV in Canada, there is concern that both Ae. albopictus and CHIKV will become established, particularly under projected climate change. We developed risk maps for autochthonous CHIKV transmission in Canada under recent (1981–2010) and projected climate (2011–2040 and 2041–2070).Methods
The risk for CHIKV transmission was the combination of the climatic suitability for CHIKV transmission potential and the climatic suitability for the presence of Ae. albopictus; the former was assessed using a stochastic model to calculate R0 and the latter was assessed by deriving a suitability indicator (SIG) that captures a set of climatic conditions known to influence the ecology of Ae. albopictus. R0 and SIG were calculated for each grid cell in Canada south of 60°N, for each time period and for two emission scenarios, and combined to produce overall risk categories that were mapped to identify areas suitable for transmission and the duration of transmissibility.Findings
The risk for autochthonous CHIKV transmission under recent climate is very low with all of Canada classified as unsuitable or rather unsuitable for transmission. Small parts of southern coastal British Columbia become progressively suitable with short-term and long-term projected climate; the duration of potential transmission is limited to 1–2 months of the year.Interpretation
Although the current risk for autochthonous CHIKV transmission in Canada is very low, our study could be further supported by the routine surveillance of Ae. albopictus in areas identified as potentially suitable for transmission given our uncertainty on the current distribution of this species in Canada. https://doi.org/10.1289/EHP669Free full text

Assessment of the Probability of Autochthonous Transmission of Chikungunya Virus in Canada under Recent and Projected Climate Change
Abstract
Background:
Chikungunya virus (CHIKV) is a reemerging pathogen transmitted by Aedes aegypti and Aedes albopictus mosquitoes. The ongoing Caribbean outbreak is of concern due to the potential for infected travelers to spread the virus to countries where vectors are present and the population is susceptible. Although there has been no autochthonous transmission of CHIKV in Canada, there is concern that both Ae. albopictus and CHIKV will become established, particularly under projected climate change. We developed risk maps for autochthonous CHIKV transmission in Canada under recent (1981–2010) and projected climate (2011–2040 and 2041–2070).
Methods:
The risk for CHIKV transmission was the combination of the climatic suitability for CHIKV transmission potential and the climatic suitability for the presence of Ae. albopictus; the former was assessed using a stochastic model to calculate
Findings:
The risk for autochthonous CHIKV transmission under recent climate is very low with all of Canada classified as unsuitable or rather unsuitable for transmission. Small parts of southern coastal British Columbia become progressively suitable with short-term and long-term projected climate; the duration of potential transmission is limited to 1–2 months of the year.
Interpretation:
Although the current risk for autochthonous CHIKV transmission in Canada is very low, our study could be further supported by the routine surveillance of Ae. albopictus in areas identified as potentially suitable for transmission given our uncertainty on the current distribution of this species in Canada. https://doi.org/10.1289/EHP669
Introduction
Chikungunya is a reemerging tropical arboviral disease transmitted by Aedes (Ae.) mosquitoes. Chikungunya virus (CHIKV) was first isolated from human sera and mosquitoes in the Makonde Plateau of the Southern Province of Tanganyika (present day Tanzania) (Robinson 1955; Ross 1956). CHIKV disease is typically characterized by fever, headache, fatigue, and debilitating polyarthralgia and myalgia (Pialoux et al. 2007; Rezza et al. 2007; Robinson 1955). Symptoms generally resolve within 7–10 days with the exception of polyarthralgia that may persist for several months to years (Brighton et al. 1983; Fourie and Morrison 1979; Javelle et al. 2015). Accordingly, the term chikungunya was applied to the disease and roughly translates as “that which bends up” the joints in the local language of the Makonde people (Robinson 1955; Ross 1956). Infrequently, the disease has been suspected to cause complications in severe cases with underlying medical conditions, including death (Economopoulou et al. 2009; Renault et al. 2007). There are no vaccines and treatment is supportive. Asymptomatic cases are rare and clinical manifestations so very characteristic for clinical diagnosis (Ayu et al. 2010; Fourie and Morrison 1979; Higgs and Vanlandingham 2015; Lumsden 1955). Post-infection immunity is life-long (Lumsden 1955; Pialoux et al. 2007).
CHIKV circulates via two distinct transmission cycles: a) a sylvatic enzootic cycle transmitted by a wide range of Aedes mosquitoes and among wild primate reservoirs in Africa with occasional spillover to humans; and b) an urban human–mosquito–human epidemic cycle observed in Asia and the Indian subcontinent (Kendrick et al. 2014) transmitted by two main vectors, Ae. aegypti and Ae. albopictus (Diallo et al. 1999; Jupp and McIntosh 1988). Until recently, the virus was restricted to Africa, Asia, and the Indian subcontinent where sporadic and isolated outbreaks are reported (Burt et al. 2012; Pialoux et al. 2007; Rougeron et al. 2015; Schwartz and Albert 2010). CHIKV appeared to subside in the 1980s and 1990s only to reemerge in urban outbreaks in Asia and Africa, initiating a large outbreak in 2005–2006 involving millions in the Indian Ocean Islands and southern and central India (Burt et al. 2012; Kalantri et al. 2006; Pialoux et al. 2007; Weaver 2014). The unexpected reemergence of CHIKV in the Indian Ocean region was associated with the mutation of the virus that facilitated virus replication in, and transmission by, Ae. albopictus mosquitoes (Thiberville et al. 2013; Tsetsarkin et al. 2007). Consequently, the mutation supported the geographic expansion of CHIKV into sub-Saharan Africa, Southeast Asia, and Europe (Thiberville et al. 2013). Autochthonous outbreaks of CHIKV in Europe were first documented in Italy in 2007 (Rezza et al. 2007), and in France in 2010 (Gould et al. 2010) and 2014 (Delisle et al. 2015). These outbreaks were initiated by infected travelers returning from CHIKV-endemic countries to regions in Europe where Ae. albopictus is present (Weaver 2014). More recently, the first cases of autochthonous transmission of CHIKV in the Caribbean were reported in December 2013 on the island of Saint Martin (Pan American Health Organization and World Health Organization 2013). The outbreak has subsequently expanded and is currently ongoing with approximately 1.8 million probable and 65,000 confirmed autochthonous cases reported across 47 countries and territories in the Caribbean, Central America, and South America (Pan American Health Organization 2016; Vega-Rúa et al. 2015). Although the outbreak in the Caribbean is caused by an Asian strain that is thought not to be efficiently transmitted by Ae. albopictus (Morrison 2014; Weaver 2014), and the principal vector is likely Ae. aegypti, there is potential for the spread of imported cases by Ae. albopictus (Higgs and Vanlandingham 2015; Vega-Rúa et al. 2015). Associated with the Caribbean outbreak, 11 autochthonous cases of CHIKV were reported in 2014 in Florida where local Ae. aegypti and Ae. albopictus populations are established (Centers for Disease Control and Prevention 2015; Higgs and Vanlandingham 2015).
To date, there has been no local transmission of CHIKV in Canada due to the absence (to our knowledge) of reproducing populations of Ae. aegypti and Ae. albopictus. The cooler Canadian climate is likely a limiting factor for the establishment of these species, particularly Ae. aegypti, which is thought to require a tropical or subtropical climate to survive (Christophers 1960). It may be unlikely that Ae. aegypti will become established in Canada even if temperatures continue to increase due to climate change (Capinha et al. 2014; Khormi and Kumar 2014). However, Ae. albopictus is a cold-tolerant invasive species with the ability to overwinter in a temperate climate (Nicholson et al. 2014), which raises the possibility of the establishment of this species in southern parts of Canada. Occasional Ae. albopictus mosquitos have been found in southern Ontario, although these are thought to be “adventitious” individuals rather than evidence of reproducing populations (Giordano et al. 2015; Public Health Ontario 2013). The species is, however, found in the United States where Ae. albopictus is thought to be endemic to some southeastern states (Hahn et al. 2016; Kraemer et al. 2015; Ogden et al. 2014; Petersen et al. 2016; Waldock et al. 2013). The rapid rate at which Ae. albopictus has spread and established across the United States and parts of Africa and Europe suggests that there is potential for this species to become more widely distributed in the United States and perhaps Canada, particularly under projected climate change (Enserink 2008).
For autochthonous CHIKV transmission to occur in a previously nonendemic location, four conditions must be met: introduction of CHIKV via an infected traveler (condition C1), a susceptible human population (condition C2), climatic suitability for a competent vector (condition C3) and climatic suitability for CHIKV transmission potential by that vector (condition C4) (Ogden et al. 2015). Canada is one of the leading destination countries for travelers returning from CHIKV-endemic countries over the summer months (Khan et al. 2014). In 2014, there were 320 confirmed and 159 probable CHIKV cases returning to Canada, up from 1–20 cases per year in previous years (Drebot et al. 2014; Pan American Health Organization 2016). Because CHIKV infections can cause high viremia and a significant proportion (20%) of infected returned Canadian travelers are viraemic at the time of seeking medical treatment (Drebot et al. 2014), condition C1 is likely currently met although worth noting is that the majority of Canadians travel to CHIKV-affected countries during winter when virus transmission risk is lowest in Canada (Statistics Canada 2016). Although there is no population immunity to CHIKV in Canada, it may be that Canadian residents spend enough of their time during the summer months indoors in air-conditioned buildings and homes that the frequency of mosquito bites is too low to maintain person-to-person transmission. However, the endemic (and sometimes epidemic) transmission of West Nile virus resulting in human cases during the summer months in Canada lends support for the existence of condition C2. Although conditions C1 and C2 are important for autochthonous CHIKV transmission, this study focused on the ecological risk factors essential for endemic CHIKV transmission in Canada (conditions C3 and C4). The current climatic suitability for the presence of Ae. albopictus in Canada (condition C3) was identified as unsuitable with the exception for southern coastal British Columbia and in south central and southeastern Canada, but northward expansion is possible with anticipated climate change (Ogden et al. 2014). However, we do not know the current and future climatic suitability for CHIKV transmission in Canada (condition C4), specifically, the effect of temperature on virus survival and replication within mosquitoes, mosquito survival beyond the extrinsic incubation period (EIP) (the time required for the development of CHIKV to spread from the mosquito’s gut to the salivary glands where the virus can be transmitted), and virus transmissibility between humans and mosquitoes. In this study we explore the potential for autochthonous, but not necessarily sustained, transmission of CHIKV in Canada. We used a stochastic mathematical model parameterized for Ae. albopictus under climatic conditions in the warmest months of the year in locations across Canada. We then combined the climatic suitability for CHIKV transmission potential in the warmest months of the year (condition C4) with climatic suitability indicators for the endemic presence of Ae. albopictus (condition C3) to produce risk maps identifying areas in Canada most suitable for autochthonous CHIKV transmission under recent and projected climate.
Methods
Climatic Suitability for Chikungunya Virus Transmission Potential
Transmission potential for CHIKV was explored by modeling the basic reproductive number (
The expected number of human infections arising from a single infected human in a completely susceptible population is therefore:
Table 1 is a summary of the parameters used in the calculation of
Table 1
Assumptions, distributions and mathematical equations used to estimate parameters in the calculation of the basic reproductive number (
| Parameter (label) | Description, assumptions, and references | Sampling distribution | Mathematical equation |
|---|---|---|---|
| Daily biting rate ( | The number of bites on a human, per mosquito, per day. Parameter values for Ae. albopictus in other studies include estimates of 0.31 per day observed in Macao, China (Almeida et al. 2005) to a range of 0.19 to 0.39 per day in modeling studies (Christofferson et al. 2014; Manore et al. 2014). We assume a modal value of 0.31 blood meals per day for Ae. albopictus in Canada (SD 0.04). | Pert (0.19, 0.31, 0.39) | — |
| Human-to-mosquito transmissibility ( | The probability of a mosquito acquiring CHIKV from an infectious human during a single blood meal. | Pert (0.37, 0.40, 0.95) | — |
| Mosquito-to-human transmissibility ( | The probability of a human acquiring CHIKV from an infected mosquito during a single blood meal. | Pert (0.5, 0.65, 0.8) | — |
| Duration of the human infectious period ( | The period of time in days when infected humans can infect mosquitoes with CHIKV. The viraemic period for CHIKV is up to 8 days, with viral load peaking during the first 3 days of illness and declining from days 4 to 8 (Appassakij et al. 2013). It is also assumed that humans are infectious a day or two prior to becoming ill (Lam et al. 2001; Liumbruno et al. 2008).We assume a mean viraemic period of 6 days (SD 1.1). | Gamma (30, 0.2) | — |
| Average adult mosquito lifespan in days ( | The life expectancy of adult Ae. albopictus at temperature ( | - | |
| Extrinsic incubation period ( | The mean EIP (
| ||
| Proportion of mosquitoes surviving the EIP ( | Temperature-dependent Ae. albopictus survival is calculated as follows, | ||
| Mosquito density per human ( | Under ideal weather, mosquito density is proportional to the minimal mortality where | ||
Note: SD, standard deviation.
Figure 1 describes the uncertainty of the parameters on the predicted
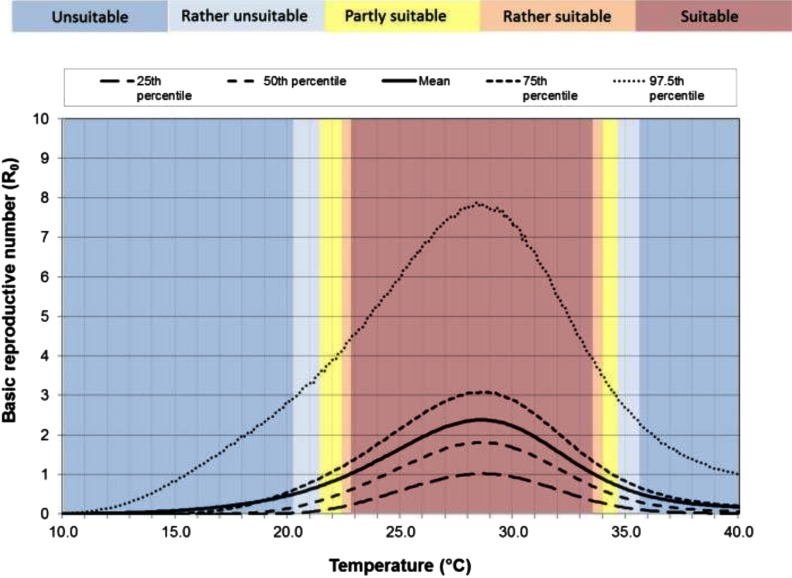
Distribution of
Mean monthly temperature (
Bias Correction of Climate Models
Data from a simulation of one regional climate model (RCM) for the time periods 2011–2040 and 2041–2070 were used to explore CHIKV transmission potential under short-term and long-term projected climate change, respectively. The Canadian Regional Climate Model version 5 (CRCM5) (Hernández-Díaz et al. 2013; Laprise et al. 2013; Martynov et al. 2013; Šeparović et al. 2013) was selected because this RCM has been extensively evaluated over North America. The CRCM5 has shown to have been substantially improved compared to previous Canadian RCMs in terms of seasonal mean statistics for both temperature and precipitation comparable to other modern RCMs (Martynov et al. 2013) and has the greatest skill among other RCMs for simulated precipitation (Diaconescu et al. 2016). The simulations used have horizontal grid meshes of
We used the Linear Scaling (LS) bias correction method (White and Toumi 2013) in order to adjust RCM time series with correction values based on the differences between mean observed (gridded ANUSPLIN) values and RCM simulation. The LS method aims to perfectly match the monthly mean of corrected values with that of observed ones (Lenderink et al. 2007). It operates with monthly correction values based on the differences between observed and raw data (raw RCM simulated data in this case). The LS method was applied to the CRCM5 simulation over the historical period as well as on the RCP4.5 and RCP8.5 future simulations. Comparison between the bias-corrected climate data from the CRCM5 model driven by CanESM2 under RCP4.5 and RCP8.5 and climate data from other RCMs driven by CanESM2 or the Irish Centre for High-End Computing EC Earth climate model (ICHEC-EC-EARTH) under RCP4.5 and RCP8.5 over two time periods (2011–2040 and 2041–2070) across Canada showed that the bias-corrected climate data from CRCM5-CanESM2-RCP4.5 and CRCM5-CanESM2-RCP8.5 used in this study were not far outliers compared to other models and did not deviate significantly from the ensemble mean (see Figure S1). The bias-corrected minimum and maximum temperature for each month and each CRCM5 grid were used to obtain
Climatic Suitability for the Presence of Aedes albopictus
We used the linear index of precipitation and air temperature suitability described by a sigmoidal function (SIG) to assess the climatic suitability for Ae. albopictus. The SIG index was originally developed to assess the climatic suitability of Ae. albopictus in Europe (Caminade et al. 2012; European Center for Disease Prevention and Control 2009) and was found to be a good fit to the current distribution of this species in the United States (Ogden et al. 2014). The SIG index is defined by three components: a) January mean temperatures (
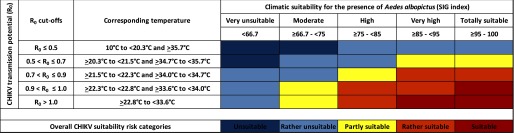
Climatic Suitability for Potential Autochthonous CHIKV Transmission in Canada
The risk categories for CHIKV transmission potential (
Sensitivity Analysis
We assessed the sensitivity of our assessments to the selection of parameter values in the transmission model by mapping the risk for autochthonous CHIKV transmission when using parameter values for the 75th percentile value of
Results
Risk Classification for Chikungunya Virus Transmission Potential
Our model of climatic suitability for CHIKV transmission potential indicated optimal suitability when the mean monthly temperature of the warmest month of the year is between
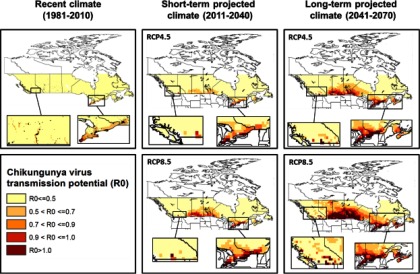
Risk maps for autochthonous CHIKV transmission in Canada based solely on CHIKV transmission potential (
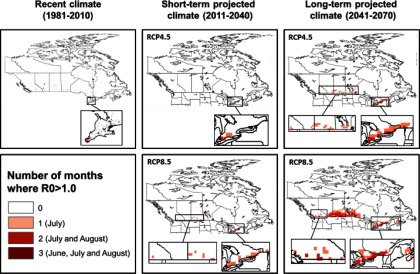
Duration in months where mean
When using transmission model parameter values for the 75th percentile value of
Risk Classification for Autochthonous Chikungunya Virus Transmission in Canada
When climatic suitability risk categories of CHIKV transmission potential (
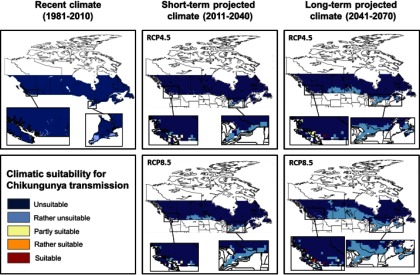
Risk maps for autochthonous CHIKV transmission in Canada combining the climatic suitability for CHIKV transmission potential (
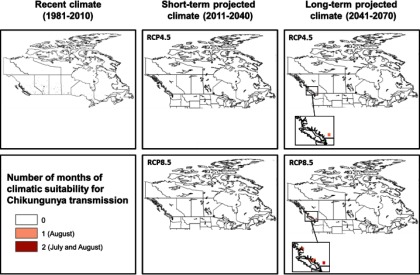
Duration in months for potential autochthonous CHIKV transmission in Canada combining the climatic suitability for CHIKV transmission potential (
The implications of using the 75th percentile value of
Discussion
In this study we investigated the climatic suitability for autochthonous CHIKV transmission in Canada. The objective was to quantitatively assess the current and future climatic suitability for CHIKV transmission under two greenhouse gas emissions scenarios (RCP4.5 and RCP8.5) using simulations from RCM to provide insight into where, when and for how long local transmission of CHIKV might occur in Canada. To achieve this, we assessed the climatic suitability for CHIKV transmission potential (
Our study suggests that Canada is currently not climatically suitable for autochthonous CHIKV transmission. Although we have the climatic suitability for limited CHIKV transmission over the summer period, the long and harsh winters impede survival of mosquito eggs and subsequently the establishment of the two known CHIKV vectors, Ae. aegypti and Ae. albopictus. The former has a minimum egg survival temperature threshold of
Our study did identify the potential for parts of southern coastal British Columbia to become progressively suitable for CHIKV transmission under short-term and long-term projected climate, particularly driven by a high emission scenario (RCP8.5). The duration of the transmission season, although short, is expected to expand from 1 month in a very small area in British Columbia in the short-term to 2 months covering a larger area in British Columbia in the long-term. This would be sufficient to sustain short-term autochthonous CHIKV transmission were pathogen and vector to co-occur (Reiskind et al. 2008). Aedes albopictus has not been detected in British Columbia although routine surveillance in this province has been sporadic and targeted to Culex spp. vectors of West Nile virus (communication, M. Morshed, May 2017, BCCDC). Our findings suggest that CHIKV and mosquito surveillance in the identified risk areas in British Columbia may be prudent, particularly as Ae. albopictus has been found in at least one county in the adjacent state of Washington (Hahn et al. 2016). However, if other species of Canadian-endemic mosquitoes turn out to be competent vectors for CHIKV, or if Ae. albopictus becomes more adapted to a cooler environment, the area of risk would expand further north and inland than currently expected and surveillance of the pathogen and vector should also be considered in southern Ontario, Québec, and the Canadian Prairies. Worth noting is that our study only considered two greenhouse gas emission scenarios (RCP4.5 and RCP8.5) representing intermediate and high emission scenarios, respectively. Implementation of the recent 2016 Paris Climate Agreement puts Canada on track for the RCP4.5 path, in which case the risk maps for CHIKV transmission under the RCP4.5 scenario would be the most likely projection. Under this scenario, we would expect reduced areas to be suitable for CHIKV transmission and a shorter duration of transmission in southern coastal British Columbia, and much lower risk for the rest of Canada compared to the RCP8.5 scenario. However, if the agreement fails, Canada will be on track for the RCP8.5 path, and the RCP8.5 projections will be the most likely.
While our findings are not surprising given the small number of cases of autochthonous transmission of CHIKV in North America, our study is the first to quantify the risk of CHIKV transmission in Canada in terms of when, where, and for how long transmission may occur, which is useful for forming public health policy. Furthermore, this study shows where northern/southern temperature regions will likely be under different climate projections, this is particularly useful for indicating where the borderline for exotic vector-borne pathogens transmitted by Ae. albopictus will likely be under climate change.
The work presented in this study could have implications for other mosquito-borne pathogens sharing the same vectors as CHIKV. At the time of writing, the outbreak of Zika virus (ZIKV) in the Americas and the Caribbean was causing concern due to the potential for returning Canadian travelers to spread the disease in the susceptible Canadian population (Fonseca et al. 2014; Musso et al. 2016; Petersen et al. 2016). ZIKV is an emerging disease transmitted by Ae. aegypti, Ae. albopictus mosquitoes and other Aedes spp. mosquitoes (Grard et al. 2014; Ledermann et al. 2014; Musso et al. 2014; Musso and Gubler 2016; Petersen et al. 2016). With additional data input specific to ZIKV such as the relationship between temperature and the EIP of ZIKV in the vectors, and the duration of the human ZIKV viraemia, the research presented here could be updated rapidly to assess the climatic suitability for autochthonous mosquito-borne ZIKV transmission in Canada. We do not currently have ZIKV-specific transmission parameters, but given that the epidemiology of ZIKV is similar to CHIKV and dengue, they are transmitted by the same vectors and they appear to co-circulate (Campos et al. 2015; Cao-Lormeau and Musso 2014; Musso and Gubler 2016), it is likely that Canada is currently not climatically suitable for autochthonous mosquito-borne ZIKV transmission, nor would we expect the risk for transmission to increase significantly with short-term and long-term projected climate. This conclusion is not surprising given ZIKV (and CHIKV) have historically been restricted to tropical and subtropical biomes (Petersen et al. 2016; Weaver and Lecuit 2015). This does not preclude autochthonous ZIKV transmission in Canada via secondary transmission routes such as sexual transmission; which was first reported in April 2016 (Public Health Agency of Canada 2016). Similar to our risk assessment for CHIKV, we cannot rule out other competent ZIKV vectors that may already be established in Canada. Routine surveillance of ZIKV and potential ZIKV vectors should be considered in southern Ontario, Québec, the Canadian Prairies, and southern coastal British Columbia.
There are a number of limitations in this quantitative risk assessment; one of the main assumptions is that climatic conditions can accurately identify suitable habitat for vectors and thus classify risk areas for mosquito-borne diseases. This does not account of the possibility that vectors evolve over time and that changes in their distribution may not be exclusively linked to climatic conditions (Fischer et al. 2014). Although there are many other factors that contribute to the overall risk, ecological risk is considered a primary driver for where CHIKV transmission may occur and climate-driven species distribution models, including models for Ae. albopictus, have been shown to predict the distribution of mosquitoes and the diseases that they can transmit with acceptable accuracy in a public health context (Brady et al. 2014; Caminade et al. 2012; European Center for Disease Prevention and Control 2009; Fischer et al. 2011; Ogden et al. 2014). Our study focused on climatic conditions during the warmest months of the year in Canada because they present the highest risk for virus transmission; however, as travel to CHIKV-affected countries by Canadians peak in winter (Statistics Canada 2016), the risk of viraemic travelers returning to Canada during the summer is lower. The impact of this reduced risk was not explored in this study because we focused on the ecological risk factors essential for CHIKV transmission rather than behavioral factors, but risk for autochthonous CHIKV transmission in Canada is likely influenced by this factor. We also calculated mean monthly temperature (
There are some data quality issues in this study. Data inputs for the CHIKV transmission model were based on very few CHIKV studies to date with some substitution of dengue data for CHIKV given the similarities between the epidemiology of the two viruses, their shared vectors and cocirculation (Campos et al. 2015; Cao-Lormeau and Musso 2014; Musso and Gubler 2016). There are few field estimates of biting rates and the numbers of mosquitoes per human, and how human behaviors and interventions (mosquito avoidance, mosquito control), infrastructure and environment in Canada (where the majority of Canadians live in an urbanized setting, i.e., air-conditioned homes, lack of stagnant water) affect these is unknown. We also do not have precise information on where Canadian travelers might be returning home from CHIKV-affected countries. However, we used a stochastic model, drawing from a range of probability distributions that were informed by a recent and comprehensive scoping review (S. Garasia et al. unpublished data, 2016) to account for uncertainty in the input parameters. Sensitivity analysis indicate risk maps produced using the 75th percentile
Data quality issues with the climate models used include uncertainty in climate scenarios from one single model and one bias correction approach used to drive the models, and only using data up until 2010. As the risk maps do not incorporate the five most recent years of climate data, the current risks identified is likely an underestimation of the risk given the climate in Canada over the last 5 years has been systematically warmer in the majority of the country compared to previous decades (1980s and 1990s) and climate baseline (Government of Canada 2016). Although the climate data from the bias-corrected models in this study were not far outliers compared to other RCMs and did not deviate significantly from the ensemble mean (see Figure S1), we note that over the summer months the models predicted higher temperatures and drier summers than the ensemble mean. The former is likely to result in a conservative estimate in the risk maps, whereas the latter may result in the under-prediction of the suitability for Ae. albopictus. Future research is needed to explore the impact of variations in climate model outputs on projected distributions by using model ensembles (IPCC 2013).
With the data we have at present, the current risk of autochthonous CHIKV transmission in Canada appears to be very low, and risk is restricted to very small parts of Canada under short-term and long-term projected climate. While our findings are not surprising, our study is the first to quantify the risk of CHIKV transmission in Canada which is useful for forming public health policy by identifying the risk of incursion of exotic vector-borne pathogens that are currently endemic to tropical and subtropical regions, into countries at high latitudes with climate change. This study identifies that southern Canada may be the very northern limit for transmission of these pathogens with climate change. Other factors need to be explored however, which include understanding when and where Canadian travelers are likely to return, infrastructure in Canada that may support vector populations in what would be expected to be climatically unsuitable regions, and whether or not there are other competent vectors in Canada. Further research to close the gap on our current understanding of CHIKV and CHIKV vectors, improved surveillance on Ae. albopictus in North America, and enhanced climate projection models (using model ensembles) will allow us to better predict the current and future risk of transmission of CHIKV and other exotic vector-borne pathogens in Canada.
Acknowledgments
We would like to thank L. Sushama and K. Winger [Centre pour l’étude et la simulation du climat à l’échelle régionale (ESCER), Université du Québec à Montréal (UQAM)], who provided the CRCM5 simulations. We also acknowledge the support of Compute Canada national HPC platform and the Calcul Québec regional HPC platform to run the CRCM5 model.
References
- Almeida AP, Baptista SS, Sousa CA, Novo MT, Ramos HC, Panella NA, et al. 2005. Bioecology and vectorial capacity of Aedes albopictus (Diptera: Culicidae) in Macao, China, in relation to dengue virus transmission. J Med Entomol 42(3):419–428, PMID: 15962796. [Abstract] [Google Scholar]
- Appassakij H, Khuntikij P, Kemapunmanus M, Wutthanarungsan R, Silpapojakul K. 2013. Viremic profiles in asymptomatic and symptomatic chikungunya fever: a blood transfusion threat? Transfusion 53(10 Pt 2):2567–2574, PMID: 23176378, 10.1111/j.1537-2995.2012.03960.x. [Abstract] [CrossRef] [Google Scholar]
- Arora VK, Scinocca JF, Boer GJ, Christian JR, Denman KL, Flato GM, et al. 2011. Carbon emission limits required to satisfy future representative concentration pathways of greenhouse gases. Geophys Res Lett 38(5):L05805, 10.1029/2010GL046270. [CrossRef] [Google Scholar]
- Ayu SM, Lai LR, Chan YF, Hatim A, Hairi NN, Ayob A, et al. 2010. Seroprevalence survey of Chikungunya virus in Bagan Panchor, Malaysia. Am J Trop Med Hyg 83(6):1245–1248, PMID: 21118929, 10.4269/ajtmh.2010.10-0279. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bonizzoni M, Gasperi G, Chen X, James AA. 2013. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends Parasitol 29(9):460–468, PMID: 23916878, 10.1016/j.pt.2013.07.003. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brady O. 2013. Model of Adult Aedes albopictus Survival/Mortality at Different Temperatures. https://figshare.com/articles/Model_of_adult_Aedes_albopictus_survival_mortality_at_different_temperatures/865035. [Europe PMC free article] [Abstract]
- Brady OJ, Golding N, Pigott DM, Kraemer MU, Messina JP, Reiner RC Jr, et al. 2014. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasit Vectors 7:338, 10.1186/1756-3305-7-338. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brady OJ, Johansson MA, Guerra CA, Bhatt S, Golding N, Pigott DM, et al. 2013. Modelling adult Aedes aegypti and Aedes albopictus survival at different temperatures in laboratory and field settings. Parasit Vectors 6:351, 10.1186/1756-3305-6-351. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Brighton SW, Prozesky OW, de la Harpe AL. 1983. Chikungunya virus infection. a retrospective study of 107 cases. S Afr Med J 63(9):313–315, PMID: 6298956. [Abstract] [Google Scholar]
- Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. 2012. Chikungunya: a re-emerging virus. Lancet 379(9816):662–671, PMID: 22100854, 10.1016/S0140-6736(11)60281-X. [Abstract] [CrossRef] [Google Scholar]
- Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, Baylis M, et al. 2012. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface 9(75):2708–2717, PMID: 22535696, 10.1098/rsif.2012.0138. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Campos GS, Bandeira AC, Sardi SI. 2015. Zika virus outbreak, Bahia, Brazil. Emerging Infect Dis 21(10):1885–1886, PMID: 26401719, 10.3201/eid2110.150847. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cao-Lormeau VM, Musso D. 2014. Emerging arboviruses in the Pacific. Lancet 384(9954):1571–1572, PMID: 25443481, 10.1016/S0140-6736(14)61977-2. [Abstract] [CrossRef] [Google Scholar]
- Capinha C, Rocha J, Sousa CA. 2014. Macroclimate determines the global range limit of Aedes aegypti. Ecohealth 11(3):420–428, PMID: 24643859, 10.1007/s10393-014-0918-y. [Abstract] [CrossRef] [Google Scholar]
- Carrington LB, Seifert SN, Willits NH, Lambrechts L, Scott TW. 2013. Large diurnal temperature fluctuations negatively influence aedes aegypti (diptera: Culicidae) life-history traits. J Med Entomol 50(1):43–51, PMID: 23427651, 10.1603/ME11242. [Abstract] [CrossRef] [Google Scholar]
- Centers for Disease Control and Prevention. 2015. Chikungunya: Information for Vector Control Programs. http://www.cdc.gov/chikungunya/pdfs/CHIKV_VectorControl.pdf.
- Chan M, Johansson MA. 2012. The incubation periods of Dengue viruses. PLoS One 7(11):e50972, PMID: 23226436, 10.1371/journal.pone.0050972. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Christofferson RC, Chisenhall DM, Wearing HJ, Mores CN. 2014. Chikungunya viral fitness measures within the vector and subsequent transmission potential. PLoS One 9(10):e110538, PMID: 25310016, 10.1371/journal.pone.0110538. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Christofferson RC, Mores CN. 2011. Estimating the magnitude and direction of altered arbovirus transmission due to viral phenotype. PLoS One 6(1):e16298, PMID: 21298018, 10.1371/journal.pone.0016298. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Christophers SR. 1960. Aedes Aegypti (L.), the Yellow Fever Mosquito. its Life History, Bionomics, and Structure. New York, New York:Cambridge University Press. [Google Scholar]
- Delisle E, Rousseau C, Broche B, Leparc-Goffart I, L’Ambert G, Cochet A, et al. 2015. Chikungunya outbreak in Montpellier, France, September to October 2014. Euro Surveill 20(17):21108, 10.2807/1560-7917.ES2015.20.17.21108. [Abstract] [CrossRef] [Google Scholar]
- Diaconescu EP, Gachon P, Laprise R, Scinocca JF. 2016. Evaluation of precipitation indices over North America from various configurations of regional climate models. Atmosphere-Ocean 54(4):418–439, 10.1080/07055900.2016.1185005. [CrossRef] [Google Scholar]
- Diallo M, Thonnon J, Traore-Lamizana M, Fontenille D. 1999. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg 60(2):281–286, PMID: 10072152. [Abstract] [Google Scholar]
- Drebot MA, Holloway K, Zheng H, Ogden NH. 2014. Travel-related chikungunya cases in Canada. Canada Communicable Disease Report 41(1):2–5. [Europe PMC free article] [Abstract] [Google Scholar]
- Dumont Y, Chiroleu F, Domerg C. 2008. On a temporal model for the Chikungunya disease: modeling, theory and numerics. Math Biosci 213(1):80–91, PMID: 18394655, 10.1016/j.mbs.2008.02.008. [Abstract] [CrossRef] [Google Scholar]
- Economopoulou A. et al. 2009. Atypical Chikungunya virus infections: clinical manifestations, mortality and risk factors for severe disease during the 2005–2006 outbreak on Réunion. Epidemiol Infect 137:(4):534–541, 10.1017/S0950268808001167. [Abstract] [CrossRef] [Google Scholar]
- Enserink M. 2008. Entomology. a mosquito goes global. Science 320(5878):864–866, PMID: 18487167, 10.1126/science.320.5878.864. [Abstract] [CrossRef] [Google Scholar]
- European Center for Disease Prevention and Control. 2009. Development of Aedes albopictus Risk Maps, Technical Report 0905. 0905. Stockholm.
- Fischer D, Thomas SM, Beierkuhnlein C. 2010. Temperature-derived potential for the establishment of phlebotomine sandflies and visceral leishmaniasis in Germany. Geospat Health 5(1):59–69, PMID: 21080321, 10.4081/gh.2010.187. [Abstract] [CrossRef] [Google Scholar]
- Fischer D, Thomas SM, Neteler M, Tjaden NB, Beierkuhnlein C. 2014. Climatic suitability of aedes albopictus in europe referring to climate change projections: comparison of mechanistic and correlative niche modelling approaches. Euro Surveill 19(6):20696, 20696, 10.2807/1560-7917.ES2014.19.6.20696. [Abstract] [CrossRef] [Google Scholar]
- Fischer D, Thomas SM, Niemitz F, Reineking B, Beierkuhnlein C. 2011. Projection of climatic suitability for Aedes albopictus Skuse (Culicidae) in Europe under climate change conditions. Global and Planetary Change 78:54–64, 10.1016/j.gloplacha.2011.05.008. [CrossRef] [Google Scholar]
- Fischer D, Thomas SM, Suk JE, Sudre B, Hess A, Tjaden NB, et al. 2013. Climate change effects on Chikungunya transmission in Europe: geospatial analysis of vector's climatic suitability and virus’ temperature requirements. Int J Health Geogr 12:51, PMID: 24219507, 10.1186/1476-072X-12-51. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fonseca K, Thomas SM, Suk JE, Sudre B, Hess A, Tjaden NB, et al. 2014. First case of Zika virus infection in a returning Canadian traveler. Am J Trop Med Hyg 91(5):1035–1038, PMID: 25294619, 10.4269/ajtmh.14-0151. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Fourie ED, Morrison JG. 1979. Rheumatoid arthritic syndrome after chikungunya fever. S Afr Med J 56(4):130–132, PMID: 494034. [Abstract] [Google Scholar]
- Giordano BV, Gasparotto A, Hunter FF. 2015. A checklist of the 67 mosquito species of Ontario, Canada. J Am Mosq Control Assoc 31(1):101–103, PMID: 25843183, 10.2987/14-6456R.1. [Abstract] [CrossRef] [Google Scholar]
- Gould EA, Gallian P, De Lamballerie X, Charrel RN. 2010. First cases of autochthonous dengue fever and chikungunya fever in France: from bad dream to reality! Clin Microbiol Infect 16(12):1702–1704, PMID: 21040155, 10.1111/j.1469-0691.2010.03386.x. [Abstract] [CrossRef] [Google Scholar]
- Government of Canada. 2016. Climate Data and Scenarios for Canada: Synthesis of Recent Observation and Modelling Results. Gatineau, QC, Canada:Environment Canada. [Google Scholar]
- Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, et al. 2014. Zika virus in Gabon (Central Africa)—2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 8(2):e2681, PMID: 24516683, 10.1371/journal.pntd.0002681. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hahn MB, Eisen RJ, Eisen L, Boegler KA, Moore CG, McAllister J, et al. 2016. Reported distribution of Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus in the United States, 1995–2016 (Diptera: Culicidae). J Med Entomol, 10.1093/jme/tjw072. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Hernández-Díaz L, Laprise R, Sushama L, Martynov A, Winger K, Dugas B. 2013. Climate simulation over CORDEX Africa domain using the fifth-generation Canadian Regional Climate Model (CRCM5). Clim Dyn 40:1415–1433, 10.1007/s00382-012-1387-z. [CrossRef] [Google Scholar]
- Higgs S, Vanlandingham D. 2015. Chikungunya virus and its mosquito vectors. Vector Borne Zoonotic Dis 15(4):231–240, PMID: 25674945, 10.1089/vbz.2014.1745. [Abstract] [CrossRef] [Google Scholar]
- Hutchinson MF, McKenney DW, Lawrence K, Pedlar JH, Hopkinson RF, Milewska E, et al. 2009. Development and testing of Canada-wide interpolated spatial models of daily minimum–maximum temperature and precipitation for 1961–2003. J Appl Meteor Climatol 48:725–741, 10.1175/2008JAMC1979.1. [CrossRef] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). 2013. Climate Change 2013: the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. New York, NY:Cambridge University Press. [Google Scholar]
- Javelle E, Ribera A, Degasne I, Gaüzère BA, Marimoutou C, Simon F. 2015. Specific management of post-chikungunya rheumatic disorders: a retrospective study of 159 cases in Reunion Island from 2006–2012. PLoS Negl Trop Dis 9(3):e0003603, PMID: 25760632, 10.1371/journal.pntd.0003603. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Johansson MA, Arana-Vizcarrondo N, Biggerstaff BJ, Gallagher N, Marano N, Staples JE. 2012. Assessing the risk of international spread of yellow fever virus: a mathematical analysis of an urban outbreak in Asuncion, 2008. Am J Trop Med Hyg 86(2):349–358, PMID: 22302873, 10.4269/ajtmh.2012.11-0432. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Johansson MA, Powers AM, Pesik N, Cohen NJ, Staples JE. 2014. Nowcasting the spread of chikungunya virus in the Americas. PLoS One 9(8):e104915, PMID: 25111394, 10.1371/journal.pone.0104915. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jupp PG, McIntosh BB. 1988. Chikungunya virus disease In: The Arboviruses: Epidemiology and Ecology. Monath TP, ed. Boca Raton, Florida:CRC Press, 137–157. [Google Scholar]
- Kalantri SP, Joshi R, Riley LW. 2006. Chikungunya epidemic: an Indian perspective. Natl Med J India 19(6):315–322, PMID: 17343016. [Abstract] [Google Scholar]
- Kendrick K, Stanek D, Blackmore C. Centers for Disease Control and Prevention (CDC). 2014. Notes from the field: transmission of chikungunya virus in the continental United States—Florida, 2014. MMWR Morb Mortal Wkly Rep 63(48):1137. [Europe PMC free article] [Abstract] [Google Scholar]
- Khan K, Bogoch I, Brownstein JS, Miniota J, Nicolucci A, Hu W. et al. 2014. Assessing the origin of and potential for international spread of chikungunya virus from the Caribbean. PLoS Curr 6, PMID: 4055609, 10.1371/currents.outbreaks.2134a0a7bf37fd8d388181539fea2da5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Khormi HM, Kumar L. 2014. Climate change and the potential global distribution of Aedes aegypti: spatial modelling using GIS and CLIMEX. Geospat Health 8(2):405–415, PMID: 24893017, 10.4081/gh.2014.29. [Abstract] [CrossRef] [Google Scholar]
- Kraemer MU, Sinka ME, Duda KA, Mylne A, Shearer FM, Brady OJ, et al. 2015. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data 2:150035, PMID: 26175912, 10.1038/sdata.2015.35. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lam SK, Chua KB, Hooi PS, Rahimah MA, Kumari S, Tharmaratnam M, et al. 2001. Chikungunya infection—an emerging disease in Malaysia. Southeast Asian J Trop Med Public Health 32(3):447–451, PMID: 11944696. [Abstract] [Google Scholar]
- Laprise R, Hernández-Díaz L, Tete K, Sushama L, Šeparović L, Martynov A, et al. 2013. Climate projections over CORDEX Africa domain using the fifth-generation Canadian Regional Climate Model (CRCM5). Clim Dyn 41(11):3219–3246, 10.1007/s00382-012-1651-2. [CrossRef] [Google Scholar]
- Ledermann JP, Guillaumot L, Yug L, Saweyog SC, Tided M, Machieng P, et al. 2014. Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl Trop Dis 8(10):e3188, PMID: 25299181, 10.1371/journal.pntd.0003188. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lenderink G, Buishand A, van Deursen W. 2007. Estimates of future discharges of the river Rhine using two scenario methodologies: direct versus delta approach. Hydrol Earth Syst Sci 11:1145–1159, 10.5194/hess-11-1145-2007. [CrossRef] [Google Scholar]
- Lima A, Lovin DD, Hickner PV, Severson DW. 2016. Evidence for an overwintering population of Aedes aegypti in Capitol Hill neighborhood, Washington, DC. Am J Trop Med Hyg 94(1):231–235, PMID: 26526922, 10.4269/ajtmh.15-0351. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Liumbruno GM, Calteri D, Petropulacos K, Mattivi A, Po C, Macini P, et al. 2008. The Chikungunya epidemic in Italy and its repercussion on the blood system. Blood Transfus 6(4):199–210, PMID: 19112735. [Europe PMC free article] [Abstract] [Google Scholar]
- Lumsden WH. 1955. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. II. General description and epidemiology. Trans R Soc Trop Med Hyg 49(1):33–57, PMID: 14373835. [Abstract] [Google Scholar]
- Manore CA, Hickmann KS, Xu S, Wearing HJ, Hyman JM. 2014. Comparing dengue and chikungunya emergence and endemic transmission in A. aegypti and A. albopictus. J Theor Biol 356:174–191, PMID: 24801860, 10.1016/j.jtbi.2014.04.033. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Martynov A, Laprise R, Sushama L, Winger K, Šeparović L, Dugas B. 2013. Reanalysis-driven climate simulation over CORDEX North America domain using the Canadian Regional Climate Model, version 5: model performance evaluation. Clim Dyn 41:2973–3005, 10.1007/s00382-013-1778-9. [CrossRef] [Google Scholar]
- McKenney DW, Hutchinson MF, Papadopol P, Lawrence K, Pedlar J, Campbell K, et al. 2011. Customized spatial climate models for North America. Bull Amer Meteor Soc 92:1611, 10.1175/2011BAMS3132. [CrossRef] [Google Scholar]
- Morrison TE. 2014. Reemergence of chikungunya virus. J Virol 88(20):11644–11647, PMID: 25078691, 10.1128/JVI.01432-14. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Musso D, Baud D, Gubler DJ. 2016. Zika virus: what do we know? Clin Microbiol Infect 22(6):494–496, PMID: 27067096, 10.1016/j.cmi.2016.03.032. [Abstract] [CrossRef] [Google Scholar]
- Musso D, Gubler DJ. 2016. Zika virus. Clin Microbiol Rev 29(3):487–524, PMID: 27029595, 10.1128/CMR.00072-15. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Musso D, Nilles EJ, Cao-Lormeau VM. 2014. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 20(10):O595–O596, PMID: 24909208, 10.1111/1469-0691.12707. [Abstract] [CrossRef] [Google Scholar]
- Nicholson J, Ritchie SA, Russell RC, Zalucki MP, Van Den Hurk AF. 2014. Ability for Aedes albopictus (Diptera: Culicidae) to survive at the climatic limits of its potential range in eastern Australia. J Med Entomol 51(5):948–957, PMID: 25276922. [Abstract] [Google Scholar]
- Ogden NH, Lindsay LR, Coulthart M. 2015. Is there a risk of chikungunya transmission in Canada? Canada Communicable Disease Report 41(1):11–14. [Europe PMC free article] [Abstract] [Google Scholar]
- Ogden NH, Milka R, Caminade C, Gachon P. 2014. Recent and projected future climatic suitability of north america for the asian tiger mosquito Aedes albopictus. Parasites Vectors 7:532, 10.1186/s13071-014-0532-4. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pan American Health Organization. 2016. Number of Reported Cases of Chikungunya Fever in the Americas, by Country or Territory 2013–2016. Cumulative Cases. Epidemiological Week 11. http://www.paho.org/hq/index.php?option=com_topics&view=readall&cid=5927&Itemid=40931&lang=en.
- Pan American Health Organization and World Health Organization. 2013. Epidemiological alert. Chikungunya fever. 9 December 2013.
- Petersen LR, Jamieson DJ, Powers AM, Honein MA. 2016. Zika virus. N Engl J Med 374:1552–1563, 10.1056/NEJMra1602113. [Abstract] [CrossRef] [Google Scholar]
- Pialoux G, Gaüzère BA, Jauréguiberry S, Strobel M. 2007. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis 7(5):319–327, PMID: 17448935, 10.1016/S1473-3099(07)70107-X. [Abstract] [CrossRef] [Google Scholar]
- Public Health Agency of Canada. 2016. Statement from the Chief Public Health Officer of Canada and Ontario’s Chief Medical Officer of Health on the First Positive Case of Sexually Transmitted Zika Virus. http://news.gc.ca/web/article-en.do?nid=1056379&_ga=1.218498143.730471110.1461176631.
- Public Health Ontario. 2013. Vector-Borne Diseases 2013 Summary Report. Toronto, Ontario:Public Health Ontario. [Google Scholar]
- Reiskind MH, Pesko K, Westbrook CJ, Mores CN. 2008. Susceptibility of Florida mosquitoes to infection with chikungunya virus. Am J Trop Med Hyg 78(3):422–425, PMID: 18337338. [Europe PMC free article] [Abstract] [Google Scholar]
- Renault P, Solet JL, Sissoko D, Balleydier E, Larrieu S, Filleul L, et al. 2007. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg 77(4):727–731, PMID: 17978079. [Abstract] [Google Scholar]
- Rezza G, Nicoletti L, Angelini R, Romi R, Finarelli AC, Panning M, et al. 2007. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet 370(9602):1840–1846, PMID: 18061059, 10.1016/S0140-6736(07)61779-6. [Abstract] [CrossRef] [Google Scholar]
- Robinson MC. 1955. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans R Soc Trop Med Hyg 49(1):28–32, PMID: 14373834. [Abstract] [Google Scholar]
- Ross RW. 1956. The Newala epidemic. III. The virus: isolation, pathogenic properties and relationship to the epidemic. J Hyg (Lond) 54(2):177–191, PMID: 13346078. [Europe PMC free article] [Abstract] [Google Scholar]
- Rougeron V, Sam IC, Caron M, Nkoghe D, Leroy E, Roques P. 2015. Chikungunya, a paradigm of neglected tropical disease that emerged to be a new health global risk. J Clin Virol 64:144–152, PMID: 25453326, 10.1016/j.jcv.2014.08.032. [Abstract] [CrossRef] [Google Scholar]
- Schwartz O, Albert ML. 2010. Biology and pathogenesis of chikungunya virus. Nat Rev Microbiol 8(7):491–500, PMID: 20551973, 10.1038/nrmicro2368. [Abstract] [CrossRef] [Google Scholar]
- Šeparović L, Alexandru A, Laprise R, Martynov A, Sushama L, Winger K, et al. 2013. Present climate and climate change over North America as simulated by the fifth-generation Canadian regional climate model. Clim Dyn 41:3167–3201, 10.1007/s00382-013-1737-5. [CrossRef] [Google Scholar]
- Statistics Canada. 2016. This analysis is based on the Statistics Canada International Travel Survey (2010–2014). All computations, use and interpretation of these data are entirely that of Aamir Fazil.
- Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P, et al. 2013. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res 99(3):345–370, PMID: 23811281, 10.1016/j.antiviral.2013.06.009. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Thomas SM, Obermayr U, Fischer D, Kreyling J, Beierkuhnlein C. 2012. Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasit Vectors 5:100, [doi], 10.1186/1756-3305-5-100. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tilston N, Skelly C, Weinstein P. 2009. Pan-European Chikungunya surveillance: designing risk stratified surveillance zones. Int J Health Geogr 8:61, 10.1186/1476-072X-8-61. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. 2007. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog 3(12):e201, PMID: 18069894, 10.1371/journal.ppat.0030201. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- van Vuuren DP. et al. 2011. The representative concentration pathways: an overview. Clim Change 109(1):5–31, 10.1007/s10584-011-0148-z. [CrossRef] [Google Scholar]
- Vega-Rúa A, Lourenço-de-Oliveira R, Mousson L, Vazeille M, Fuchs S, Yébakima A, et al. 2015. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis 9(5):e0003780, PMID: 25993633, 10.1371/journal.pntd.0003780. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Waldock J, Chandra NL, Lelieveld J, Proestos Y, Michael E, Christophides G, et al. 2013. The role of environmental variables on Aedes albopictus biology and chikungunya epidemiology. Pathog Glob Health 107(5):224–241, PMID: 23916332, 10.1179/2047773213Y.0000000100. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Weaver SC. 2014. Arrival of chikungunya virus in the new world: prospects for spread and impact on public health. PLoS Negl Trop Dis 8(6):e2921, PMID: 24967777, 10.1371/journal.pntd.0002921. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Weaver SC, Lecuit M. 2015. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 372(13):1231–1239, PMID: 25806915, 10.1056/NEJMra1406035. [Abstract] [CrossRef] [Google Scholar]
- White RH, Toumi R. 2013. The limitations of bias correcting regional climate model inputs. Geophys Res Lett 40(12):2907–2912, 10.1002/grl.50612. [CrossRef] [Google Scholar]
- Yakob L, Clements AC. 2013. A mathematical model of chikungunya dynamics and control: the major epidemic on Réunion Island. PLoS One 8(3):e57448, PMID: 23554860, 10.1371/journal.pone.0057448. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Zender CS. 2015. NetCDF Operator (NCO) User Guide. A Suite of netCDF Operators Edition 4.5.1, for NCO Version 4.5.1 July 2015.
Articles from Environmental Health Perspectives are provided here courtesy of National Institute of Environmental Health Sciences
Full text links
Read article at publisher's site: https://doi.org/10.1289/ehp669
Read article for free, from open access legal sources, via Unpaywall:
https://ehp.niehs.nih.gov/doi/pdf/10.1289/EHP669
Citations & impact
Impact metrics
Article citations
Chikungunya Beyond the Tropics: Where and When Do We Expect Disease Transmission in Europe?
Viruses, 13(6):1024, 29 May 2021
Cited by: 10 articles | PMID: 34072346 | PMCID: PMC8226708
Eastern Equine Encephalitis Virus: A Scoping Review of the Global Evidence.
Vector Borne Zoonotic Dis, 21(5):305-320, 17 Dec 2020
Cited by: 18 articles | PMID: 33332203 | PMCID: PMC8086401
Review Free full text in Europe PMC
Climate Change and Inpatient Dermatology.
Curr Dermatol Rep, 9(4):201-209, 22 Aug 2020
Cited by: 3 articles | PMID: 32864193 | PMCID: PMC7442546
Review Free full text in Europe PMC
Current and Projected Distributions of Aedes aegypti and Ae. albopictus in Canada and the U.S.
Environ Health Perspect, 128(5):57007, 22 May 2020
Cited by: 20 articles | PMID: 32441995 | PMCID: PMC7263460
Could exotic mosquito-borne diseases emerge in Canada with climate change?
Can Commun Dis Rep, 45(4):98-107, 04 Apr 2019
Cited by: 13 articles | PMID: 31285699 | PMCID: PMC6587696
Go to all (11) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe.
PLoS Negl Trop Dis, 9(5):e0003780, 20 May 2015
Cited by: 75 articles | PMID: 25993633 | PMCID: PMC4439146
Vertical transmission of Indian Ocean Lineage of chikungunya virus in Aedes aegypti and Aedes albopictus mosquitoes.
Parasit Vectors, 9:227, 23 Apr 2016
Cited by: 25 articles | PMID: 27108077 | PMCID: PMC4842298
High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus.
J Virol, 88(11):6294-6306, 26 Mar 2014
Cited by: 223 articles | PMID: 24672026 | PMCID: PMC4093877
Chikungunya virus and its mosquito vectors.
Vector Borne Zoonotic Dis, 15(4):231-240, 12 Feb 2015
Cited by: 45 articles | PMID: 25674945
Review

 1
1