Abstract
Free full text

Chinese Guidelines for the Diagnosis and Management of Tumefactive Demyelinating Lesions of Central Nervous System
Associated Data
Tumefactive demyelinating lesions (TDLs),[1,2,3] previously named as tumor-like inflammatory demyelinating disease[4,5] or demyelinating pseudotumor,[6] are relatively special type of immune-mediated inflammatory demyelinating lesions in the central nervous system (CNS),[7] which mainly occur within cerebrum, but rarely in spinal cord. TDLs are so named because it mimics brain tumors with such characteristics as less severe symptoms, large lesions with perilesional edema, mass effect and/or enhancement on neuroimaging, and easily misdiagnosed as brain tumors.[8,9]
In 1993, Kepes[10] reported 31 cases of pathologically confirmed TDLs in the brain, and the lesions were proposed as a disease entity between multiple sclerosis (MS) and disseminated encephalomyelitis (DEM) after infection or vaccination. Recent studies suggest that TDLs share similar pathogenesis with MS, Balo disease, and DEM, and they overlap clinically; thus, TDLs may be a disease entity.[2,11,12]
Although brain biopsy with pathological study is a gold standard for the diagnosis of TDLs, it has the following limitations: (1) patients may have concerns about the procedure, and hospitals may not have the pathology service available; (2) dilemma of pathological results: atypical pathological profile of TDLs may exist when the lesions are accompanied with gliosis or pleomorphism, which is difficult to be distinguished from glioma;[13] (3) steroids therapy before biopsy may result in the loss of typical pathological features of primary central nervous system lymphomas (PCNSLs), and the lesions of PCNSL are often surrounded by responsive infiltration of T-cells, making PCNSL easily be misdiagnosed as TDLs; (4) repeat biopsy is needed when the initial one was done without obtaining enough brain tissue or reaching the right place.[14]
Currently, the diagnosis of TDLs mainly depends on clinical characteristics in addition to neuroimaging features. Because no diagnostic criterion or expert census about this disorder is available, the diagnosis and treatment of this disorder are not consistent among different services. Many patients did not receive appropriate management due to misdiagnosis, and some even received tumorectomy or gamma knife therapy.[15,16] During the recent years, studies of TDLs in China provided increasing evidence for the diagnosis of the disorder, and the experience for the diagnosis of this disorder is becoming mature. Thus, the experts in the communities of neuroimmunology, neuroimaging, and neuropathology formulated the guidelines for the diagnosis and management of the TDLs as a reference for various levels of hospitals in China. It is especially valuable and can be used as a reference for managing those patients for whom the biopsy cannot be performed.
CLINICAL CHARACTERISTICS
Onset of the disorder
There is a lack of data about the prevalence and incidence of TDLs. The onset of TDLs is usually acute or subacute, with less cases having chronic onset. The patients rarely have prodromal symptoms, and some patients may have a history of vaccination or cold.[2] There is no gender predominance. The onset of disease is mainly in young- and middle-aged patients though they may occur in any age.[2,7,17] The average age of onset is 35 years in the cases reported in China, while the age of onset is older in other countries. For the 15 cases reported by Kim et al.,[9] the mean age of onset was 42 years.
Disease course
Previously, experts proposed that TDLs were intermediate type in between MS and acute disseminated encephalomyelitis (ADEM).[18] Adolescent ADEM may have comorbid TDLs.[19] Poser and Brinar believed that TDL is a phenotypic variant of classic MS and so did Lolekha and Kulkantrakorn.[20] Recently, studies in China and other countries showed that most of the TDLs were monophasic, and some cases of TDLs may transform to relapsing-remitting MS (RRMS)[2,17] or take the form of recurrent TDLs. A very few cases of TDLs may overlap with neuromyelitis optica spectrum disorders (NMOSDs).[2,12,21,22,23]
Symptoms and signs
Most of the lesions of TDLs are in the brain, with less of them being in the spinal cord. In comparison with glioma, most of the patients with TDLs have more severe symptoms and signs. However, less frequently, patients with TDLs may have large lesions on neuroimaging with relatively less severe symptoms and signs, similar to glioma. Patients with TDLs often present with headaches, slurred speech, and weakness of limbs. Some patients may have cognitive and psychiatric symptoms such as memory loss, retardation, and apathy, which are easily neglected by the patients and family members. The symptoms usually deteriorate or more symptoms are presenting with the progression of the disorder, and visual impairment may occur.[24] The symptoms and signs of TDLs are related to the involved location and scope of lesions, and the symptoms may deteriorate or more symptoms may occur during an attack, but seizures occur less frequently, which more often occur in glioma. Disseminated or multiple lesions of TDLs may have impact on cognition and even cause incontinence of urine and stool.
White matters of the brain are more frequently involved in TDLs, and cortical and subcortical areas can also be involved. The lesions can be single or multiple, and often they are bilateral,[2,14] and rarely the spinal cord can also be involved simultaneously. Frontal lobes are most frequently involved, and temporal and parietal lobes, basal ganglia area, corpus callosum, and centrum semiovale can also be involved.
AUXILIARY EXAMINATION
Cerebrospinal fluid and blood tests
Cerebrospinal fluid (CSF) tests: Intracranial pressures are usually normal or slightly elevated, and proteins levels are normal or slightly or moderately elevated. Cell count is usually in normal reference range. Some cases may have mild or strongly positive oligoclonal band (OB). Levels of myelin basic protein (MBP)[2] or IgG index may elevate. Persistent positive OB with dynamic observation may indicate the possibility of MS transformation.
Serum test: NMSOD transformation may occur in very few cases with TDLs, with seropositive AQP4 antibody. Cases with positive extractable nuclear antigen antibodies tend to be more likely relapsing.
Electrophysiology
Electrophysiological studies are not specific for TDLs, but visual, brainstem, and somatic evoked potentials, served as subclinical evidence, can be used for localizing the lesions and determining the area of TDLs.
Neuroimaging
Lesions of TDLs can be classified into three types based on morphological features on neuroimaging [Figures [Figures11–7]:[2,25,26] (1) diffuse infiltrating lesions, with unclear margins, uneven enhancement, taking a diffusely infiltrating growth pattern on T2-weighted image (T2WI) [Figure [Figure1a1a and and1b];1b]; (2) ring-shaped lesions [Figure 1c]: the lesions are round or round-like, with closed-ring- or open-ring-shaped enhancement; and (3) megacystic lesions: hypointensity on T1-weighted image (T1WI) and hyperintensity on T2WI signals, with clear margin and ring-shaped enhancement [Figure 1d].
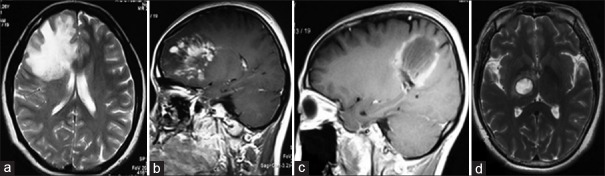
Three cases of TDLs with three different types in morphology are infiltrating (a and b), circular (c), and megacytic (d), respectively. TDLs: Tumefactive demyelinating lesions.
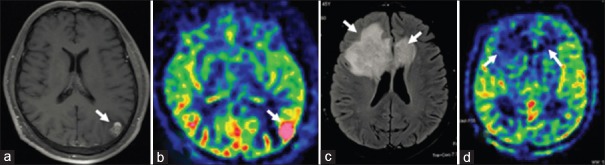
In a patient with glioblastoma, nodular enhancement in left parietal-occipital area on axial contrast-enhanced T1WI (a), and hyperfusion on ASL were detected (b); In a patient with tumefactive demyelinating lesions, butterfly-shaped subcortical lesion in between cortex and lateral ventricle, with the involvement of genu of corpus callosum, on axial fluid-attenuated inversion recovery T2WI (c), and no elevation of perfusion bilaterally on ASL, were found (d). ASL: Arterial spin labeling; T1WI: T1-weighted images; T2WI: T2-weighted images.
Head computed tomography
On plain head computed tomography (CT), most of the TDLs are hypodense [Figure 2a], and few lesions are isodense [Figure 3b], without obvious enhancement on contrast-enhanced imaging.[2,14,17,27]
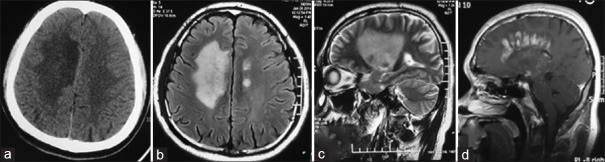
Lesions of TDLs on axial CT: Large patchy hypodense signal in bilateral semiovale (a); Lesions of TDLs on axial fluid-attenuated inversion recovery T2WI: Large patchy hyperintense signal in right semiovale, and multiple patchy hyperintense signals in left semiovale (b); Lesion of TDLs on sagittal T2WI: large patchy hyperintense signal in right hemisphere (c); Lesion of TDLs with contrast enhancement on sagittal T1-weighted images: “comb”-shaped enhancement which was vertical to lateral ventricle (d). TDLs: Tumefactive demyelinating lesions; T2WI: T2-weighted images; CT: Computed tomography.
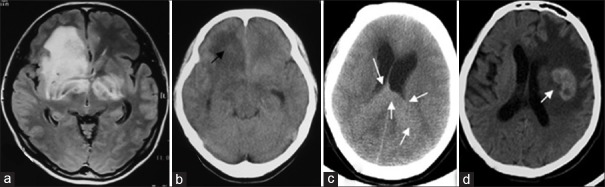
Lesion of TDLs showed large “butterfly”-shaped T2 signal on axial MRI (a); and the same lesion showed large patchy and slightly hypodense signal on axial CT (b); Grade III astrocytoma within corpus callosum, diffuse hyperintense signal was detected in between splenium of corpus callosum and bilateral parietal and occipital area (c); In patient with PCNSL (diffused large B-cell lymphoma), “kidney”-shaped focus was detected in left basal ganglion area on axial head CT (d). TDLs: Tumefactive demyelinating lesions; PCNSL: Primary central nervous system lymphoma; CT: Computed tomography; MRI: Magnetic resonance imaging.
Brain magnetic resonance imaging
Plain magnetic resonance imaging (MRI): Lesions of TDLs usually show hypointensity on T1WI and hyperintensity on T2WI on plain MRI[4] and are larger than they are on CT.[28] In general, in 70–100% of the patients with TDLs, the lesions show hyperintensity on T2WI, with clear margins [Figure 5b], and hypointensity on T2WI may exist in the margin of some lesions [Figure 4a].[2,26,29] Most of the lesions of TDLs have mass effect [Figures [Figures1a,1a, ,2b,2b, ,2c,2c, and and3a],3a], but less severe than that of brain tumor, and edema around the lesions is often observed.[27,30] In acute or subacute phase, the edema is mainly cytotoxic and shows high signal on diffusion-weighted imaging (DWI) [Figure 4b].[7] The lesions may decrease or diminish within several weeks after the treatment with steroids.
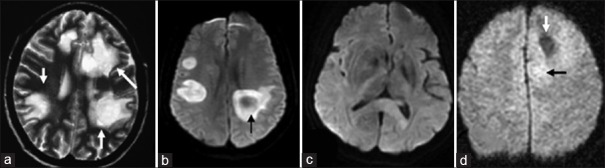
Multiple round-shaped lesions of TDLs show “fried egg” sign on T2WI (a); Lesions of TDLs showed bilateral perilateral ventricle hyperintense signal on DWI, with circular diffuse restriction (b); Lesion of PCNSL showed diffuse hyperintense signals in splenium of corpus callosum on DWI (c); Patient with anaplastic astrocytoma grade III, patchy hypointense signal surrounded by diffused hyperintense signal was detected in right frontal lobe on DWI (d). TDLs: Tumefactive demyelinating lesions; PCNSL: Primary central nervous system lymphoma; DWI: Diffusion-weighted images; T2WI: T2-weighted images.
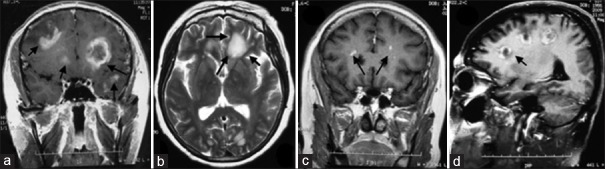
In a patient with TDLs, “closed-ring”- and “open ring”-shaped enhancements, with the opening toward cortex, were detected on contrast-enhanced axial T1WI. In addition, patchy or nodular enhancement was also detected (a, 22 days after the onset); In another patient with TDLs, “cloudy-patchy” hyperintensity on T2WI surrounded by hypointense margin in frontal horn of bilateral ventricles and occipital lobes on axial T2WI (b, arrow, 30 days after the onset), and patchy dot enhancement was found in frontal horns of bilateral ventricles on coronal contrast-enhanced T1WI (c, arrow, 10 days after the onset); “C”-shaped enhancement was detected in frontal horns of bilateral ventricles and occipital lobes on sagittal contrast-enhanced T1WI, with the former opening toward lateral ventricles and the latter opening toward the cortex (d, arrow, 30 days after the onset). TDLs: Tumefactive demyelinating lesions; T1WI: T1-weighted images; T2WI: T2-weighted images.
Contrast-enhanced brain MRI: Due to the breakdown of blood-brain barrier, in the acute and subacute phases of TDLs, various patterns such as nodular-, closed-ring-, open-ring-, and flame-shaped enhancement are noted on gadolinium-diethylenetriaminepentaacetic acid contrast-enhanced MRI.[31,32,33] Open-ring-shaped enhancement (also named “C-shaped” enhancement, Figure 5a) is most characteristic, i.e., perilesional discontinuous semi-ring-shaped enhancement.[3,7,13,30,34,35,36] In addition, for some lesions of TDLs, the dilated venules show “comb” structure [Figures [Figures1b1b and and2a2a],[29,37] which is vertical to lateral ventricles and often observed in acute or subacute phase, and this imaging feature is relatively specific for TDLs and not observed in brain tumor.[2]
In China, a study[2] of 60 cases with TDLs showed that the lesions of TDLs were dynamic in consistent with the disease progression: (a) in acute phase (≤3 weeks after the onset),[38] the lesions showed patchy or nodular enhancement [Figure 5c]; (b) in subacute phase (4–6 weeks after the onset), the lesions gradually evolved to “open-ring”-, “closed-ring”- or “rosette”-shaped enhancement, combined with patchy enhancement [Figure 5d]; (c) in chronic phase (>7 weeks after the onset), the lesions still demonstrated “open-ring”- or “closed-ring”-shaped enhancement, and the enhancement gradually became weakly patchy or vanished.
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) may reflect the metabolism in the tissues of a lesion and is valuable for the differential diagnosis of TDLs with PCNSL.[39,40] TDLs on MRS show as follows:[41,42] elevation of choline (Cho) peak, reduction of N-acetylarginine (NAA) peak, and most of the lesions have some elevation of lactate peak [Figure 6a].
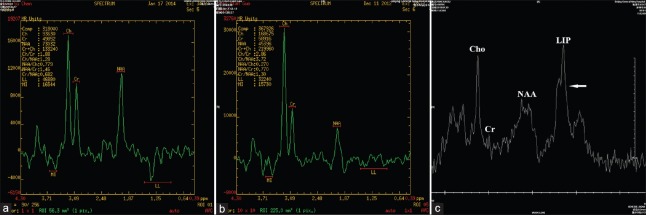
In a patient with TDLs, marked elevation of Cho peak value, slight depression of NAA peak value, with Cho/NAA = 4.7, elevation of lactate peak value (TE = 144) and elevation of β, γ-Glx peak value were detected on 1H-MRS (a). In a patient with anaplastic astrocytoma Grade III, elevation of Cho peak value, depression of NAA peak value, with Cho/NAA = 6.1, and visible lactate peak were detected on 1H-MRS (b). In a patient with PCNSL (diffuse large B-cell lymphoma), elevation of Cho peak value, with Cho/Cr = 8.0, NAA within normal limit, and high lip peak were detected on 1H-MRS (c). TDLs: Tumefactive demyelinating lesions; PCNSL: Primary central nervous system lymphomas; NAA: N-acetylarginine.
Perfusion-weighted imaging
They can be used for the differential diagnosis of TDLs with brain tumors.[39,43] Hyperperfusion is usually observed in glioma [Figure [Figure7a7a and and7b],7b], which does not occur in TDLs [Figure [Figure7c7c and and7d7d].[24]
PATHOLOGY
The lesions involve mainly white matters in TDLs,[44] and cortical and subcortical areas can also be involved [Figure 1a]. Pathological features of TDLs are as follows:[13,28,45,46,47,48] (1) loss of tissue structure and demyelination are detected using hematoxylin and eosin (HE) and myeline staining, respectively; (2) axonal and immunohistochemical neurofilament staining reveal axon preservation in the area of demyelination; (3) HE and immunohistochemical staining of CD68 demonstrate phagocytosis myelin by macrophages within the lesions; in the acute phase of the disorder, Luxol fast blue staining reveals that macrophages are filled with debris of myelin in the cytoplasm; (4) perivessel “cuffing” lymphocytes and infiltration can be observed in and around the lesions, and lymphocytes are mainly T-cells; (5) HE and immunohistochemical staining of glial fibrillary acidic protein (GFAP) demonstrate various degrees of astrocytosis in the lesions, and the astrocytes have prominent cytoplasm, with eccentric nuclei and multiple asterisk processes using GFAP or Holzer staining; (6) in most of the lesions, scattered Creutzfeldt cells can be observed (eccentric and enlarged astrocyte),[17] which are characterized by abundant cytoplasm, weak staining, loss of nuclei membrane, irregular chromosome called “aborted karyokinesis,” and the lesion is easily misdiagnosed as glioma. Creutzfeldt cell does not provide diagnostic value for TDLs but is helpful for the diagnosis of TDLs in combination with pathological demyelination; (7) the pathological features are changing with the clinical courses of TDLs.[49] In acute phase (≤3 weeks after the onset), the pathological result is consistent with the acute stage of inflammatory responses: inflammatory activities are active in the lesions, with massive loss of myelin and various degrees of axonal swelling. In subacute phase (4–6 weeks after the onset), the pathological characteristics are consistent with the chronic stage of inflammatory responses: clear margin of the lesions, relative axonal preservation, and macrophages containing myelin debris being aggravated in a radiative pattern around the lesions. In chronic phase (>7 weeks after the onset), smoldering or no activity of inflammation is the main pathological feature: partial remyelination in the lesions, no active inflammation and less inflammatory cells found in the core of the lesions, macrophages and microglia being in the peripheral area of the lesions, and degradated myelin seldom found in these cells. Gradual remyelination is the main picture in the nonactive inflammation stage of the lesions [Supplementary Material 1].
SUPPLEMENTARY MATERIAL
1. Commonly used staining for tumefactive demyelinating lesions in pathological studies:
Caution
(1) Limitations of brain biopsy and pathological study should be considered. Some patients with brain tumors may be misdiagnosed as TDLs due to the atypical pathological feature. As exacerbation of the disease occurs during the follow-ups, the patients are finally diagnosed with brain tumors after repeating or even multiple brain biopsy. Thus, for the patients with atypical pathological or neuroimaging findings, repeat pathological and neuroimaging studies are important. (2) Studies in other countries found that prebiopsy steroids use is one of the common factors causing atypical pathology, especially for PCNSL, so such that prebiopsy steroids should be avoided.[50] (3) The location for biopsy is another factor that may determine the yield of pathological study, and thus, sample from the lesion area with strong contrast enhancement on MRI is appropriate because it reflects the immune activities in the lesion.
DIAGNOSTIC CRITERIA
Based on the clinical presentations, results of laboratory workups, neuroimaging, and pathological studies, the diagnosis of TDLs includes items which are basic, supportive, warning, and exclusive. Three categories of the diagnosis of TDLs are recommended [Supplementary Material 2]:
SUPPLEMENTARY MATERIAL
2. Flowchart for the diagnosis of tumefactive demyelinating lesions.
Pathologically definite TDLs: typical pathology of TDLs, without other findings to exclude the diagnosis.
Clinical definite TDLs: must satisfy the followings: (1) no evidence exists for excluding the diagnosis; (2) all basic diagnostic criteria met; (3) four out of six of the supportive items met; (4) no warning items exist.
Clinical probable TDLs: the followings need to be satisfied: (1) no exclusion item exist; (2) all basic diagnostic criteria satisfied; (3) at least four of the six supportive items satisfied; (4) warning item(s) exist(s), which can be countered by supportive items: (a) one warning item exists, at least one supportive item should exist; (b) two warning items exist, at least two supportive items should exist; and (c) more than two warning items are not allowed.
Details of diagnosis criteria
Basic criteria
Persistent symptoms and signs >24 h, progression within a period of time, with or without neurological deficits.[51]
Brain MRI (≥1.5T): one or multiple lesions, at least one lesion with mass effect, with or without edema, and the size of the lesion in the long dimension ≥2 cm.[2,8,31,38]
Mass effect rating scale:[29] (a) mild: sulcal effacement; (b) moderate: ventricular compression; (c) severe: midline shift, or uncal herniation, or subfalcine herniation.
Edema rating scale:[29] (a) mild: <1 cm; (b) moderate: 1–3 cm; (c) severe: >3 cm.
Patient's clinical presentations and the results of laboratory and neuroimaging cannot be explained by other intracranial lesions.
Supportive items
For the clinical symptoms and signs,[2,15,53,54] three of the four items need to be satisfied: (1) young adults or adults onset; (2) acute or subacute onset; (3) headache as the initial symptom; (4) the severity of the disease is consistent with neuroimaging findings (for some infectious diseases, the clinical symptoms and signs are more obvious than neuroimaging findings, while the glioma is the opposite).
For laboratory workups,[2] three of the five items need to be satisfied: (1) normal or mild elevation of intracranial pressure (usually ≤240 mmH2O); (2) normal or mildly elevated cell count (usually ≤50/mm3); (3) normal or mildly to moderately elevated protein in CSF (usually ≤10,000 mg/L); (4) positive CSF-OB and/or elevated MBP; (5) positive serum AQP4.
For neuroimaging,[2,7,15,26] one of the following two items needs to be satisfied: (1) multiple foci, but not miliary, two hemispheres involved; (2) clear margin of the lesion (sometimes hypointense margin on T2WI).
Dynamics of the lesions on contrast-enhanced MRI develop in different clinical stages (≤3 weeks, 4–6 weeks, and >7 weeks):[2,8] the same lesion shows “nodular”- or “patchy”- to “circular”- (“open-ring-shaped,” “rosette-shaped,” “flame-shaped”) shaped enhancement, and then the enhancement reduced gradually.
Lesion with “ring”-shaped enhancement in morphology is detected on contrast-enhanced MRI,[2,17,25,54,55,56] with the following features: the “ring” is not continuous, with one or multiple openings, and thus showing “open-ring”-, “C”- or inverse “C”-shaped enhancement.
Positive “comb” sign: “comb”-shaped dilated venules within the paraventricular lesions on contrast-enhanced MRI.[2,25]
Warning items
The diagnosis of TDLs is less likely if the followings exist:
One of the following clinical features exists: (1) age of onset >60 years; (2) insidious onset, with course of disease longer than 1 year; (3) more severe findings on neuroimaging, and less severe symptoms and signs exist;[8] (4) meningeal irritation sign; (5) fever lasts >24 h, without other known etiologies.
Bleeds and necrosis within the lesion or hypointense or mixed hyper- and hypo-signals in the lesion on DWI.[8,57,58,59]
Contrast-enhanced MRI: the lesion with appearance of regularity, smooth outer wall, and closed “ring” in shape.[8]
MRS: Cho/NAA ≥2 or high peak of lip in the region of interest.[8,39,60]
Relapse of the disease whiting 3 months after the treatment with high dose of steroids.
Exclusion criteria
Tumor cells in CSF study.[61]
Hyperdense lesion on the head CT (calcification, bleeds, and spongiform vascular malformation are not included).[9,52]
Contrast-enhanced MRI: (1) typical findings of PCNSL:[62,63,64] even patchy-shaped enhancement, “notch” or “closed fist” signs; (2) typical findings of glioma:[8,58] basilar artery “embedding” sign; (3) other typical findings of tumor or nontumor lesions.
Arterial spin labeling (ASL) or perfusion-weighted imaging (PWI): obvious high perfusion in a lesion.[39,43]
Positron emission tomography-CT: high metabolism in a lesion.[65,66]
Definitely diagnosed noninflammatory demyelinating disease, i.e., tumor, infection, or angiitis.
DIFFERENTIAL DIAGNOSIS
Astrocytoma
(1) Clinical characteristics: for astrocytoma, mass effect is prominent on neuroimaging, and the symptoms are relatively mild in comparison with TDLs. The reason behind this is that glioma cells grow slowly along and in between nerve fibers and cause little damage of neurons and their fibers.[8,32] Statistical analysis showed that about 25% of patients with TDLs presented with headaches and are easily misdiagnosed as brain tumors, and 20% of patients with astrocytoma have seizures as initial presentations, while seizures as initial presentation of TDLs were seldom reported.[8,33] (2) Head CT: more than half of astrocytoma show hyperdense or isodense lesions [Figure 3c], while over 98% of TDLs show low-dense lesions, which are significant for the differential diagnosis.[52] (3) Plain brain MRI: in comparison with TDLs [Figures [Figures1a1a and and4a],4a], astrocytoma shows slightly hypointense or isointense signals on T1WI and vague margin on T2WI [Figure 8a],[8,32] and the lesion has prominent mass effect, significant perilesional edema and shift of midline even when the tumor is not large; some lesions of astrocytoma show increasingly high signals on DWI with the progression of the disease. For high grade of astrocytoma with necrosis, bleeds, and cyst, low or mixed signals can be observed within the high signal on DWI [Figure 4d],[8,57,58,59] while the signals of TDLs on DWI become weaker gradually with the progression of the disorder;[5] (4) contrast-enhanced MRI: astrocytoma shows diverse patterns of enhancement and the patterns are mainly nodular, massive, or foggy-like, based on different pathological types or WHO grades, and glioblastoma is characterized by becoming more easily become cystic, bleeding, and necrotic on neuroimaging;[8,32] (5) function MRI (fMRI): fMRI including MRS and ASL can be used for the differential diagnosis. Glioblastoma may have high lip peak, and astrocytoma may have Cho/NAA ≥2;[8,39,60] thus, the significantly elevated levels of lip and Cho/NAA are clinically meaningful [Figure 6b]. Some foci of glioma show hyperperfusion on PWI or ASL, which is more obvious for high-grade glioma [Figure [Figure7a7a and and7b7b],[39,43] while the lesions of TDLs frequently show isoperfusion or mildly hypoperfusion; (6) specific neuroimaging signs: (a) “comb” sign on contrast-enhanced MRI [Figures [Figures1b1b and and2d]2d] is relatively specific for TDLs;[2,25] (b) “wrapped basilar artery” sign in pons highly indicated astrocytoma [Figure 8d].[8]
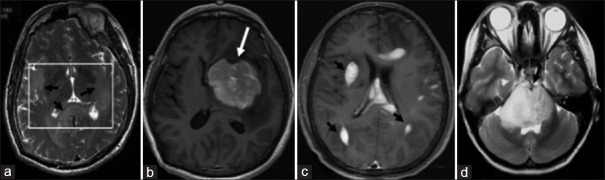
In a patient with anaplastic astrocytoma grade III, diffuse hyperintense lesions on T2WI with vague margin in splenium of corpus callosum on and bilateral temporal lobes on axial T2WI (a) were detected; in a patient with PCNSL (diffuse large B cell lymphoma), large round mass with enhancement and “notch sign” on the top in left basal ganglia area (b) were found; in another patient with PCNSL (diffuse large B cell lymphoma), multiple peri- and intra-lateral ventricular lesions, with even enhancement and “rain drop” sign or “sharp horn” sign on axial contrast-enhanced T1WI were observed (c); in a patient with grade II diffuse astrocytoma, diffuse hyperintense on T2WI in pons and marked swelling of the brain stem, within which anterior part of basilar artery being wrapped, were identified on axial T2WI (d). PCNSL: Primary central nervous system lymphoma. T1WI: T1-weighted images; T2WI: T2-weighted images.
Primary central nervous system lymphoma
(1) Clinical features: patients with PCNSL usually present with cognition impairment including memory loss as initial symptoms, and some of the patients may present with vision decline. However, patients with TDLs often present with headache as the initial symptom, with few patients being accompanied by vision decline;[2,61] (2) head CT: most of the PCNSLs show hyperdense or isodense lesions [Figure 3d],[52] and few PCNSLs have hypodense lesions in early stage on head CT, and the lesions can gradually become hyperdense with the progression of the disease. Endocentric enhancement (globular shaped) is usually seen on contrast-enhanced CT; (3) brain MRI scan: in comparison with PCNSL, most of the lesions of TDLs have clear margins on T2WI, with relatively limited area involved and less severe mass effect; the lesions of PCNSL usually show high signal on DWI [Figure 4c],[67] and the signal become even higher with the progression of the disorder;[32] (4) contrast-enhanced MRI: lesions of PCNSL show evenly enhanced patchy or globular signal,[50,63,68] and “notch” sign [Figure 8b], “angle” sign [Figure 8c], or raindrop-shaped appearance can be observed, which are different from the “comb” sign and dynamic changes of the lesions of TDLs; (5) fMRI: in comparison with the lesions of TDLs, the lesions of PCNSL usually show Cho/NAA ≥2 and high lip peak [Figure 6c],[39,60] which are the important characteristics for the differential diagnosis of the two disorders.
Primary angiitis of central nervous system
PACNS is an idiopathic inflammatory disorder of small arterioles, originated from CNS, and characterized by multiple lesions with mass effect.[69] The clinical and neuroimaging presentations of PACNS are difficult to be distinguished from TDLs, and they are easily mutually misdiagnosed. Pathological results of PANCS may be atypical and easily misdiagnosed as TDLs. In comparison with TDLs, some characteristics of PACNS can be used for the differential diagnosis:[70] (1) the onset is usually acute, and the lesions are closer to the cortex, and more seizures are observed; (2) due to the cortex is more often involved, gyrus-shaped enhancement is observed on contrast-enhanced MRI [Figure 9d], and sometimes, the midline structures can be involved, often bilaterally; (3) edema around the lesions and their mass effect are less severe in comparison with TDLs; (4) laboratory workups: reports from other countries show that in some 30% of PACNS, mild to moderate elevation of platelet can be observed, and p-ANCA and c-ANCA are positive, which are somewhat valuable for the differential diagnosis; (5) for some patients in acute and subacute phases of PACNS, necrosis with bleeds may occur, hyperintensity on T1WI [Figure 9c] and hypointensity on T2WI [Figure 9a] and low or mixed signals on DWI [Figure 9b] can be observed,[57,71] and the bleeds can be confirmed by SWI; (6) the response of PACNS to steroids is relative slow, and the enhancement of the lesion on MRI less likely reduces quickly with steroids treatment; (7) based on pathological features, PACNS can be classified: lymphocyte infiltrate, granulomatous, and acute necrotic types; microscopically, infiltrate and necrosis of inflammatory cells around blood vessels and occlusion of the involved blood vessel can be observed, which is distinguishable from TDLs.
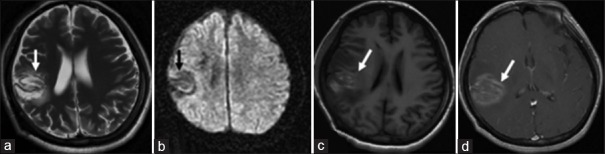
In a patient with PACNS confirmed with pathological study, round hyperintense lesion on T2WI with hypointensity on T2WI gyro-shaped signal in frontal and parietal lobes was detected on axial T2WI (a); the lesion showed gyro-shaped hypointense signal on axial diffusion-weighted imaging (b); long T1 gyro-shaped signal in frontal parietal lobes on axial T1WI was detected (c); gyro-shaped enhancement of the above lesion was detected on axial T1WI (d). PACNS: Primary angiitis of central nervous system; T1WI: T1-weighted images; T2WI: T2-weighted images.
Other
Germinoma and metastatic brain tumor can also show hyperdensity on head CT.[32] However, other signs on MRI can also be detected for germinoma: for basal ganglion area germinoma, atrophy of ipsilateral cerebral peduncle and shift of lateral ventricle toward tumor can be observed;[32] In addition, for patients with germinoma, the age of onset is early, with male predominance;[15] metastatic brain tumors are usually secondary to pulmonary or breast cancers, and multiple lesions are usually seen and located in subcortical area which has abundant blood supply. Some of the lesions may have circular shaped enhancement. Some others may have cystic-shaped enhancement. The age of onset and sex predominance are related to the primary tumors.
TREATMENT
(1) Pathological and clinical definite TDLs: the treatment for TDLs can be initiated; (2) clinical probable TDLs: biopsy is recommended based on the location of the lesion and the assessment of the risk for the biopsy. If the result of pathological study is atypical and the diagnosis of TDLs cannot be made, repeat biopsy and pathological study can be performed after finding out the reason for the unsuccessful biopsy and pathological study. The treatment plan can be work out according to the result of pathological study; (3) if patients cannot be diagnosed based on the result of pathological study and repeat biopsy cannot be done due to various reasons, steroids can be given if there is no contraindication.[72] The patients need to be reassessed with contrast-enhanced MRI after steroids. If the lesions resolved completely or mostly, glioma is very unlikely. The patient needs to be followed up on a regular basis, and if relapse or exacerbation happens within half a year, lymphoma should be considered.
TDL is a special type of demyelinating disease of CNS. Based on recent reports about its prognosis, most of the TDLs are monophasic, and few of the cases may relapse. Some of the cases may overlap with MS and NMOSD.[2,21,22,23,73,74,75,76] For relapsing TDLs, the treatment similar to MS and NMOSD can be initiated,[51,77] including the management in acute and remitting phases (disease-modifying management), neurotrophic treatment, symptomatic treatment, rehabilitation, and counseling for daily living; because most of the cases with TDLs are monophasic[2,15,17] and less likely relapse, and the lesions are relatively large, the treatment for the disorder is unique,[78] which is different from the treatment for NMOSD with “sustained low dose of steroids” and the treatment for MS with “short-term steroids treatment.”
There is such a significant difference in the treatment between NMOSD and MS that the serum AQP4 antibodies should be determined first. Positive AQP4 antibodies may indicate the transformation of TDLs to NMOSD, and patients of TDLs with positive AQP4 may have high rate of relapse and relatively more obvious neurological deficits. The treatment of TDLs in acute and/or relapse phases can be conducted based on “China NMOSD diagnosis and management guidelines” of 2016;[77] if the AQP4 antibodies are negative, the recommended managements are as follows.
Treatment of tumefactive demyelinating lesions in acute phase
Therapeutic goal: alleviate the symptoms in acute phase, shorten the disease course, improve the neurological deficits, reduce or even resolve the size of lesion to reach the remitting or cure on neuroimaging, and prevent complications.
Indication: first attack of TDLs or new attack with objective neurological deficits.
Medications and usage:
Steroids
As the first choice,[79] it can alleviate the symptoms in the acute phase of TDLs and reduce the size of lesion and enhancement on imaging. However, in comparison with MS, the lesion of TDLs is larger and symptoms are more severe, and thus, the tapering after pulse steroids treatment may last longer to avoid the relapse or exacerbation of the disorder.
Principle of the treatment with steroids: pulse treatment with high dose, slow tapering.
Approach: (a) adult: methylprednisolone 1000 mg/d, intravenous (IV) 3–4 h, 3–5 days, then tapering, cutting down half of the dose each time, with each dose continuing for 2–3 days, and when the dose being cut down to 120 mg/d, 80 mg/d, the dose form should be changed to oral prednisone 40 mg/d for 3 days, then taper in such dose as 32 mg/d for 3 days, 28 mg/d for 3 days, and one tablet should be cut down each week until discontinuation. (b) Child:[51] methylprednisolone 20–30 mg·kg−1·d−1 (≤1000 mg), IV 3–4 h, for 5 days. In consideration of adverse effects in children, short-term use is recommended. If full resolution of the disease is reached, oral prednisone 1 mg·kg−1·d−1 can be started and then cut down 5 mg every other day until discontinuation; if the symptoms alleviate slowly, the dose can be cut down in half every 2–3 days. When the dose of methylprednisolone tapers to 80 mg/d, oral prednisone is used and the dose is the same as above. Most of the TDLs are responsive to steroids and can resolve with pulse IV methylprednisolone followed by an oral prednisone taper; during the steroids tapering, if the patient has symptoms rebound or new symptoms, another round of pulse IV methylprednisolone can be tried or one course of intravenous immunoglobulin can be given (for more details see the following).[80]
Precautions: (a) steroids should be given in the morning, which is consistent with rhythm of endogenous steroids secretion and reduce the inhibition of hypothalamic-pituitary-adrenal axis; (b) high dose of steroids can cause cardiac arrhythmia so that the IV steroids should not be given too quickly, and the infusion should be finished in 3–4 h. Steroids should be hold and timely effective solutions could be provided when arrhythmia occurs; (c) other adverse effects include hypokalemia, hyperglycemia, high blood pressure, dyslipidemia, upper gastrointestinal bleeding, osteoporosis, caput femoris necrosis, etc. Simultaneous use of proton pump inhibitor and supplement of potassium, calcium, and vitamin should be considered; In addition, high dose of steroids can cause insomnia, which can be managed with zolpidem; (d) for the patient with suspected PCNSL, steroids should be avoided before biopsy because it can lead to the atypical transformation in neuroimaging and pathology, making the diagnosis complicated.[51,77]
Combined use of steroids and immunosuppressant
For patients without responding to steroids, immunosuppressants such as azathioprine, cyclophosphamide, mycophenolate mofetil, methotrexate, and tacrolimus may be considered. No evidence supports the use of them for TDLs. For detailed usage and caution for these medications, refer “China NMOSD diagnosis and management guidelines” of 2016.[77]
Intravenous immunoglobulin
No evidence supports the use for TDLs. It may be suitable for the patients with positive serum AQP4 antibodies or the patients who cannot be treated with steroids or do not respond to steroids or are not suitable for immunosuppressant, i.e., pregnancy, breastfeeding, and children. The recommended dose is 0.4 g·kg−1·d−1, IV, for 5 days as a course of treatment.[77]
Maintenance management for relapsing tumefactive demyelinating lesions
Therapeutic goal: reduce the progress and prevent the relapse of the disorder. For the patients with symptoms consistent with MS which is multiple in time and space, the management includes immunosuppressant and disease-modifying therapy (DMT),[51] and for the patients whose diagnoses not consistent with MS or NMOSD, treatment with immunosuppressant is a choice even though it is lack of evidence.
Main DMTs: The US Food and Drug Administration approved DMTs for MS include 10 medications:[51,81,82,83,84] (a)first line: Betaseron (interferon β-1b), Extavia (interferon β-1b), Rebif (interferon β-1a), Avonex (interferon β-1a), glatiramer acetate, dimethyl fumarate, teriflunomide; (b) second line: natalizumab; (c) third line: mitoxantrone. Currently, in China, the approved DMTs by the China Food and Drug Administration are Betaferon and Rebif. Cases of TDLs induced by fingolimod were reported in other countries;[85] thus, it should be used with caution for the treatment of TDLs.
Although there is lack of evidence for treating TDLs with DMT, several international studies have confirmed the efficacy (level A evidence) of Betaseron and Rebif for MS. Compared with placebo, Betaseron and Rebif both can: (a) reduce the ratio of transformation of clinical solitary syndrome to clinical definite MS; (b) significantly reduce the numbers and volume of active lesions on MRI T2WI; (c) reduce 34% of the recurrence rate of RRMS and reduce the numbers and volume of new lesions; and (d) restrain the progress of disability in patients with MS.
Recommendations
For relapsing TDLs with negative serum AQP4-IgG, DMT can be used. For approaches and precautions, see reference of “Diagnosis and treatment of MS experts consensus China 2014.”[51]
- 3.
Immunosuppressant: these groups of medications can be considered as third lines if the TDLs meet the diagnostic criteria of MS,[51] while for those TDLs not fulfilling the criteria of MS or NMOSD, these groups of medications can be used as first line.[77] Azathioprine, cyclophosphamide, and mycophenolate are commonly used. For detailed information about the approaches and precautions, please see reference of 2016 “China NMOSD diagnosis and treatment guidelines.”[77]
Neuroprotection
Vitamin B including Vitamin B1, mecobalamin, Vitamin B complex, and folic acid can be used as conventional dose. In addition, nerve growth factor, ganglioside, and citicoline can also be tried.
Symptomatic management
Depression/anxiety: medications are recommended including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), noradrenergic and specific serotonergic antidepressant (NaSSA), and serotonin 1A receptor agonist, i.e., tandospirone.[51,77]
Cognition impairment: cholinesterase inhibitor can be used.[51,77]
Headache: headache is one of the common initial symptoms. For headaches related to intracranial hypertension, mannitol or fructose-added glycerol solution can be used. Other pain-killer medications can be used for the management of headaches.
Painful spasticity: carbamazepine, oxcarbazepine, pregabalin, gabapentin, baclofen, and tizanidine can be chosen for the management of pain related to spasticity.[51,77]
Chronic pain and paresthesia: pregabalin, antidepressants/anxiolytics such as amitriptyline, 5-HT1a receptor agonist, SSRI, SNRI, or NaSSA, and tizanidine can be used as pharmacotherapy. Psychotherapy can also be used as a supplementary choice.[51,77]
Fatigue and lethargy: modafinil and amantadine can be used.[51,77]
Dysfunction of bowel and bladder: (a) for urinary incontinence, imipramine, oxybutynin, prazosin, and tamsulosin can be used; (b) catheterization can also be used for urine incontinence; (c) constipation: laxative can be used, and severe cases can be managed with enema; (d) sexual dysfunction can be managed with medications that can improve the function.[51,77]
Rehabilitation and counseling activity of daily living
After the acute phase of TDLs, some residual neurological deficits may exist such that rehabilitation (rehab) is important. Rehab for extremities, language, and swallowing should be started early under the instruction of rehab therapist. Patients should avoid factors that may trigger the relapse of inflammatory demyelinating disorders of the nervous system,[51,77] such as hot bath, exposure to high temperature under sunshine, cigarette smoking, and vaccination. At the same time, patient should keep a stable and happy mood and maintain a healthy life pattern and mild to moderate exercise. Vitamin D supplement can be given. The doctors should provide counseling for those patients with relapsing TDLs about marriage and pregnancy for female patients.
PROGNOSIS AND FOLLOW-UPS
The prognosis of TDLs, with no large sample being studied, is good in limited data. Liu et al.[2] followed up 60 cases with TDLs for 3–6 years and found that most of the cases had good prognosis, and only two patients died with the causes not related to TDLs. Most of TDLs are monophasic, and some of the cases may relapse. Some of them may transform to MS or overlap with NMO, with the former being the most. Similar results were reported in other country. The difference between our report and the reports from other country is that the frequency of relapse is lower for TDLs in ours. In our follow-up data, the highest relapse times were three, and the main findings were small patchy signals (more like MS), with few being large lesions of TDLs.
We found that some cases with TDLs were misdiagnosed even in pathological study. Patients alleviated initially after the treatment with steroids followed by relapse and deterioration with glioma or PCNSL finally diagnosed after surgery and repeat pathological studies (some of these cases showed hypodense lesions on CT in early stage and later turned hyperdense).[2] Thus, the following strategies for follow-ups are recommended: (1) follow-ups with telephone interview for all patients with TDLs (within the first 3 years after diagnosis, for pathological definite TDLs, once a year at least; for clinical definite TDL, at least once every 6 months; and for clinical probable TDLs, once every 3 months); (2) for those with relapsing TDLs, a contrast-enhanced brain MRI should be repeated every 3–6 months; (3) for those patients with the lesion reappearing or becoming larger, head CT is recommended, and repeat biopsy may be necessary.
Committee of specialists (in an order of first letter of family name): Zhong-Ping An (Tianjin Huanhu Hospital); Bi-Tao Bu (Tongji Hospital of Tongji Medical University, Huazhong Technology University); Li-Li Zeng (Shanghai Ruijin Hospital); Xiang-Jun Chen (Huashan Hospital of Fudan University); Jiang Cheng (General Hospital of Ningxia Medical University); Qi Cheng (Ruijin Hospital of Shanghai Jiao Tong University); Lan Chu (Affiliated Hospital of Guiyang Medical College); Hui-Qing Dong (Xuanwu Hospital of Capital Medical University); Yan-Hui Du (General Hospital of Ningxia Medical University); Rui-Sheng Duan (Qianfoshan Hospital of Shandong University); Cong Gao (The Second Affiliated Hospital of Guangzhou Medical University); Feng Gao (The First Hospital of Beijing University); Yang-Tai Guan (Renji Hospital of Shanghai Jiao Tong University); Li Guo (The Second Hospital of Hebei Medical University); Xue-Qiang Hu (The Third Affiliated Hospital of Sun Yat-sen University); De-Hui Huang (The PLA General Hospital); Wei-Zhong Ji (Qinghai Provincial People's Hospital); Tao Jin (The First Hospital of Jilin University); Jun Jing (Beijing Tongren Hospital of Capital Medical University); De-Hong Lu (Department of Pathology at Xuanwu hospital, Capital Medical University); Hai-Feng Li (Qilu Hospital of Shandong University); Hong-Zeng Li (Tangdu Hospital of the Fourth Military Medical University); Jian-Guo Liu (Navy General Hospital); Ze-Yu Li (The Affiliated Hospital of Inner Mongolia Medical University); Zhu-Yi Li (Tangdu Hospital of the Fourth Military Medical University); Xiao-Ping Liao (Hainan Medical College); Guang-Zhi Liu (Peking University People's Hospital); Wei-Bin Liu (The First Affiliated Hospital of Sun Yat-sen University); Lin Ma (Department of Radiology at The PLA General Hospital); Xue-An Muo (The Institute of Neurology, Guangxi Medical University); Xiao-Kun Qi (Navy General Hospital); Xin-Yue Qin (The First Affiliated Hospital of Chongqing Medical University); Wei Qiu (The Third affiliated Hospital of Sun Yat-sen University); Hong-Dang Qu (The Affiliated Hospital of Bengbu Medical College); Fu-Dong Shi (The General Hospital of Tianjin Medical University); Hong-Hao Wang (Nanfang Hospital of Southern Medical University); Jia-Wei Wang (Tongren Hospital of the Capital Medical University); Jin-Cun Wang (Xijing Hospital of the Fourth Military Medical University); Li-Hua Wang (The Second Affiliated Hospital of Harbin Medical University); Man-Xia Wang (The Second Affiliated Hospital of Lanzhou University); Wei-Zhi Wang (The Second Affiliated Hospital of Harbin Medical University);Yong-Gang Wang (Renji Hospital of Shanghai Jiao Tong University); Dong-Ning Wei (The 309th Hospital Chinese People's Liberation Army);Wei-Ping Wu (South Building of Chinese PLA General Hospital); Xiao-Mu Wu (Jiangxi Provincial People's Hospital); Bao-Guo Xiao (The Institute of Neurology, Huashan Hospital of Shanghai Fudan University); Yan Xu (Peking Union Medical College Hospital); Zhu Xu (The Affiliated Hospital of Guizhou Medical University); Xian-Hao Xu (Beijing Hospital); Gang Yu (The First Affiliated Hospital of Chongqing Medical University); Hua Zhang (Beijing Hospital); Mei-Ni Zhang (The First Hospital of Shanxi Medical University); Xing-Hu Zhang (The Affiliated Tiantan Hospital of Capital University); Xu Zhang (The First Affiliated Hospital of Wenzhou Medical University); Yu-Wu Zhao (The Sixth People's Hospital of Shanghai Jiao Tong University); Kui-Hong Zheng (Department of Radiology of Navy General Hospital); Xue-Ping Zheng (The Affiliated Hospital of Qingdao University); Hong-Yu Zhou (West China Hospital of Sichuan University); Wen-Bin Zhou (Xiangya Hospital of Central South University); Ming Ren (Xuanwu Hospital of Capital Medical University).
Financial support and sponsorship
This study was supported by the grants from Biological Medicine and Life Sciences Innovation and Cultivation of Research Projects of Beijing Municipal Science and Technology Commission (No. Z151100003915113) and Capital Foundation of Medical Developments (NO. 2009-2054).
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Prof. De-Hong Lu in Department of Pathology at Xuanwu Hospital, Prof. Qiu-Ping Gui in Department of Pathology and Prof. Lin Ma in Department of Radiology at PLA General Hospital, and Prof. Kui-Hong Zheng in Department of Imaging at Navy General Hospital for their help in confirming the Pathological and Neuroimaging data.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Footnotes
Edited by: Xin Chen
REFERENCES
Articles from Chinese Medical Journal are provided here courtesy of Wolters Kluwer Health
Citations & impact
Impact metrics
Article citations
Tumefactive demyelinating lesions: A literature review of recent findings.
Neurosciences (Riyadh), 29(3):153-160, 01 Jul 2024
Cited by: 0 articles | PMID: 38981633 | PMCID: PMC11305340
Review Free full text in Europe PMC
Clinical spectrum and prognosis of pathologically confirmed atypical tumefactive demyelinating lesions.
Sci Rep, 13(1):7773, 13 May 2023
Cited by: 1 article | PMID: 37179394 | PMCID: PMC10183015
The value of convolutional neural networks-based deep learning model in differential diagnosis of space-occupying brain diseases.
Front Neurol, 14:1107957, 02 Feb 2023
Cited by: 0 articles | PMID: 36816568 | PMCID: PMC9932812
Phenotyping variants of tumefactive demyelinating lesions according to clinical and radiological features-A case series.
Front Neurol, 14:1092373, 03 Feb 2023
Cited by: 0 articles | PMID: 36816572 | PMCID: PMC9935935
Central nervous system tumefactive demyelinating lesions: Risk factors of relapse and follow-up observations.
Front Immunol, 13:1052678, 01 Dec 2022
Cited by: 2 articles | PMID: 36532021 | PMCID: PMC9752826
Go to all (11) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Utility of proton MR spectroscopy for differentiating typical and atypical primary central nervous system lymphomas from tumefactive demyelinating lesions.
AJNR Am J Neuroradiol, 35(2):270-277, 08 Aug 2013
Cited by: 25 articles | PMID: 23928144 | PMCID: PMC7965761
Neuroimaging and clinicopathological differences between tumefactive demyelinating lesions and sentinel lesions of primary central nervous system lymphoma.
Front Immunol, 13:986473, 18 Aug 2022
Cited by: 1 article | PMID: 36059526 | PMCID: PMC9433969
MRI Findings in Tumefactive Demyelinating Lesions: A Systematic Review and Meta-Analysis.
AJNR Am J Neuroradiol, 39(9):1643-1649, 16 Aug 2018
Cited by: 23 articles | PMID: 30115676 | PMCID: PMC7655270
Review Free full text in Europe PMC
Differential imaging of atypical demyelinating lesions of the central nervous system.
Radiol Med, 126(6):827-842, 24 Jan 2021
Cited by: 11 articles | PMID: 33486703
Review




