Abstract
Free full text

Anexelekto/MER tyrosine kinase inhibitor ONO-7475 arrests growth and kills FMS-like tyrosine kinase 3-internal tandem duplication mutant acute myeloid leukemia cells by diverse mechanisms
Abstract
Nearly one-third of patients with acute myeloid leukemia have FMS-like tyrosine kinase 3 mutations and thus have poor survival prospects. Receptor tyrosine kinase anexelekto is critical for FMS-like tyrosine kinase 3 signaling and participates in FMS-like tyrosine kinase 3 inhibitor resistance mechanisms. Thus, strategies targeting anexelekto could prove useful for acute myeloid leukemia therapy. ONO-7475 is an inhibitor with high specificity for anexelekto and MER tyrosine kinase. Herein, we report that ONO-7475 potently arrested growth and induced apoptosis in acute myeloid leukemia with internal tandem duplication mutation of FMS-like tyrosine kinase 3. MER tyrosine kinase-lacking MOLM13 cells were sensitive to ONO-7475, while MER tyrosine kinase expressing OCI-AML3 cells were resistant, suggesting that the drug acts via anexelekto in acute myeloid leukemia cells. Reverse phase protein analysis of ONO-7475 treated cells revealed that cell cycle regulators like cyclin dependent kinase 1, cyclin B1, polo-like kinase 1, and retinoblastoma were suppressed. ONO-7475 suppressed cyclin dependent kinase 1, cyclin B1, polo-like kinase 1 gene expression suggesting that anexelekto may regulate the cell cycle, at least in part, via transcriptional mechanisms. Importantly, ONO-7475 was effective in a human FMS-like tyrosine kinase 3 with internal tandem duplication mutant murine xenograft model. Mice fed a diet containing ONO-7475 exhibited significantly longer survival and, interestingly, blocked leukemia cell infiltration in the liver. In summary, ONO-7475 effectively kills acute myeloid leukemia cells in vitro and in vivo by mechanisms that involve disruption of diverse survival and proliferation pathways.
Introduction
Anexelekto (AXL) is a receptor tyrosine kinase (RTK) of the TAM (Tyro3, AXL, and MER) family.1–4 Activation of AXL by growth arrest specific 6 (GAS6) induces diverse survival cascades.1–4 Recent studies have revealed that AXL regulates survival signaling in many cancers, including leukemia.1–5 High expression of AXL or GAS6 in AML patients is prognostic for poor survival outcome.6–10 The AXL/GAS6 axis promotes leukemia cell proliferation and chemoresistance.7 AXL is a key regulator of myeloid cell differentiation.2 FMS-like tyrosine kinase (FLT3) regulates leukemia cell differentiation.11–13 FLT3 mediated effects on differentiation may be mediated by AXL. Bone marrow (BM) derived mesenchymal stromal cells (MSC) protect leukemia cells from chemotherapy.14–17 FLT3 mutations involving internal tandem duplication (ITD) or point mutations (e.g., D835) are found in >30% of AML patients and are associated with poor survival.18 Furthermore, though FLT3 inhibitors are in the clinic, these agents have shown limited effectiveness, with acquired resistance being a major problem.18–20 AXL positively regulates signaling mediated by mutant FLT3 protein.7–10 Thus, disruption of AXL in cells with mutant FLT3 may disrupt signaling controlled by the mutant kinase. ONO-7475 is a novel TAM inhibitor that targets AXL in the nM range.21 In the report herein, we examine the efficacy of ONO-7475 in AML cells in both in vitro and in vivo models.
Methods
Cell lines and cell culture
MOLM13 were purchased from DSMZ (Braunschweig, Germany). MV4;11 and HL60 were purchased from ATCC (Manassas, VA, USA). OCI-AML3 were kindly provided by Mark Minden (Ontario Cancer Institute, Toronto, ON, Canada). MOLM13 luciferase (luc)/green fluorescent protein (gfp) cells were generated using lentiviral transduction. The lentiviral plasmid was generated by cloning the firefly luciferase sequence excised from pGL4.51 (Promega, Madison, WI, USA) into the pCDH-CMV-MCS-EF1-copGFP lentiviral vector (System Biosciences Inc., Mountain View, CA, USA).
MOLM13 p53 short hairpin ribonucleic acid (shRNA) cells were previously described.22 BM-MSC were acquired in accordance with regulations and protocols approved by the Investigational Review Board of the University of Texas MD Anderson Cancer Center (MDACC). Informed consent was obtained in accordance with the Declaration of Helsinki.
Reagents
ONO-7475, mouse feed containing ONO-7475, and control mouse feed were supplied by Ono Pharmaceutical Co. Ltd. (Osaka, Japan). Cytarabine (AraC) was purchased from LC Laboratories (Woburn, MA, USA). Stock solutions were prepared with dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA).
RPPA
Reverse phase protein analysis (RPPA) was performed by the RPPA Core at the University of Texas MDACC as described in the Online Supplementary Methods.
DNA synthesis and cell cycle assay
Cells were incubated with vehicle or ONO-7475 and then analyzed for their rates of DNA synthesis and cell cycle status using the Alexa Fluor 444 Click-It EdU Flow Cytometry assay kit from Molecular Probes (Carlsbad, CA, USA) per manufacturer’s instructions.
Gene expression analysis
Real-time polymerase chain reaction (qRT-PCR) was performed with an ABI 7900HT Fast Real-Time PCR System (Life Technologies, Carlsbad, CA, USA), using TaqMan assays listed in the Online Supplementary Methods, as directed by the manufacturer. RQ Manager 1.2.1 (Life Technologies) was used for data analysis.
Immunoblot analysis
Cells were incubated with vehicle or ONO-7475 and then lysed, and total protein was fractionated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS/PAGE) electrophoresis. Immunoblot analysis was performed with antibodies listed in the Online Supplementary Methods. Signals were detected with Odyssey Infrared Imaging System and quantitated by Odyssey software version 3.0 (both LI-COR Biosciences, Lincoln, NE, USA). Tubulin was used as a loading control.
Pathway analysis
String software (String 10.0; website: http://string-db.org)23 was used to determine protein associations.
Human AML xenograft in vivo model
Human xenograft experiments were approved by the Institutional Animal Care and Use Committee at the University of Texas MDACC and are described in the Online Supplementary Methods.
Immunohistochemistry
Immunohistochemistry (IHC) was performed on paraffin embedded spleen sections using cleaved Caspase 3 (Asp 175) rabbit antibody from Cell Signaling (Danvers, MA, USA) and Vectastain ABC AP kit (Vector Laboratories; Burlingame, CA, USA).
Pathologic analysis
Immunohistochemistry (IHC) was performed on paraffin embedded spleen sections using cleaved Caspase 3 (Asp 175) rabbit antibody from Cell Signaling (Danvers, MA, USA) and Vectastain ABC AP kit (Vector Laboratories; Burlingame, CA, USA).
Results
AXL inhibitor ONO-7475 kills or growth arrests FLT3-ITD AML cells
MOLM13 and MV4;11 (FLT3 ITD) and OCI-AML3 (FLT3 WT) cells were incubated with vehicle (0.1% DMSO) or varying doses of ONO-7475 for 48 hours. Viable cell number and apoptosis were measured by flow cytometry. ONO-7475 slightly reduced OCI-AML3 viable cell number and did not induce apoptosis (Figure 1A, Online Supplementary Figure S1A). FLT3 WT HL60 cells were also resistant to ONO-7475 (data not shown). ONO-7475 was effective against MOLM13 and MV4;11 cells, both of which have FLT3-ITD. For these cells, 10 nM ONO-7475 significantly reduced cell viability by >50% (P<0.0001; Figure 1A) and induced apoptosis (P<0.00001; Online Supplementary Figure S1A). Cell based tyrosine kinase inhibition assays revealed ONO-7475 is 210-fold more selective for AXL compared to FLT3 (Online Supplementary Table S1). While IC50 for 48-hour treatment of drug was 0.7 nM for AXL and 1.0 nM for MER, the IC50 for FLT3 was 147 nM (Online Supplementary Table S1). As apoptosis was significantly induced in both MOLM13 and MV4;11 cells after 48-hour treatment with a dose (10 nM; Online Supplementary Figure S1B) of drug well below the 48 hour IC50 of ONO-7475 for FLT3 (147 nM), this result suggested that the drug action is independent of FLT3. After 72 hours, 10 nM ONO-7475 nearly eliminated all viable MOLM13 cells (Figure 1B) with >70% of cells becoming apoptotic (Online Supplementary Figure S1B). BM derived MSC protect leukemia cells from chemotherapy.14–17 To simulate the effect of the BM microenvironment during chemotherapy, we tested the efficacy of ONO-7475 using an in vitro co-culture system. MOLM13 cells in monoculture or in co-culture with MSC were incubated for 72 hours with vehicle or ONO-7475. The drug potently induced apoptosis and nearly eliminated the AML cells in monoculture (Figure 1B, Online Supplementary Figure S1B). While MSC protected the AML cells from the inhibitor, treatment with a higher dose of drug abrogated most of this effect (50 nM ONO-7475, Figure 1B).
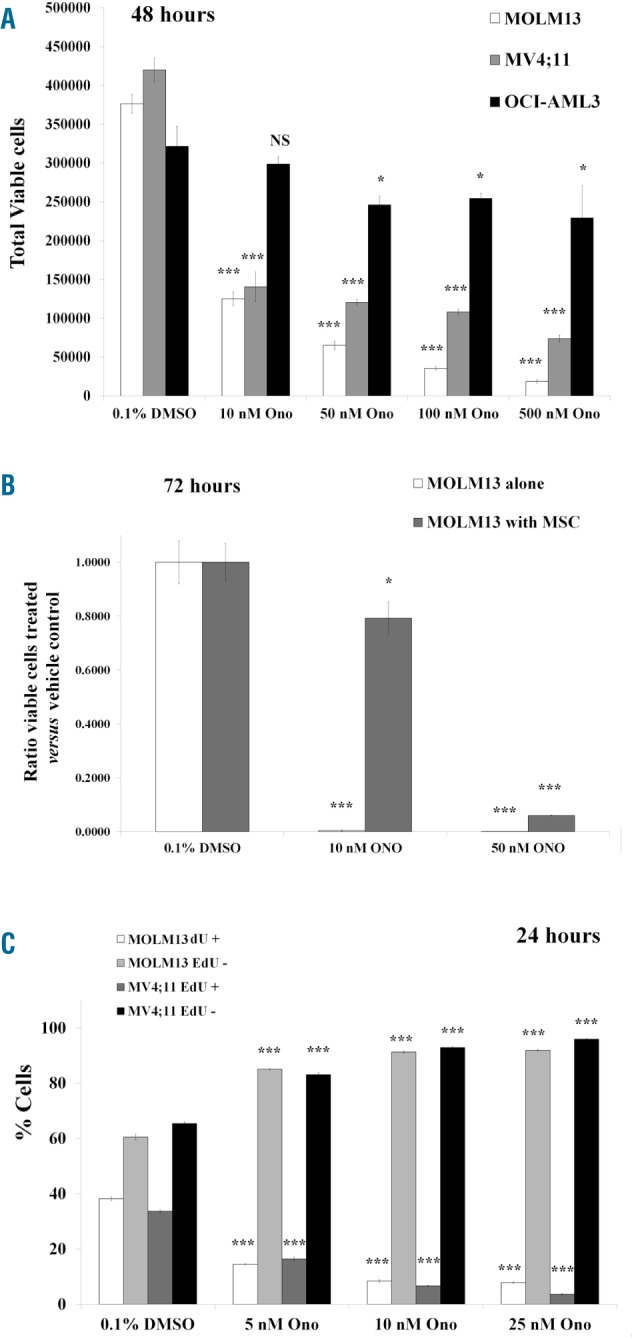
ONO-7475 effectively reduces viable FLT3-ITD AML cells even in the presence of MSC. (A) Cells were incubated with varying doses of ONO-7475 and cell viability was assessed by flow cytometry. (B) MOLM13 cells were co-cultured with MSC, incubated with ONO-7475 and cell viability was assessed by flow cytometry. (C) DNA synthesis and cell cycle were assessed by EdU Click-It assay in vehicle treated cells and cells treated with ONO-7475. Student’s t-test was performed against vehicle treated cells (*P<0.05; ***P<0.001). DMSO: dimethyl sulfoxide.
MOLM13 and MV4;11 cells were incubated with vehicle or varying doses of drug for 24 hours, and their cell cycle status and rates of DNA synthesis were assessed by flow cytometry using FX Cycle Violet staining and EdU incorporation. Low dose ONO-7475 (5 nM) suppressed DNA synthesis in both cell lines by >2-fold (Figure 1C). ONO-7475 potently induced G0/G1 arrest in both cell lines (Online Supplementary Figure S2A,B).
AraC as well as p53 reduction augments ONO-7475-induced apoptosis in MOLM13 cells. MOLM13 cells were incubated with vehicle (0.2% DMSO), 1 μM AraC alone, ONO-7475 alone (10nM or 50 nM), or a combination of the drugs for 48 hours. While AraC and ONO-7475 were effective as single agents, the combination reduced viable cell numbers (Figure 2A) and induced apoptosis (Online Supplementary Figure S3A) to a greater extent.
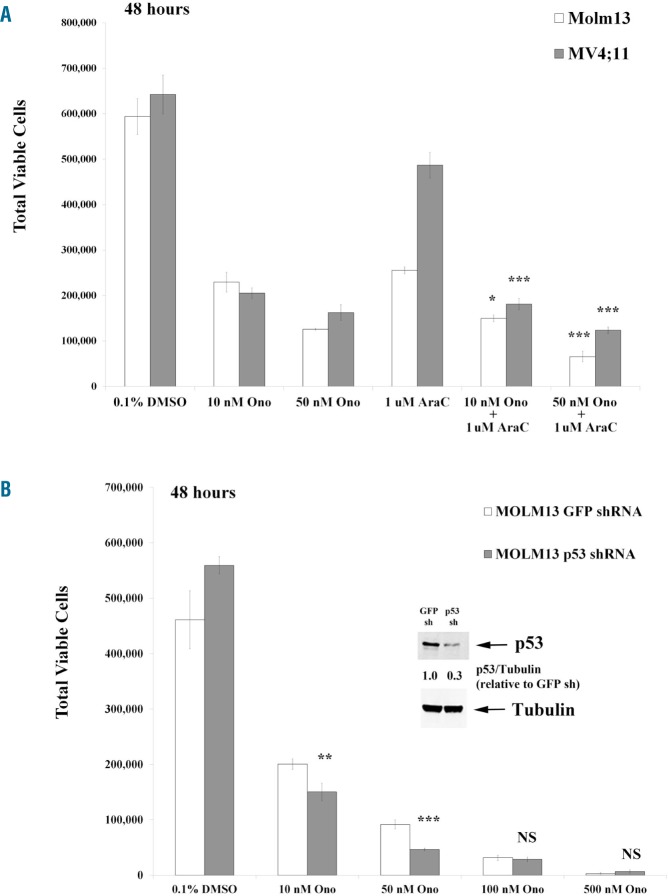
ONO-7475 augments AraC-induced killing in FLT3-ITD AML cell lines though suppression of p53 sensitizes cells to the drug. (A) Cells were incubated with varying doses of ONO-7475 and/or 1 μM AraC for 48 hours and cell viability assessed by flow cytometry. (B) MOLM13 control cells or MOLM13 p53 shRNA were incubated with ONO-7475 for 48 hours and then cell viability assessed by flow cytometry. Protein expression of p53 and Tubulin in MOLM13 GFP shRNA and MOLM13 p53 shRNA cells as determined by immunoblot is imbedded in (B). Ratio of p53 to Tubulin relative to GFP shRNA control was determined by densitometry of bands using LiCore imager. Student’s t-test was performed against AraC treated (*P<0.05; ***P<0.001) for (A) and against GFP control (**P<0.01; ***P<0.001) for (B). NS: not significant; DMSO: dimethyl sulfoxide; AraC: cytarabine; GFP: green fluorescent protein; shRNA: short hairpin ribonucleic acid.
Both MOLM13 and MV4-11 cells have wild-type p53. To determine whether the drug’s mechanism of action involves p53, we incubated MOLM13 control shRNA (GFP as non-human shRNA target) or MOLM13 p53 shRNA cells for 48 hours with varying doses of ONO-7475 for 48 hours. Expression of p53 in cells with p53 shRNA was ~30% of that of cells with GFP control shRNA (Figure 2B). Surprisingly, the p53 knockdown cells were more sensitive to the inhibitor than the control cells. A dose of 10 nM ONO-7475 reduced the viability of control cells by ~55% (Figure 2B) without inducing apoptosis (Online Supplementary Figure S2B), whilst, however, reducing the viability of knockdown cells by >70% (Figure 2B) and inducing some apoptosis (~20%; Online Supplementary Figure S2B). Only at doses >100 nM were the control and knockdown cells similarly sensitive (Figures 2B, Online Supplementary Figure S3B), suggesting a pro-survival role for p53 in this scenario in AXL-regulated cascades.
Inhibition of AXL suppresses survival and proliferation signaling and induces apoptotic proteins in FLT3-ITD but not WT FLT3 AML cells
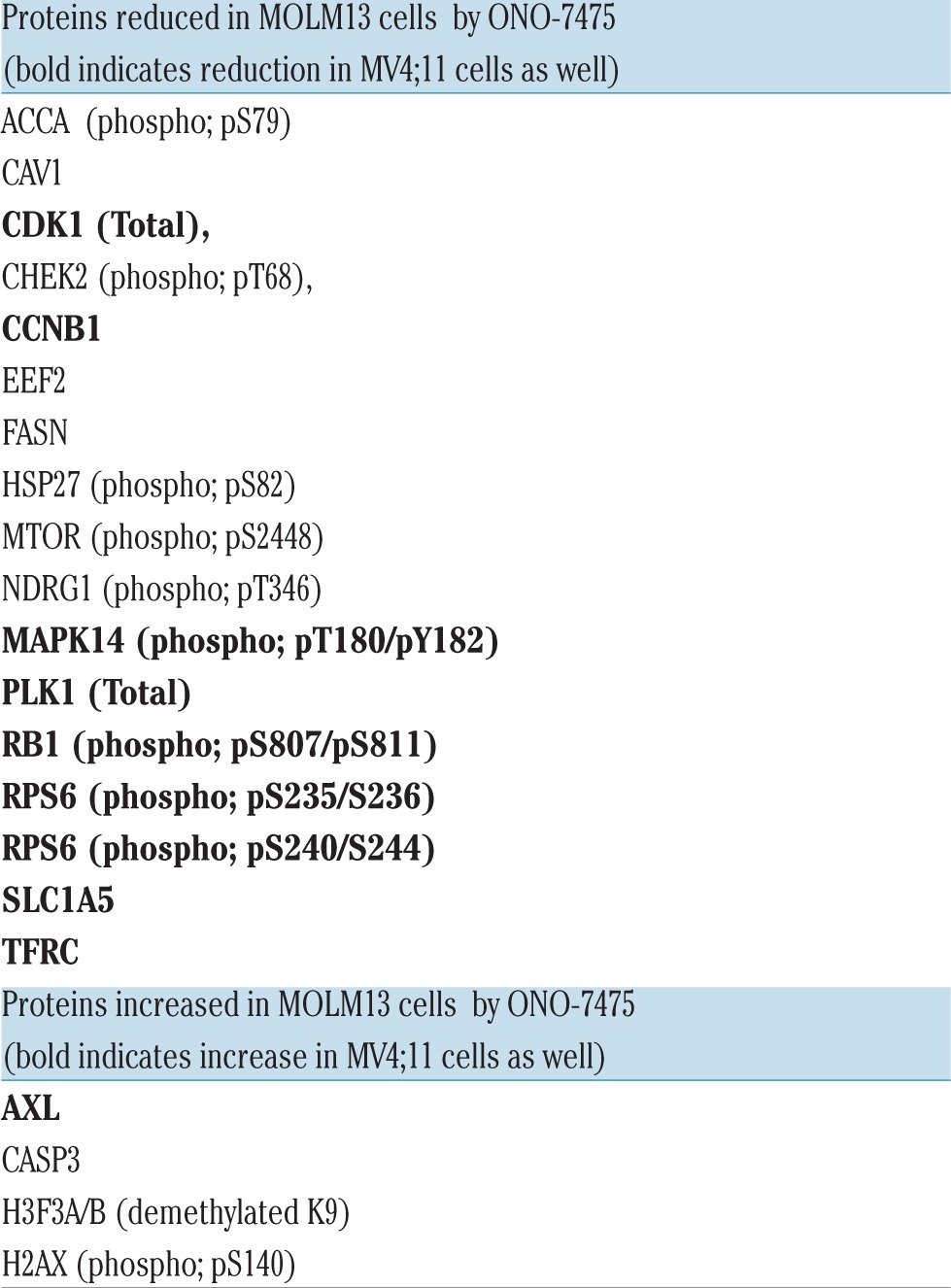
RPPA was performed by the MDACC Proteomics Core to analyze MOLM13, MV4:11, OCI-AML3, and HL60 cells that were treated with vehicle or ONO-7475 for 24 hours. Proteins surveyed by RPPA are listed in Online Supplementary Table S2. Trypan blue staining of collected cells revealed that cells were >95% viable in all cases, except that of MOLM13 cells treated with 100 nM ONO-7475, which were ~88% viable. There was no significant change (i.e., 2-fold) in the level of protein observed in either FLT3 WT cell line (OCI-AML3 or HL60; Online Supplementary Figure S4A). Protein changes of at least 2-fold were most prominent in MOLM13 cells, with some of the same proteins showing similar changes in MV4;11 cells (Online Supplementary Figure S4A-C; Online Supplementary Figure S5A,B). Proteins suppressed in MOLM13 and MV4;11 cells are listed in Table 1. RPPA identified a number of molecules involved in signal transduction and cell cycle regulation. Only four proteins were induced by ONO-7475 in MOLM13 cells, however, one of these was AXL, which was also induced in MV4;11 cells (Online Supplementary Figure S5A,B; Table 1). Interestingly, total AXL protein is induced by the drug in both cell lines, suggesting the presence of a feedback loop involving the activity of the protein and its level of expression.
Table 1.
Proteins affected by ONO-7475 in MOLM13 and MV4;11 cells as identified by RPPA.
Protein association network analysis was performed using STRING 10.0 23 on proteins identified as suppressed in MOLM13 cells (Figure 3A). The analysis suggests strong interconnection between various proteins that are distinctly suppressed by the drug, with only ACACA, NDRG1, and SLC1A5 showing no interconnection. There appears to be strong cross-talk between proteins involved in the cell cycle (Figure 3A).
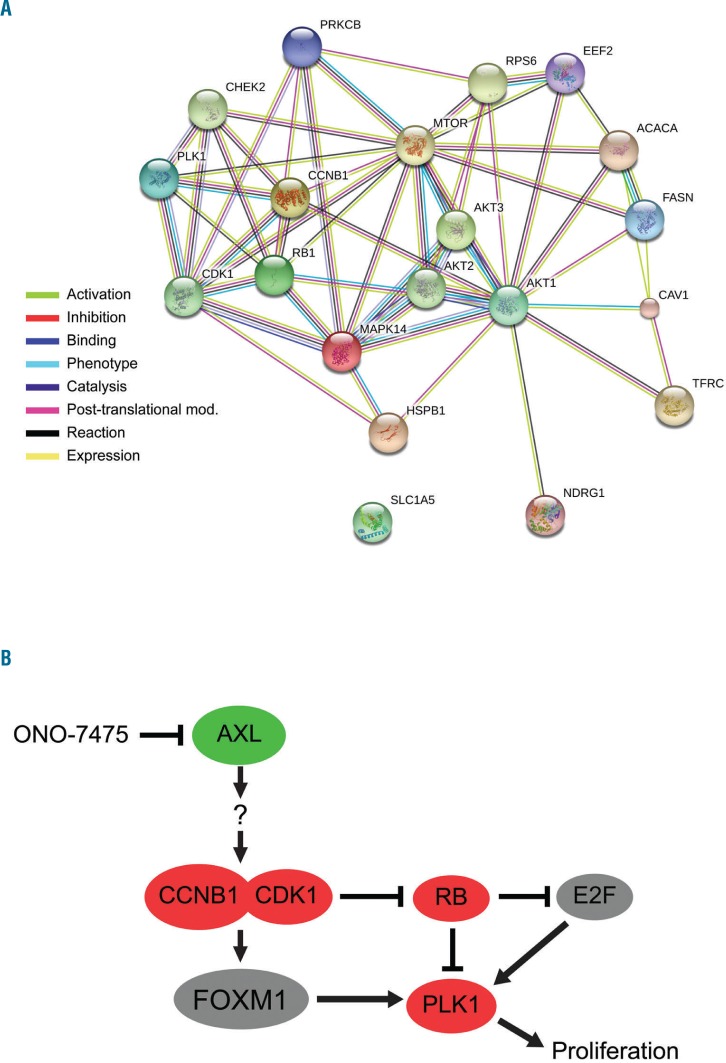
Proteins identified by RPPA as suppressed by ONO-7475 suggest common pathways and a model of cell cycle blockade involving CDK1/CCNB1, RB, and PLK1. String analysis was performed on proteins suppressed by >2-fold according to RPPA. Network is depicted in (A). A model hypothesizing ONO-7475 blocks CDK1/CCNB1 to inhibit RB phosphorylation resulting in the activation of RB, which in turn suppresses PLK1, is depicted in (B).
The effects of AXL on PLK1, RB, or CDK1 in leukemia cells have yet to be reported. PLK1 is regulated in part via transcriptional mechanisms.24–27 Figure 3B depicts a model of AXL regulation of PLK1. ONO-7475 suppression of CDK1 and Cyclin B1 could inhibit PLK1 expression via the activation of RB. Immunoblot analysis confirmed the effect of ONO-7475 on RPPA candidate target proteins, including PLK1, p-RB, p-S6 ribosomal protein, p-p38, and AXL (Figure 4A,B). Consistent with RPPA results (Online Supplementary Figure S4B,C), immunoblot analysis demonstrated that the AXL inhibitor potently suppressed PLK-1 in both cell lines (Figure 4A). Little, if any, PLK1 was detected when either were treated with 50 nM or 100 nM drug. In addition, as predicted by RPPA (Online Supplementary Figure S5A,B), ONO-7475 induced AXL and blocked RB phosphorylation in both cell lines, even at the lowest dose (Figure 4A). The drug reduced total RB in MOLM13 cells, and, to a lesser extent, in MV4;11 cells (Figure 4A). As shown in Figure 4C, ONO-7475 potently suppressed both Cyclin B1 and CDK1 in both cell lines even at a low dose (10 nM) ONO-7475. Immunoblot validation of the suppression of Cyclin B1, CDK1, PLK1 and the inhibition of RB phosphorylation supports the model proposed in Figure 3B.

ONO-7475 suppresses various positive regulators of survival and cell cycle in FLT3-ITD AML cell lines. MOLM13 and MV4;11 were incubated with varying doses of ONO-7475 for 24 hours. Immunoblot analysis was performed as described in Methods. Ratio of protein signal to that of Tubulin loading control or to total protein for phospho-protein was determined by densitometry using LiCor imager. Veh: vehicle.
In MOLM13 cells, the AXL inhibitor inhibited the phosphorylation of S6, and p38 phosphorylation was reduced due to reduction of total p38 (Figure 4B). Though not detected by RPPA, p-ERK and MCL-1 were examined by immunoblot. AXL is known to induce ERK,2,3,28 and ERK is a positive regulator of MCL-1.29,30 ONO-7475 reduced ERK phosphorylation (~50% at 10 nM; Figure 4B) in MOLM13 cells. ONO-7475 suppressed MCL-1 (>80% at 10 nM) in MOLM13 cells but was less effective in MV4;11 cells (50% reduction of MCL-1 at 100 nM; Figure 4A).
AXL inhibition by siRNA inhibits FLT3 phosphorylation.9 Treatment with ONO-7475 reduced FLT3 phosphorylation in both MOLM13 and MV4;11 cells (Online Supplementary Figure S6). An ONO-7475 dose of 10 nM greatly reduced p-FLT3 in MOLM13 cells and nearly eliminated it in MV4;11 cells, suggesting that AXL inhibition may prevent FLT3 activation (Online Supplementary Figure S6).
To determine whether AXL modulated its target genes at the transcriptional level, we incubated MOLM13 and MV4;11 cells with vehicle or drug for 24 hours, then extracted total RNA, reverse transcribed it and performed qRT-PCR to measure the abundance of transcripts for PLK-1, CDK1, CCNB1 and B2M. ONO-7475 reduced the messenger (m)RNA of each cell cycle regulator relative to that of B2M in both cell lines. A dose of 10 nM ONO-7475 reduced PLK-1 mRNA ~60% and 50 nM drug reduced PLK-1 mRNA by ~80% in MOLM13 cells (Figure 5A). Both doses more potently suppressed PLK-1 in MV4;11 cells (Figure 5A). The drug reduced CDK1 (Figure 5B) and CCNB1 (Figure 5C) in both cell lines by at least 60% at 10 nM dose. These data suggest that AXL positively regulates transcription of these cell cycle regulators in FLT3-ITD AML cells. Moreover, we found that ONO-7475 promoted an increase in the level of the AXL transcript. In both cell lines AXL mRNA relative to that of B2M was increased by ~3.5-fold with 10 nM drug and by >6-fold in MOLM13 cells and >8-fold in MV4;11 cells with 50 nM drug after 24 hours (Online Supplementary Figure S7). These results suggest a feedback mechanism whereby AXL inhibition induces AXL transcription.
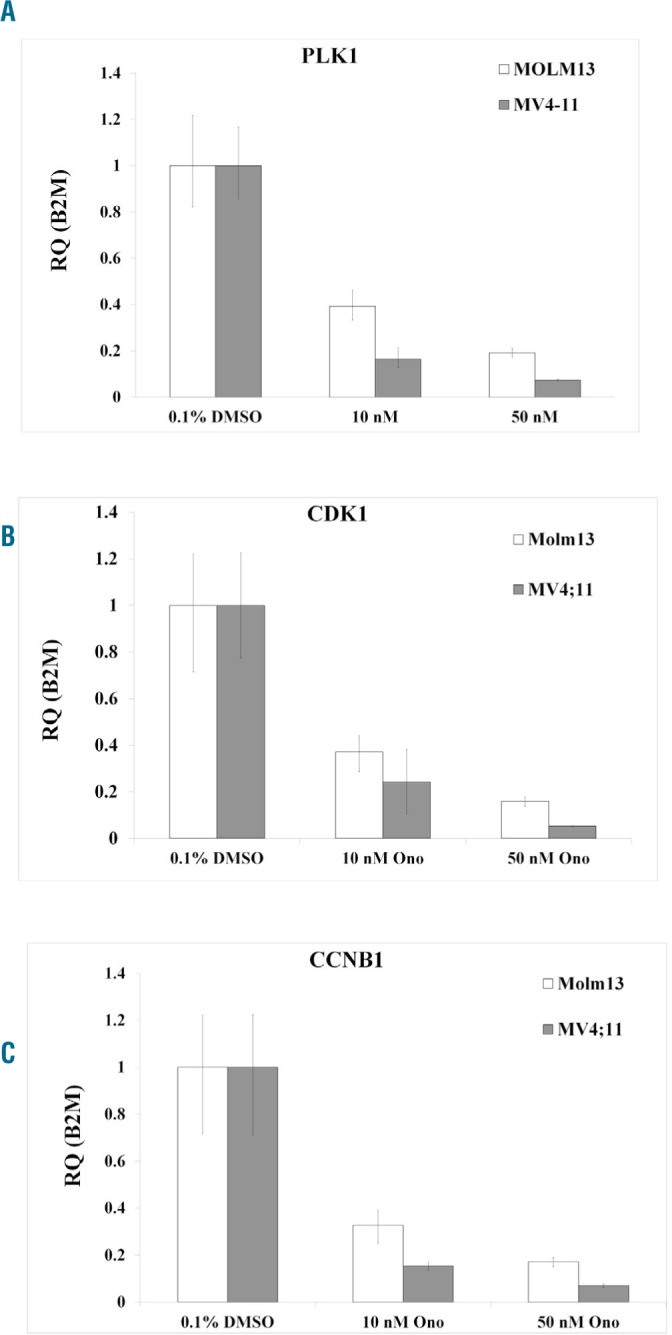
ONO-7475 suppresses PLK1, CDK1, and CCNB1 in MOLM13 and MV4;11 cells. MOLM13 and MV4;11 were incubated with varying doses of ONO-7475 for 24 hours. RNA from cells was extracted and reverse transcribed, and the abundance of the transcripts for PLK-1 (A), CDK1 (B), and CCNB1 (C), and B2M (A–C) were determined by qRT-PCR. Gene expression levels were normalized to B2M. DMSO: dimethyl sulfoxide.
ONO-7475 is effective in a murine in vivo xenograft model using MOLM13 cells
To test the efficacy of ONO-7475 in a human AML xenograft model, the initial experiment utilized a relatively low dose of drug (i.e., 6 mg/kg) which was introduced into mouse food. The manufacturer estimates 0.004% is roughly 6 mg/kg daily consumption of drug (T. Yasuhiro and T. Yoshizawa, Ono Pharmaceutical Co. LTD, personal communication). MOLM13 luc/gfp were injected into NOD scid γ (NSG) mice. Mice were given control feed for one week and then split into groups that continued control feed (N=5) or feed with 0.004% ONO-7475 (N=5). Mice given feed with ONO-7475 exhibited significantly (P=0.0047) longer median survival compared to mice given control feed (Figure 6A, Online Supplementary Table S3). In vivo imaging system (IVIS) imaging was performed weekly to determine the leukemic burden. On Day 14, three out of five of the mice given food containing ONO-7475 exhibited reduced leukemic burden compared to the mice which were fed control food (Figure 6B). Sternum (for BM) and liver were collected from a moribund mouse from the control group and a moribund mouse from the 0.004% ONO-7475 group and H&E staining was performed (Online Supplementary Figure S8A) As shown in Online Supplementary Figure S8A, there were fewer mitotic bodies (red arrows) and more apoptotic cells (yellow arrows) observed in tissues of the mouse fed drug compared to the mouse fed normal diet. Spleen was collected from these animals for IHC staining of cleaved Caspase 3 to visualize apoptosis (Online Supplementary Figure S8B). There were more cells exhibiting cleaved Caspase 3 in the spleen of the mouse that was fed drug. Next, efficacy of a higher dose (i.e., 0.013%, which is roughly 20 mg/kg daily) of ONO-7475 was tested. MOLM13 luc/gfp cells were introduced into NSG mice. Mice were fed control feed for one week and then split into groups that continued control feed (N=9) or feed containing 0.013% ONO-7475 (N=10). Mice fed 0.013% ONO-7475 exhibited significantly (P=0.0062) longer median survival compared to mice given control feed (Online Supplementary Figure S9, Online Supplementary Table S3). Sternum (for BM) and liver were collected when mice became moribund, and H&E staining was performed (Figure 7A). Mitotic bodies and apoptotic cells were visualized in samples. While leukemia cells were present in samples from both control and ONO-7475 fed mice, mitotic bodies (red arrows) were higher in control mice while apoptotic cells (yellow arrows) were more prominent in leukemia cells in BM from mice fed the drug (Figure 7A). To quantitate apoptotic cells, IHC using antibody to cleaved Caspase 3 was performed on spleen sections. More apoptotic cells were observed in spleen sections of mice given drug compared to control mice (Figure 7B). Apoptotic cells and total AML cells were counted and the percentage determined for each set of mice. There were significantly more apoptotic cells in spleen sections of mice fed ONO-7475 compared to mice given control food (>2-fold; P=0.010; Figure 7C).
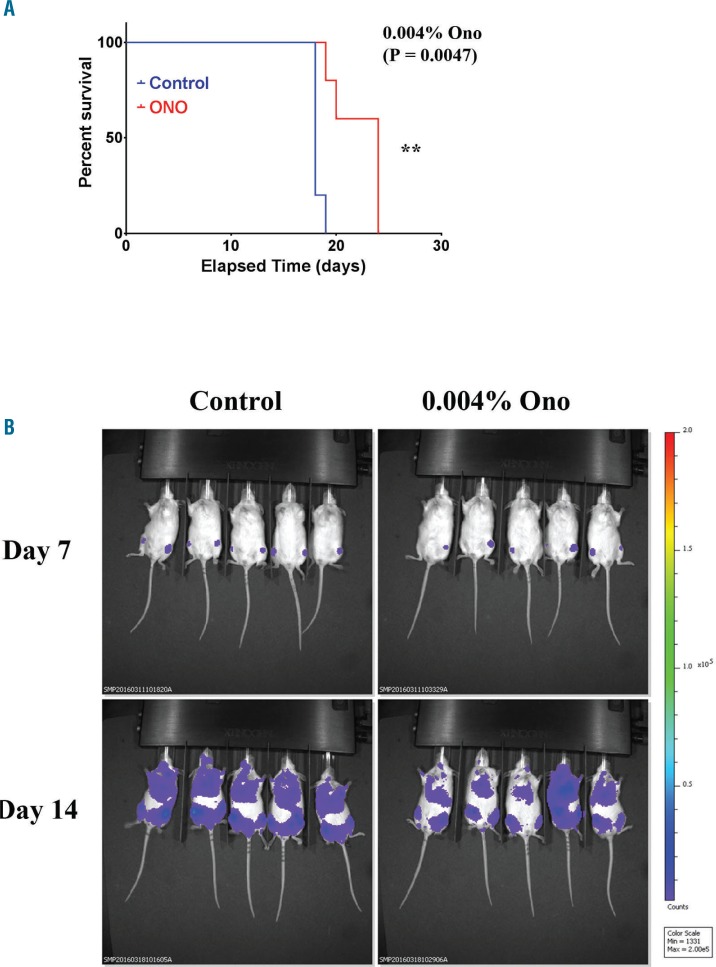
ONO-7475 prolongs survival of mice bearing MOLM13 leukemia cells. MOLM13 luc/gfp cells were injected into NSG mice, and mice received either control diet or diet containing 0.004% ONO-7475. (A) Survival analysis of mice fed 0.004% drug using GraphPad software. (B) Imaging of mice fed control feed or feed with 0.004% ONO-7475 taken at Day 7 and Day 14.
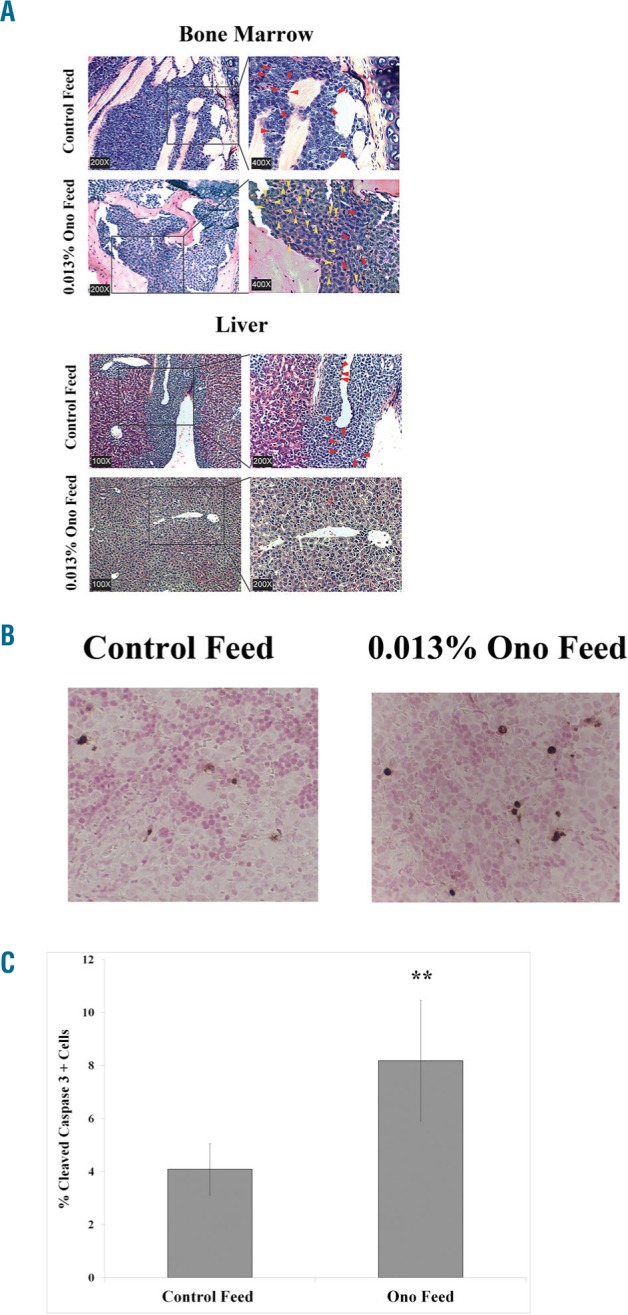
ONO-7475 induces apoptosis of MOLM13 leukemia cells in vivo and inhibits metastasis to the liver. MOLM13 luc/gfp cells were introduced into NSG mice and mice received either control feed or feed containing 0.013% ONO-7475. (A) H&E stain of BM and liver tissue. Mitotic cells (red arrows) and apoptotic cells (yellow arrows) are indicated. (B) Representative IHC using antibody against cleaved Caspase 3 to identify apoptotic cells in spleen. (C) Total apoptotic cells counted in spleen IHC samples probed with antibody against cleaved Caspase 3 from mice fed control diet (N=3) or 0.013% ONO-7475 (N=3). Statistical significance was determined by Student’s t-test (*P<0.05).
An interesting effect of the drug in the in vivo model involves leukemia infiltration in the liver. Mice fed ONO-7475 showed little, if any, AML cells in the liver, whereas AML cells infiltrated the livers in control mice (Figure 7A).
Discussion
In this study, we found FLT3 WT OCI-AML3 and HL60 cells were resistant to ONO-7475, while FLT3-ITD cell lines MOLM13 and MV4;11 were sensitive (Figure 1A and Online Supplementary Figure S1A). ONO-7475 induced apoptosis in MOLM13 and MV4;11 cells. At low dose (5 nM), ONO-7475 effectively growth arrests both cell lines (Online Supplementary Figure S2A,B). ONO-7475 is a TAM kinase inhibitor, so a role for MER in ONO-7475-induced effects is possible. MER is aberrantly expressed in AML.31 We tested MER expression in the AML cell lines and found it to be abundant in OCI-AML3, minimal in MV4;11, and undetectable in MOLM13 cells even after ONO-7475 treatment (Online Supplementary Figure S10A). AXL was detectable in MOLM13 cells, and, to a lesser extent, in HL60 cells, but not in OCI-AML3 cells (Online Supplementary Figure S10B). Short term treatment of recombinant human GAS6 (500 ng/ml for one hour) did not induce AXL in the OCI-AML3 cells (Online Supplementary Figure S10B). These data suggest that the effects of the drug in these AML cells are mediated only through AXL.
The microenvironment is central to the role of AXL in drug resistance.7–10 In our in vitro model of the leukemia microenvironment, MSC protected MOLM13 cells from the inhibitor, though this effect was overcome with a higher drug dose (Figure 1B and Online Supplementary Figure S1B). AXL mediates drug resistance involving a number of RTK in diverse cancers.1–5,7,9,10 ONO-7475 suppressed FLT3 phosphorylation (Online Supplementary Figure S6), which is consistent with AXL as a positive regulator of mutant FLT3.9 RPPA revealed that a distinct set of proteins was altered by ONO-7475 in FLT3-ITD but not FLT3 WT cells (Online Supplementary Figure S2A). ONO-7475 reduced p-ERK and MCL-1 in MOLM13 cells even at a low dose (Figures 4A,B). MCL-1 is critical for AML cell survival.31,32 FLT3-ITD induces MCL-1 expression in AML stem cells,32 so perhaps MCL-1 is central for AXL-mediated survival in FLT3 ITD cells. The mechanism by which AXL affects proliferation in FLT3 ITD cells is unclear.10 ONO-7475 suppresses DNA synthesis (Figure 1C, Online Supplementary Figure S2A,B) and potently suppresses PLK-1 (Figure 4A and Figure 5A, respectively). As PLK-1 is required for mitotic entry in cancer cells,24–27 this may account for the blockade of DNA synthesis by the drug. Although ONO-7475 more potently induces apoptosis in MOLM13 cells (Online Supplementary Figure S1B), it acts to a greater extent in MV 4;11 cells by inducing growth arrest (Online Supplementary Figure S2A,B). These findings suggest that the physiologic response to the drug will depend on the nature of the pathways affected. Consistent with a model where ONO-7475 activation of RB suppresses PLK-1 to growth arrest cells (Figure 3C), the drug more potently blocked RB phosphorylation and PLK-1 expression in MV4;11 cells compared to MOLM13 cells (Figure 4A). ONO-7475 blocks Cyclin B1 and CDK1 expression, suggesting a possible mechanism by which the drug blocks RB phosphorylation. Wilson and colleagues observed that AXL suppression inhibits CDK1 phosphorylation, but they did not observe a reduction of CDK1 expression.33 In the study herein, gene expression of both CDK1 (Figure 5B) and CCNB1 (Figure 5C) were suppressed by the drug as observed by qRT-PCR. The possible regulation by AXL of the CDK1/RB/PLK1 axis is novel and suggests that AXL has an important function in regulating cell cycle in FLT3-ITD AML cells. Consistent with a role for AXL in cell cycle regulation, Kariolis et al. found that blockade of AXL in AML cells with AXL decoy receptor MYD1-72 was associated with cell cycle arrest.34 Importantly, MYD1-72 killed FLT3 ITD AML cells or GAS6 exposed FLT3 WT cells, suggesting that AXL is a viable target for AML therapy.34 Currently, a number of agents that can inhibit AXL such as gilteritinib (ASP2215) and BGB324 (R48) are in clinical trials for cancers, including AML.35,36 The results of the trial using gilteritinib as a single agent for therapy for refractory or relapse AML were promising. The agent was well tolerated; 90% of patients exhibited inhibition of FLT3 phosphorylation and 40% achieved some response.36 Preclinical studies have shown that gilterinib is effective in in vitro and in vivo models of AML.37,38 Gilteritinib targets both FLT3 and AXL with IC50 is in the nM range (0.29 nM and 0.7 nM, respectively).35,37 BGB324 is another promising agent that targets AXL.35 A recent study found that epigenetic modifying agents induced AXL, and BGB324 could sensitize resistant cells to a combination of decitabine and vorinostat.39 Thus, AXL inhibition may be beneficial at many levels for AML therapy. ONO-7475 is over 200-fold more selective for AXL compared to FLT3 (Online Supplementary Table S1), consequently, perhaps resistance mechanisms that are observed with other FLT3 inhibitors might be avoided with this agent. We are currently testing ONO-7475 in combination with traditional FLT3 inhibitors to test this possibility.
ONO-7475 was able to synergize with AraC (Figure 2A and Online Supplementary Figure S3A), thus, the AXL inhibitor could be combined with standard chemotherapy. We examined if ONO-7475 affected the level of p53 and found little effect (data not shown), albeit we did not examine p53 cellular localization or phosphorylation status. We tested the effect of ONO-7475 on MOLM13 cells with suppressed p53 expression. Interestingly, knockdown of p53 sensitized the cells to low doses of the drug (Figure 2B and Online Supplementary Figure S3B). FLT3 mutations and p53 mutations appear to be mutually exclusive in AML;40 perhaps p53 has some survival function in FLT3 mutated cells, even if the precise mechanism remains unclear.
The in vivo study using ONO-7475 in an AML xenograft model with MOLM13 luc/gfp cells in NSG mice suggests the drug is efficacious for AML therapy, at least in FLT3-ITD AML. Both low dose and high dose ONO-7475 significantly prolonged the survival of mice bearing MOLM13 cells (Figure 6A and Online Supplementary Figure S8; Online Supplementary Table S2). Increased apoptosis in leukemia cells in treated mice was verified by IHC examining Cleaved Caspase 3 levels. There was >2-fold more apoptotic cells in the spleens of mice fed ONO-7475 compared to mice given control feed (Figures 7B,C). Interestingly, mice on control feed showed moderate to severe infiltration of AML cells in the liver, whereas mice fed high dose ONO-7475 showed minimal presence of MOLM13 cells in the liver (Figure 7A). The in vivo data are encouraging as the drug supports prolonged survival and may have beneficial effects in leukemic infiltration in the liver.
In summary, ONO-7475 potently arrests growth and induces apoptosis of FLT3-ITD AML cells. The drug acts via diverse mechanisms, including suppression of the CDK1/RB/PLK1 axis. Importantly, the drug prolonged mouse survival and suppressed AML cell infiltration in the liver in the AML xenograft model. These combined results suggest that ONO-7475 is efficacious for AML therapy and warrants pursuit for development of the drug in the clinic.
Supplementary Material
Acknowledgments
We thank Tomoko Yasuhiro, Kohei Tanaka, and Toshio Yoshizawa from Ono Pharmaceutical Company Ltd., Osaka, Japan for providing drug and technical advice for these studies.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/12/2048
Funding
This work was supported in part by funds from Ono Pharmaceutical Company Ltd. (PR). MDACC Flow Core (MA) and RPPA Core are funded by the National Institutes of Health (CA-016672).
References
Articles from Haematologica are provided here courtesy of Ferrata Storti Foundation
Full text links
Read article at publisher's site: https://doi.org/10.3324/haematol.2017.168856
Read article for free, from open access legal sources, via Unpaywall:
http://www.haematologica.org/content/haematol/102/12/2048.full.pdf
Citations & impact
Impact metrics
Article citations
MERTK Inhibition as a Targeted Novel Cancer Therapy.
Int J Mol Sci, 25(14):7660, 12 Jul 2024
Cited by: 0 articles | PMID: 39062902 | PMCID: PMC11277220
Review Free full text in Europe PMC
AXL as immune regulator and therapeutic target in Acute Myeloid Leukemia: from current progress to novel strategies.
Exp Hematol Oncol, 13(1):99, 04 Oct 2024
Cited by: 0 articles | PMID: 39367387 | PMCID: PMC11453060
Review Free full text in Europe PMC
Drug tolerant persister cell plasticity in cancer: A revolutionary strategy for more effective anticancer therapies.
Signal Transduct Target Ther, 9(1):209, 14 Aug 2024
Cited by: 0 articles | PMID: 39138145 | PMCID: PMC11322379
Review Free full text in Europe PMC
Effects of Combined Therapeutic Targeting of AXL and ATR on Pleural Mesothelioma Cells.
Mol Cancer Ther, 23(2):212-222, 01 Feb 2024
Cited by: 2 articles | PMID: 37802502 | PMCID: PMC10831449
TAM family kinases as therapeutic targets at the interface of cancer and immunity.
Nat Rev Clin Oncol, 20(11):755-779, 04 Sep 2023
Cited by: 13 articles | PMID: 37667010
Review
Go to all (14) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
AXL/MERTK inhibitor ONO-7475 potently synergizes with venetoclax and overcomes venetoclax resistance to kill FLT3-ITD acute myeloid leukemia.
Haematologica, 107(6):1311-1322, 01 Jun 2022
Cited by: 12 articles | PMID: 34732043
MZH29 is a novel potent inhibitor that overcomes drug resistance FLT3 mutations in acute myeloid leukemia.
Leukemia, 31(4):913-921, 24 Oct 2016
Cited by: 12 articles | PMID: 27773927
Cabozantinib is selectively cytotoxic in acute myeloid leukemia cells with FLT3-internal tandem duplication (FLT3-ITD).
Cancer Lett, 376(2):218-225, 06 Apr 2016
Cited by: 23 articles | PMID: 27060207
Gilteritinib: An FMS-like tyrosine kinase 3/AXL tyrosine kinase inhibitor for the treatment of relapsed or refractory acute myeloid leukemia patients.
J Oncol Pharm Pract, 26(5):1200-1212, 26 Apr 2020
Cited by: 0 articles | PMID: 32338136
Review
Funding
Funders who supported this work.
NCI NIH HHS (2)
Grant ID: P30 CA016672
Grant ID: R01 CA207204





