Abstract
Background
In the CYD14 and CYD15 Phase 3 trials of the CYD-TDV dengue vaccine, estimated vaccine efficacy (VE) against symptomatic, virologically confirmed dengue (VCD) occurring between months 13 and 25 was 56.5% and 60.8%, respectively.Methods
Neutralizing antibody titers to the 4 dengue serotypes in the CYD-TDV vaccine insert were measured at month 13 in a randomly sampled immunogenicity subcohort and in all VCD cases through month 25 (2848 vaccine, 1574 placebo) and studied for their association with VCD and with the level of VE to prevent VCD.Results
For each trial and serotype, vaccinees with higher month 13 titer to the serotype had significantly lower risk of VCD with that serotype (hazard ratios, 0.19-0.43 per 10-fold increase). Moreover, for each trial, vaccinees with higher month 13 average titer to the 4 serotypes had significantly higher VE against VCD of any serotype (P < .001).Conclusions
Neutralizing antibody titers postdose 3 correlate with CYD-TDV VE to prevent dengue. High titers associate with high VE for all serotypes, baseline serostatus groups, age groups, and both trials. However, lowest titers do not fully correspond to zero VE, indicating that other factors influence VE.Free full text

Neutralizing Antibody Correlates Analysis of Tetravalent Dengue Vaccine Efficacy Trials in Asia and Latin America
Abstract
Background
In the CYD14 and CYD15 Phase 3 trials of the CYD-TDV dengue vaccine, estimated vaccine efficacy (VE) against symptomatic, virologically confirmed dengue (VCD) occurring between months 13 and 25 was 56.5% and 60.8%, respectively.
Methods
Neutralizing antibody titers to the 4 dengue serotypes in the CYD-TDV vaccine insert were measured at month 13 in a randomly sampled immunogenicity subcohort and in all VCD cases through month 25 (2848 vaccine, 1574 placebo) and studied for their association with VCD and with the level of VE to prevent VCD.
Results
For each trial and serotype, vaccinees with higher month 13 titer to the serotype had significantly lower risk of VCD with that serotype (hazard ratios, 0.19–0.43 per 10-fold increase). Moreover, for each trial, vaccinees with higher month 13 average titer to the 4 serotypes had significantly higher VE against VCD of any serotype (P < .001).
Conclusions
Neutralizing antibody titers postdose 3 correlate with CYD-TDV VE to prevent dengue. High titers associate with high VE for all serotypes, baseline serostatus groups, age groups, and both trials. However, lowest titers do not fully correspond to zero VE, indicating that other factors influence VE.
Although a satisfactory immune correlate of protection (CoP)—a biomarker measuring an immune response to vaccination that is strongly statistically associated with vaccine efficacy (VE) [1]—has not been established, a leading hypothesis is that a dengue vaccine must elicit functional neutralizing antibodies against all 4 serotypes to achieve high efficacy to prevent infection and disease. Indeed, neutralizing antibodies are an established CoP (not necessarily mechanistic) for related flavivirus vaccines [2]. The ideal dengue vaccine would protect individuals seronegative to dengue, as well as individuals partially immune to dengue or to nondengue flaviviruses; these categories may have different CoPs.
Some studies have identified neutralizing antibody titers associated with protection from polymerase chain reaction-confirmed dengue infection [3] or symptomatic, virologically confirmed dengue (VCD) [4, 5], but others have suggested that the humoral response alone is not reliably predictive [6–8]. Moreover, CD4+ and CD8+ T cells contribute to protection against disease-accompanying infections with heterotypic dengue viruses [9–13], highlighting the complexity of dengue and the need to identify reliable CoPs.
CYD-TDV (Dengvaxia, Sanofi Pasteur) is a licensed recombinant, live, attenuated tetravalent dengue vaccine [14]. In 2 Phase 3 trials, CYD14 in 2- to 14-year-olds in Asia (NCT01373281) and CYD15 in 9- to 16-year-olds in Latin America (NCT01374516), estimated VE of CYD-TDV was 56.5% and 60.8% for the prevention of symptomatic, VCD of any serotype (DENV-Any) between 28 days after the third injection at 12 months through 25 months [15, 16]. Vaccine efficacy significantly varied by serotype, estimated at 50.0% (50.3%), 35.0% (42.3%), 78.4% (74.0%), and 75.3% (77.7%) against DENV-1, -2, -3, and -4 in CYD14 (CYD15) [15, 16]. However, the proportion of vaccine recipients with month 13 50% plaque reduction neutralization test (PRNT50) responses [17] above the lower limit of quantitation (LLOQ) (93.8%, 98.5%, 96.7%, and 96.8% in CYD14; 94.9%, 98.5%, 98.4%, and 98.1% in CYD15 for DENV-1, -2, -3, and -4) far exceeded the VE estimates [15, 16]. This discordance between VE rates and neutralization response rates, also seen in [7], indicated that PRNT50 neutralization response is not a completely valid CoP. However, this aggregate data analysis provides less information about CoPs than an analysis incorporating individual-level titer data. Accordingly, a case-cohort study was conducted to investigate the association of month 13 neutralizing antibody titers with dengue occurrence through month 25 and with the level of VE to prevent dengue, for each trial and pooled for 9- to 16-year-olds, given the CYD-TDV target population of 9 and older [18].
Prevaccination neutralizing antibody titers measure prior dengue infection and modify month 13 titers [19] and VE [15, 16], with baseline seropositive individuals having greater VE. There was low statistical precision for assessing how VE varies with month 13 titers separately in the baseline seropositive and seronegative subgroups, because 86.6% of DENV-Any endpoint cases did not have baseline samples collected that would allow classifying them in these baseline subgroups. We include this analysis but focus on assessing how VE varies with month 13 titers in all participants and in age subgroups, where age positively correlates with dengue seroprevalence [20–25] and with VE [15, 16].
METHODS
CYD14 and CYD15
In harmonized designs, healthy children were randomly assigned (2:1) to vaccine or placebo and vaccinated at months 0, 6, and 12. Randomization was site- and age-stratified (2–5, 6–11, 12–14 years, CYD14; 9–11, 12–16 years, CYD15); participants were followed with active surveillance for dengue through month 25. All correlates analyses were based on the primary dengue study endpoint, DENV-Any, and the serotype-constituent endpoints DENV-1, -2, -3, -4, assessed previously for VE [15, 16].
Case-Cohort Sampling Design
Participants enrolled in the first 2–4 months were randomly assigned to an immunogenicity subset for neutralization response assessment at months 0 (before Dose 1), 7, 13, and 25. Month 13 neutralization responses were also measured from all cases, defined as participants who experienced the dengue primary endpoint DENV-Any by month 25, constituting a case-cohort design.
Month 13 titers were assessed as correlates of risk (CoRs) and CoPs in participants who had not experienced the DENV-Any endpoint by month 13. All analyses were based on dengue cases and dengue-free controls with month 13 neutralization data. Controls for analyses of each dengue serotype were defined by not having that serotype dengue endpoint by month 25; participants experiencing the dengue endpoint with another serotype were included. Controls for analyses of the DENV-Any endpoint were defined by not having the DENV-Any endpoint by month 25.
50% Plaque Reduction Neutralization Test Assay
The PRNT50 titers were measured by Sanofi Pasteur using CYD-TDV dengue strains [17]. The optimized, validated assay detects antibody neutralization-mediated reduction in virus infectivity [17]. Serial 2-fold dilutions of heat-inactivated serum (initial dilution 1:5) were mixed with a constant challenge-dose of virus and inoculated onto Vero cell monolayers. Cells were incubated under a carboxymethyl cellulose overlay for 4 days, after which they were fixed and immunostained with serotype-specific antienvelope antibodies, followed by alkaline phosphatase-conjugated secondary antibodies. Dengue-infected cells appear as visible plaques after incubation with a colorimetric substrate. The PRNT50 is the reciprocal of the highest serum dilution at which ≥50% of dengue challenge virus (in plaque counts) was neutralized compared with the challenge virus control wells. Plaque counts were calculated for all 12 serum dilutions to ensure a dose-response relationship. Values less than the LLOQ (10) were set to 5.
Study Oversight
Sanofi Pasteur sponsored and funded the trials and reviewed the manuscript before submission. All relevant ethics review boards approved the protocols; parents/guardians provided written informed consent, and older children provided written informed assent before participation, according to local regulations [15, 16].
Statistical Analysis
The analysis plan (Supplementary Material) was finalized before data analysis. For each trial, CoRs and CoPs [1, 26] for the DENV-1–4 endpoints and the DENV-Any endpoint were assessed following the analysis plan. We also applied the trial-specific analysis plan to the trial-pooled data set of 9- to 16-year-olds. The analyses of the DENV-k endpoint for a given serotype k assessed titer to serotype k as a CoR and CoP for DENV-k. The analyses of the DENV-Any endpoint assessed average titer as a CoR and CoP for DENV-Any. The average titer for an individual is the geometric mean of her/his 4 PRNT50 values (1 per serotype). Average titer is a single summary biomarker that can be assessed as a CoR and CoP of DENV-Any with greater precision than the correlates analyses of the serotype-specific DENV endpoints [27].
The CoR analyses were conducted using logistic regression and Cox proportional-hazards models accounting for the case-cohort sampling [28, 29]. The results were almost identical; only the Cox results are reported. All analyses right-censored participants at month 25 or at dropout if it occurred earlier. All analyses controlled for the baseline covariates sex, protocol-specified age categories, and country.
The CoR analyses were conducted separately in the vaccine and placebo groups, with results reported as estimated dengue hazard ratios (HRs) (with 95% confidence intervals [CIs]) for medium vs low and high vs low categories of neutralization response, with low, medium, and high defined by tertiles of all neutralization titers combined over the vaccine and placebo groups and the 4 serotypes. Wald tests were used to test for HRs differing from 1. Similar analyses were conducted for HRs per 10-fold increase in quantitative titers.
The CoPs were assessed using the VE curve-effect modification framework [1, 26, 30], which studies how VE varies over vaccinated subgroups defined by month 13 neutralization titers. Two methods [31, 32] were used (for 31, see Supplementary Material), each using either hinge [33] or linear logistic regression models of dengue risk conditional on titer and baseline covariates. A hinge model was used if a likelihood ratio test supported a better fit (P < .05) and if there were enough dengue endpoints to support this more flexible model. This process selected hinge logistic models [33] for method [31] (except for DENV-4 in CYD14) and linear logistic models for method [32]. Hinge models specify that interindividual variability in titers at the lowest values near the LLOQ does not affect dengue risk, which is plausible because much of this variability reflects PRNT50 technical measurement error. Both methods [31, 32] provide pointwise and simultaneous bootstrap-based Wald 95% CIs about the VE curve and test whether VE varies across titer subgroups. Method [34] was used to assess how VE varied with baseline average titer and method [35] was used to assess how VE varied by month 13 titers of vaccinees within baseline seropositive and seronegative subgroups. P values for testing each serotype-specific titer as a CoR were adjusted over the 4 serotypes using family-wise error rate (Holm-Bonferroni [36]) and false-discovery rate (Q values [37]) adjustment, separately for each treatment group and each trial. The same multiplicity adjustments were made for the serotype-specific VE curves. All P values and Q values are 2-sided.
RESULTS
Figure 1 shows the number of study participants with neutralization data. With controls and dengue cases defined under Methods, month 13 titers were measured from n = 1879 controls (1275 vaccine, 604 placebo) in CYD14 and n = 1884 controls (1275 vaccine, 609 placebo) in CYD15. Month 13 titers were measured from n = 244 cases occurring after month 13 through month 25 (115 vaccine, 129 placebo) in CYD14 and n = 415 cases (183 vaccine, 232 placebo) in CYD15, representing 99.6% and 99.8% of the total DENV-Any cases. Because month 0 samples were collected only for the immunogenicity subset, month 0 neutralization responses were available for 99.7% of controls but only n = 52 (21.3%) cases in CYD14 and n = 36 (8.7%) cases in CYD15. Of the 2123 (2299) participants with month 13 neutralization data in CYD14 (CYD15), 99.6% (99.4%) received all 3 immunizations.
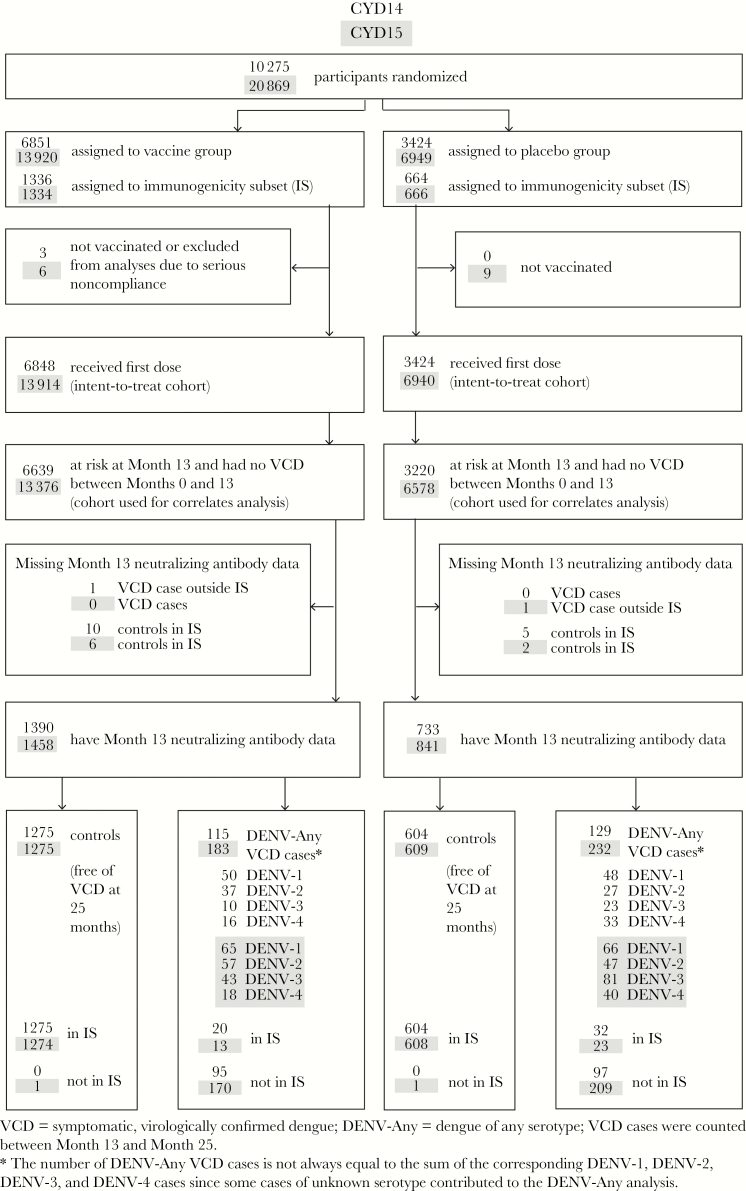
Sample selection for the case-cohort studies. Participants enrolled in the CYD14 and CYD15 studies were vaccinated at months 0, 6, and 12, and neutralizing antibody titers at month 13 were evaluated as correlates of risk and protection. The analysis datasets consisted of all participants at risk at month 13 who did not experience symptomatic, virologically confirmed dengue (VCD) before month 13 and who had month 13 neutralizing antibody data. Cases refer to participants with documented symptomatic VCD that occurred between month 13 and month 25; controls refer to participants with no documented symptomatic VCD throughout the first 25 months of the trials.
Titers were significantly higher in CYD15 than CYD14 in both treatment groups (Figures 2 and and33 and Supplementary Figure S1) (Holm, P < .001), presumably because participants in CYD14 were younger (2–14 years vs 9–16 in CYD15) and CYD15 had a higher frequency of dengue seropositivity. In children 9–16 years old, titer distributions were similar (Supplementary Figures S2 and S3).
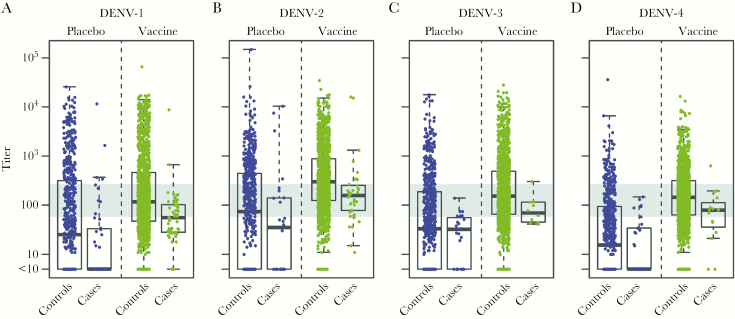
Distributions of serotype-specific month 13 50% plaque reduction neutralization test (PRNT50) neutralization titer variables in dengue cases and controls among vaccine and placebo recipients in the month 13 at-risk cohorts in CYD14 for all age groups. Neutralization titers to (A) DENV-1, (B) DENV-2, (C) DENV-3, (D) DENV-4 in CYD14. Box plots show the 25th percentile (lower edge of the box), 50th percentile (horizontal line in the box), and 75th percentile (upper edge of the box), with participants stratified according to dengue case/control status and treatment assignment. The whiskers indicate the most extreme data points, which are no more than 1.5 times the interquartile range from the box. Low, medium, and high are the bottom, middle, and upper third of the month 13 PRNT50 titers of the month 13 at-risk cohort pooling over DENV1-4 and over the vaccine and placebo groups within each trial. Medium response is indicated by the gray horizontal bar (medium response titer = 58.0–266.0 in CYD14). Case = symptomatic, virologically confirmed dengue (VCD) primary endpoint between month 13 and month 25; Control = no VCD primary endpoint through month 25.
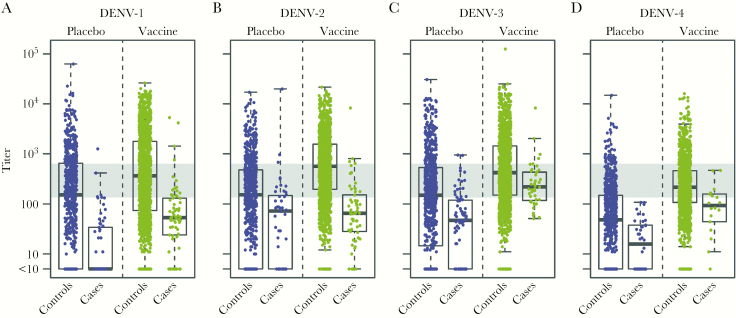
Distributions of serotype-specific month 13 50% plaque reduction neutralization test (PRNT50) neutralization titer variables in dengue cases and controls among vaccine and placebo recipients in the month 13 at-risk cohorts in CYD15 for all age groups. Neutralization titers to (A) DENV-1, (B) DENV-2, (C) DENV-3, (D) DENV-4 in CYD15. Box plots show the 25th percentile (lower edge of the box), 50th percentile (horizontal line in the box), and 75th percentile (upper edge of the box), with participants stratified according to dengue case/control status and treatment assignment. The whiskers indicate the most extreme data points, which are no more than 1.5 times the interquartile range from the box. Low, medium, and high are the bottom, middle, and upper third of the month 13 PRNT50 titers of the month 13 at-risk cohort pooling over DENV1-4 and over the vaccine and placebo groups within each trial. Medium response is indicated by the gray horizontal bar (medium response titer = 135.0–631.0 in CYD15). Case = symptomatic, virologically confirmed dengue (VCD) primary endpoint between month 13 and month 25; Control = no VCD primary endpoint through month 25.
In the CoR analyses by low, medium, and high, the consistent dose-response pattern of HR estimates support consistent inverse CoRs in each trial and treatment group (Table 1 and Supplementary Table S1). In vaccine recipients, in each trial, the hazards of DENV-Any, DENV-1, and DENV-2 significantly decreased over low, medium, and high subgroups (Figure 4 and Supplementary Figures S4A and B andS5A and B; P ≤ .001; Holm, P < .005). The pattern was similar for DENV-3 and DENV-4, albeit not significant in CYD14, possibly due to fewer dengue endpoints (Supplementary Figures S4C and D and S5C and D). In the CoR analyses by quantitative titers, in each trial and treatment group, the hazards of dengue decreased with average titer and serotype-specific titer, more strongly in the vaccine group (all interaction test P < .01) where the estimated HRs ranged from 0.19 to 0.43 per 10-fold titer increase over all DENV endpoint analyses (9 of 10 P < .01, 7 of 8 Holm P < .03) (Table 1 and Supplementary Figures S4–S7). This differential association by treatment group suggests that vaccine-induced immunity is not equivalent to natural dengue virus-induced immunity.
Table 1.
Hazard Ratios of DENV-Any, DENV-1, DENV-2, DENV-3, DENV-4 Between Months 13 and 25 Across Month 13 Neutralization Titer Subgroups (Low, Medium, Higha) and Per 10-Fold Titer Increase in the Vaccine and Placebo Groups of Individuals Free of VCD Through Month 13 in the CYD14 and CYD15 Studies for All Age Groups From Cox Regression Models
| Comparison | Titer Variable, DENV Endpoint | |||||
|---|---|---|---|---|---|---|
| CYD14 (n = 1390 Vaccine Recipients) | CYD14 (n = 733 Placebo Recipients) | |||||
| Hazard Ratio (95% CI) | P Value | Global P Valueb (HolmP Value)c | Hazard Ratio (95% CI) | P Value | Global P Valueb (Holm P Value)c | |
| Average Titerd, DENV-Any | ||||||
| Med vs low | 0.37 (0.23–0.59) | <.001 | <.001 (−) | 0.69 (0.43–1.10) | .12 | .04 (−) |
| High vs low | 0.10 (0.05–0.19) | <.001 | <.001 (−) | 0.46 (0.24–0.88) | .02 | .04 (−) |
| Per 10-fold increase | 0.25 (0.17–0.38) | <.001 | − (−) | 0.75 (0.57–1.00) | .05 | − (−) |
| DENV-1 Titer, DENV-1 | ||||||
| Med vs low | 0.60 (0.33–1.10) | .10 | .001 (.003) | 0.37 (0.14–1.00) | .05 | .004 (.02) |
| High vs low | 0.06 (0.01–0.28) | <.001 | .001 (.003) | 0.20 (0.07–0.57) | .003 | .004 (.02) |
| Per 10-fold increase | 0.39 (0.25–0.62) | <.001 | − (<.001) | 0.47 (0.31–0.71) | <.001 | − (<.001) |
| DENV-2 Titer, DENV-2 | ||||||
| Med vs low | 0.96 (0.38–2.45) | .93 | .001 (.004) | 0.66 (0.23–1.91) | .45 | .11 (.11) |
| High vs low | 0.21 (0.07–0.64) | .006 | .001 (.004) | 0.29 (0.09–0.93) | .04 | .11 (.11) |
| Per 10-fold increase | 0.43 (0.25–0.75) | .003 | − (.009) | 0.81 (0.52–1.25) | .34 | − (.43) |
| DENV-3 Titer, DENV-3 | ||||||
| Med vs low | 0.61 (0.16–2.39) | .48 | .29 (.42) | 0.93 (0.35–2.47) | .88 | .02 (.07) |
| High vs low | 0.16 (0.02–1.58) | .12 | .29 (.42) | 0.00 (0.00–0.73) | .01 | .02 (.07) |
| Per 10-fold increase | 0.39 (0.13–1.15) | .09 | − (.09) | 0.70 (0.40–1.23) | .22 | − (.43) |
| DENV-4 Titer, DENV-4 | ||||||
| Med vs low | 0.76 (0.27–2.20) | .62 | .21 (.42) | 0.75 (0.29–1.95) | .56 | .05 (.10) |
| High vs low | 0.14 (0.02–1.25) | .08 | .21 (.42) | 0.00 (0.00–0.85)e | .02e | .05 (.10) |
| Per 10-fold increase | 0.30 (0.13–0.73) | .008 | − (0.02) | 0.53 (0.29–0.99) | .05 | − (.14) |
| Comparison | Titer Variable, DENV Endpoint | |||||
|---|---|---|---|---|---|---|
| CYD15 (n = 1458 Vaccine Recipients) | CYD15 (n = 841 Placebo Recipients) | |||||
| Hazard Ratio (95% CI) | P Value | Global P Valueb (Holm P Value)c | Hazard Ratio (95% CI) | P Value | Global P Valueb (Holm P Value)c | |
| Average Titerd, DENV-Any | ||||||
| Med vs low | 0.25 (0.17–0.36) | <.001 | <.001 (−) | 0.18 (0.12–0.27) | <.001 | <.001 (−) |
| High vs low | 0.04 (0.02–0.08) | <.001 | <.001 (−) | 0.03 (0.01–0.12) | <.001 | <.001 (−) |
| Per 10-fold increase | 0.19 (0.14–0.27) | <.001 | − (−) | 0.40 (0.32–0.50) | <.001 | − (−) |
| DENV-1 Titer, DENV-1 | ||||||
| Med vs low | 0.31 (0.16–0.62) | .001 | <.001 (<.001) | 0.11 (0.04–0.30) | <.001 | <.001 (<.001) |
| High vs low | 0.06 (0.02–0.17) | <.001 | <.001 (<.001) | 0.02 (0.00–0.17) | <.001 | <.001 (<.001) |
| Per 10-fold increase | 0.32 (0.23–0.45) | <.001 | − (<.001) | 0.26 (0.18–0.39) | <.001 | − (<.001) |
| DENV-2 Titer, DENV-2 | ||||||
| Med vs low | 0.14 (0.07–0.29) | <.001 | <.001 (<.001) | 0.49 (0.24–1.01) | .06 | .01 (.01) |
| High vs low | 0.04 (0.01–0.10) | <.001 | <.001 (<.001) | 0.21 (0.06–0.72) | .01 | .01 (.01) |
| Per 10-fold increase | 0.20 (0.13–0.30) | <.001 | − (<.001) | 0.71 (0.51–1.01) | .06 | − (.06) |
| DENV-3 Titer, DENV-3 | ||||||
| Med vs low | 0.91 (0.45–1.85) | .80 | .001 (.002) | 0.29 (0.16–0.53) | <.001 | <.001 (<.001) |
| High vs low | 0.13 (0.04–0.40) | <.001 | .001 (.002) | 0.05 (0.01–0.22) | <.001 | <.001 (<.001) |
| Per 10-fold increase | 0.43 (0.26–0.72) | .001 | − (.002) | 0.45 (0.34–0.61) | <.001 | − (<.001) |
| DENV-4 Titer, DENV-4 | ||||||
| Med vs low | 0.38 (0.14–1.01) | .05 | .01 (.01) | 0.00 (0.00–0.33)e | <.001e | <.001 (<.001) |
| High vs low | 0.00 (0.00–0.66)e | .009e | .01 (.01) | 0.00 (0.00–1.31)e | .10e | <.001 (<.001) |
| Per 10-fold increase | 0.25 (0.11–0.56) | .001 | − (.002) | 0.44 (0.26–0.73) | .002 | − (.004) |
Abbreviations: CI, confidence interval; DENV, dengue virus; PRNT50, 50% plaque reduction neutralization test; VCD, symptomatic, virologically confirmed dengue.
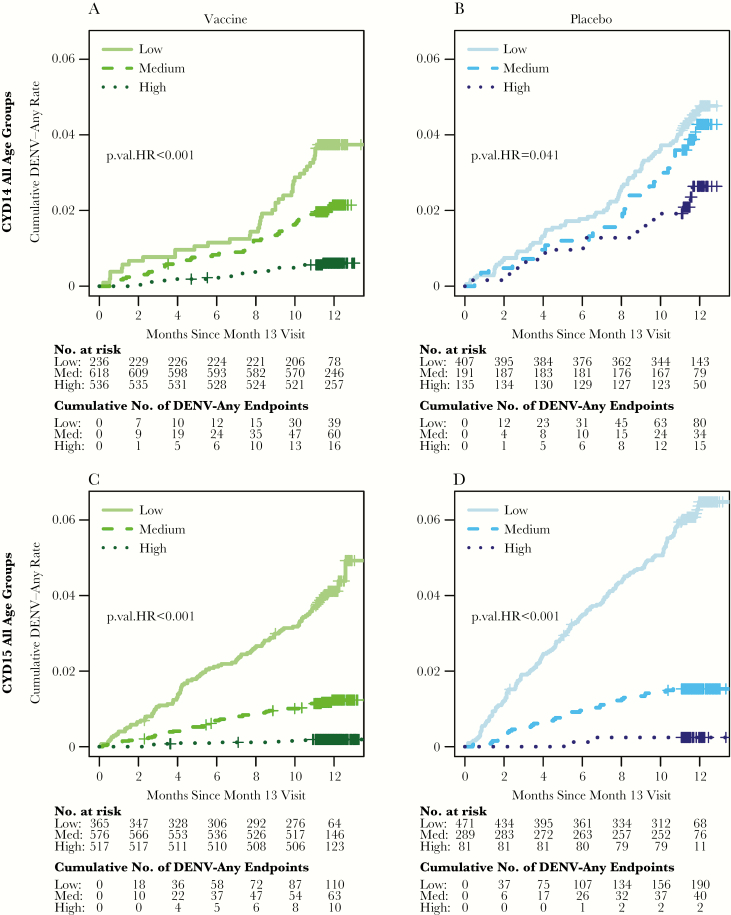
Cumulative incidence of symptomatic, virologically confirmed dengue (VCD) of any serotype (DENV-Any) by month 13 average titer subgroups (low, medium, high) for the vaccine and placebo groups in CYD14 for all age groups (A and B) and CYD15 for all age groups (C and D). Two-sided P values test for different hazard rates (HR) of dengue across the 3 subgroups. Low, medium, and high are the bottom, middle, and upper third of the month 13 50% plaque reduction neutralization test (PRNT50) titers of the month 13 at-risk cohort pooling over DENV1-4 and over the vaccine and placebo groups within each trial. Medium response titer = 58.0–266.0 in CYD14 and 135.0–631.0 in CYD15.
Using method [31], CYD14 vaccine recipients (all ages) with average titer at no seroresponse (defined as month 13 titer less than the LLOQ for all 4 serotypes) or at 82, 500, and 10000 had estimated VE against DENV-Any of 2.3%, 47.4%, 78.1%, and 96.1%, respectively (Figure 5A). These estimates for CYD15 were 22.8%, 35.9%, 78.8%, and 98.3%, respectively (Figure 5B), and in 9- to 16-year-olds pooled from both trials, the estimates were 34.6% (95% CI, 7.4–53.9), 50% (95% CI, 39.4–58.7), 80% (95% CI, 74.6–84.3), and 97.5% (95% CI, 95.5–98.6) (Figure 5C). The increase of each VE curve in Figure 5A–C was significant (P < .001). All serotype-specific estimated VE curves increased with homologous titers in each trial and pooled in 9- to 16-year-olds (Supplementary Figures S8–S10, Supplementary Table S2); the increase was significant for DENV-1 (P = .03, Holm P = .08) and DENV-2 (P < .001, Holm P < .001) in CYD15 and for DENV-1 (P = .005, Holm P = .01), DENV-2 (P < .001, Holm P < .001), and DENV-4 (P = .04, Holm P = .08) in 9- to 16-year-olds pooling over the trials. The alternative VE curve method [32] yielded similar conclusions, albeit with wider CIs (Supplementary Figure S11).
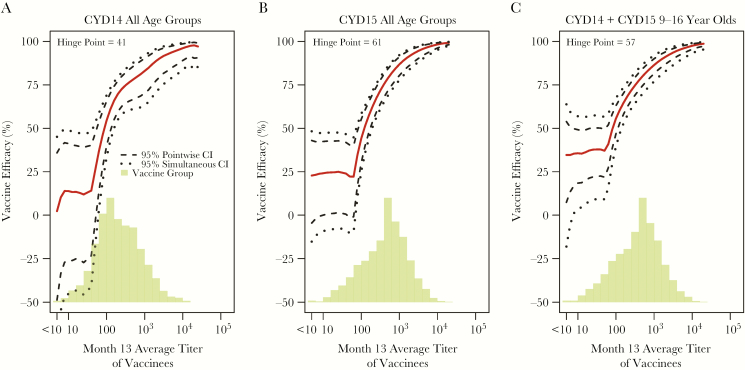
Estimated vaccine efficacy (VE) against symptomatic, virologically confirmed dengue of any serotype (DENV-Any) between months 13 and 25 by month 13 average titer with 95% pointwise and simultaneous confidence intervals (CI) and histogram of average titer in the vaccine group for the month 13 at-risk cohort. The following estimated VE curves are shown: (A) CYD14 all age groups; (B) CYD15 all age groups; (C) CYD14 + CYD15 9- to 16-year-olds. For A–C, 0.14%, 0.21%, 0.19% vaccine recipients, respectively, had no seroresponse (month 13 titer less than the lower limit of quantitation for all available serotypes).
Comparing serotype-specific VE curves among serotypes in the low (bottom tertile) homologous titer subgroups, some VE curves significantly differed in the low range (DENV-2 vs DENV-1, -3, -4 in CYD15, and DENV-2 vs DENV-1 and DENV-4 and DENV-1 vs DENV-4 in 9- to 16-year-olds pooling over the trials Supplementary Table S3), with lower VE against DENV-2. The DENV-2 VE curves across the complete age range in each trial (Supplementary Figures S8 and S9) suggest possible negative VE for vaccine recipients without anti-DENV-2 titers (ie, PRNT50 below the LLOQ). However, the simultaneous 95% confidence bands for DENV-2 VE include 0%, and an inference of negative VE is based on a very small number of vaccine-recipient DENV-2 cases (0 of 87 in CYD14 and 5 of 264 in CYD15).
Separate DENV-Any VE curves for the CYD14 age subgroups 2- to 5-year-olds, 6- to 11-year-olds, and 12- to 14-year-olds showed estimated VE consistently above 50% (75%) for average titers above 100 (1000) (Supplementary Figure S12). At the LLOQ, there was a nonsignificant dose-response trend toward VE increasing with age, with estimated VE near zero for 2- to 5-year-olds. For CYD15, the VE curves were similar for 9- to 11-year-olds and 12- to 16-year-olds (Supplementary Figure S13). The DENV-Any VE curves by average titer for CYD14 9- to 14-year-olds versus CYD15 9- to 16-year-olds were similar (Supplementary Figure S14). Comparing VE curves for CYD14 versus CYD15 in the low (bottom tertile) and highest (top 20%) homologous titer subgroups of 9- to 16-year-olds, VE in the highest range was only significantly different for DENV-2 (P = .007, Holm P = .06) and in the low range only for DENV-Any (P = .01) (Supplementary Table S4). The differences in ages and related differences in prior dengue exposure may explain these differences in the VE curves between the trials.
The DENV-Any VE curves by month 13 average titer for baseline seropositive and baseline seronegative subgroups in 9- to 16-year-olds pooled across trials were similar, with estimated VE approximately 25% for vaccine recipients with no seroresponse at month 13 (Supplementary Figure S15). For vaccinees with month 13 average titers of 500 and 10000, respectively, estimated VE was 79.3% and 97.3% for the baseline seropositive subgroup compared with 70.4% and 91.8% for the baseline seronegative subgroup.
Vaccine efficacy against DENV-Any from month 0 to 25 increased with baseline average titer (P = .01 in CYD14, P = .04 in CYD15, P = .006 in CYD14 + CYD15 9- to 16-year-olds, and P < .001 in CYD14 + CYD15 all ages) (Figure 6A–D). In CYD14 (CYD15), estimated VE increased from 32% (49%) for dengue seronegative participants to 99% (97%) for participants with maximal baseline average titers (Figure 6A and andB);B); the stronger effect modification in CYD14 is likely due to the inclusion of younger children. Vaccine efficacy against DENV-Any increased more strongly with month 13 average titer than with baseline average titer (Figures 5 and and6),6), indicating that postvaccination titers were a stronger modifier of VE. Supplementary Table S5 lists VE estimates for baseline subgroups and month 13 vaccinee subgroups at the PRNT50 values <10, 100, 500, 1000, and 10000.
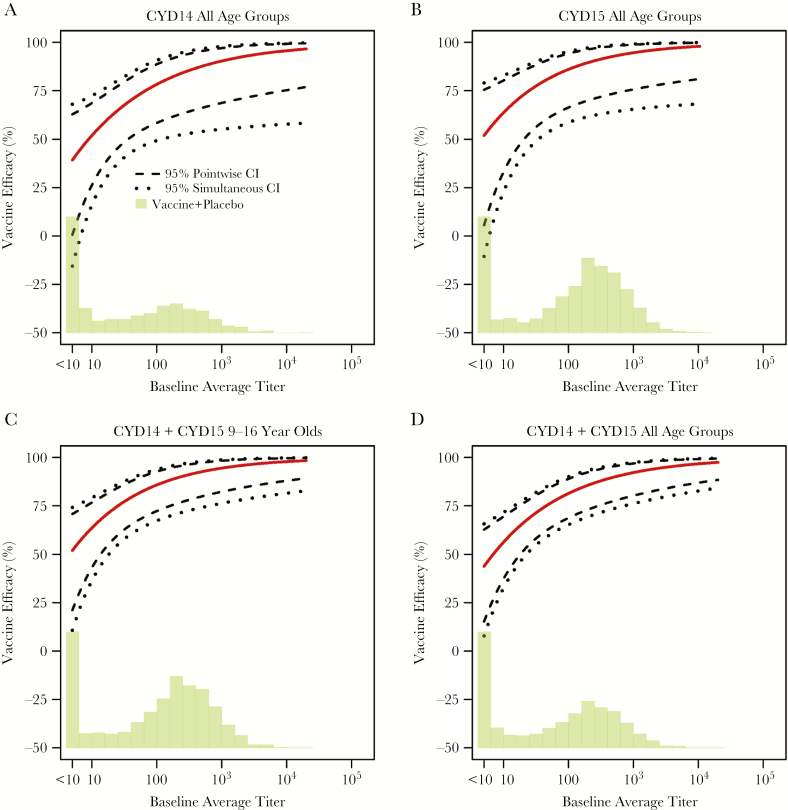
Estimated vaccine efficacy (VE) against symptomatic, virologically confirmed dengue of any serotype (DENV-Any) between months 0 and 25 by baseline average titer with 95% pointwise and simultaneous confidence intervals (CI) and histogram of baseline average titer in the vaccine and placebo groups combined. The following estimated VE curves are shown: (A) CYD14 all age groups; (B) CYD15 all age groups; (C) CYD14 + CYD15 9- to 16-year-olds; D, CYD14 + CYD15 all age groups. For A–D, 32.9%, 20.5%, 20.6%, 26.6% participants, respectively, were baseline seronegative (month 0 titer less than the lower limit of quantitation for all available serotypes).
DISCUSSION
We conducted a correlates analysis to assess how PRNT50 titers after 3 immunizations with CYD-TDV (month 13) associated with VCD and with VE against VCD, for VCD outcomes occurring between month 13 and 25; in the future, it will be important to study PRNT50 correlates of longer-term dengue outcomes. Higher PRNT50 titers after 3 immunizations with CYD-TDV were associated with a lower rate of VCD, for each trial and serotype. In addition, estimated VE against VCD of each serotype monotonically increased with homologous neutralization titer, and VE against VCD of any serotype (DENV-Any) increased with average titer. The DENV-Any endpoint analysis had the greatest statistical precision and supported zero or low VE for vaccine recipients with no seroresponse (month 13 titer less than the LLOQ for all 4 serotypes) to dengue, and VE increased to above 95% for vaccine recipients with highest average titers. These results were similar between the 2 trials, except that vaccine recipients with no seroresponse had VE near zero in CYD14 but approximately 25% in CYD15. Moreover, in 9- to 16-year-olds pooled over the trials, vaccine recipients with no seroresponse had approximately 35% VE against DENV-Any, indicating that PRNT50 titers are not a completely valid CoP according to the definition of [38], which requires VE for vaccine recipient nonresponders to be 0%. Violation of this requirement implies that the immune response marker does not fully mediate the vaccine’s effect on the dengue outcome [39]. This result supports that other factors besides PRNT50 titers (eg, [9–13, 40, 41]) played a role in VE. However, high titers were a remarkably consistent marker of high VE, holding across all serotypes, age groups, and both trials, suggesting that PRNT50 could potentially be used for extrapolation of VE for bridging to other populations where high titers are observed. Moreover, both baseline seronegative and baseline seropositive subgroups with high postdose 3 titers had high estimated VE. Although these last results had relatively low precision, they support that baseline seronegative vaccine recipients still received protection from the CYD-TDV vaccine if they had a high neutralization response to vaccination.
Estimated VE increased more with anti-DENV-1 and anti-DENV-2 titers than with anti-DENV-3 and anti-DENV-4 titers, and CYD15 vaccine recipients with homologous titers below 135.0 (bottom tertile) had significantly lower VE against DENV-2 than against DENV-1, DENV-3, and DENV-4. These results support that vaccine recipients with no month 13 response to DENV-2 had no VE against DENV-2, whereas vaccine recipients with no month 13 response to DENV-3 or no month 13 response to DENV-4 did have partial beneficial VE against DENV-3 and DENV-4, respectively. These results indicate that—like for DENV-Any—other factors besides PRNT50 titers played a role in VE against DENV-3 and DENV-4, such as qualitative functional differences in the antibodies to the different serotypes [19, 42]. Moreover, the observation in both trials that DENV-2 titers were significantly higher than DENV-4 titers, yet estimated DENV-4 VE was significantly higher than DENV-2 VE (75% vs 35%, P = .009 in CYD14; 78% vs 42%, P = .005 in CYD15), indicates that PRNT50 titers are not a completely valid CoP, suggesting potential qualitative differences in the humoral immune responses or that protective levels of neutralizing responses differ by serotype [3, 6].
As shown in Supplementary Figures S16 and S17, all vaccinated cases with no month 13 seroresponse to at least 1 serotype (n = 14 in CYD14, n = 43 in CYD15) had a month 13 seroresponse to at least 1 serotype different from the case-causing serotype. Most of the 58 vaccinated cases had a DENV-1 (n = 20) or DENV-2 (n = 26) endpoint, where the former had a lower frequency of month 13 seropositivity to the case-causing serotype (7 of 20 [35%] for DENV-1 vs 22 of 26 [85%] for DENV-2). This may suggest a distinct feature of DENV-2 with less protective immunity indicated by seroresponse compared with other serotypes.
In CYD14, estimated VE against DENV-Any was consistently high across the subgroups 2–5, 6–11, 12–14 years with high average titers (eg, VE at least 75% for average titers above 1000, as noted above). However, at the lowest titers, the data supported VE near zero for 2- to 5-year-olds and increasing with age category. This suggests that achieving high titers to vaccination is especially important in the youngest children to attain VE. The ability of high-titer responses to eliminate an age effect suggests that the molar excess of antibody compensates for qualitative differences in the humoral response. A potential explanation for these age differences is the increasing rate of dengue seropositivity at baseline with age (Supplementary Figure S18) [42].
In contrast to the month 13 titer results, in both trials, fold-rises in titer from baseline to month 13 were not associated with dengue in either treatment group nor with VE (Supplementary Figures S19 and S20). This suggests that it is not the new neutralization response over baseline generated by the vaccine that predicts dengue or VE, but rather the absolute titer achieved after vaccination. The opposite result was seen in a herpes zoster VE trial [30].
For several licensed vaccines, an “absolute correlate” [2] is available for which 100% VE can be predicted by an immune response biomarker exceeding a threshold. Such an absolute correlate was not observed (7 vaccine recipients had breakthrough dengue disease despite having very high neutralization titers), classifying neutralization titers as a “relative correlate” [2].
CONCLUSIONS
Our findings are consistent with 2 models of CYD-TDV VE. In a “quantitative model,” the lower protection in month 13 seronegative individuals and the youngest children with low month 13 titers derives from a subpotent neutralization response. In a “qualitative model,” the lower protection derives from qualitative differences in the vaccine-induced antibodies by serotype and serostatus before vaccination; presumably, the correspondingly low proportion of potent antibodies with the needed qualities is insufficient to confer complete protection. These models could be tested and refined by adding other immunological assays to the assessment of month 13 correlates.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Figure 7
Supplementary Figure 8
Supplementary Figure 9
Supplementary Figure 10
Supplementary Figure 11
Supplementary Figure 12
Supplementary Figure 13
Supplementary Figure 14
Supplementary Figure 15
Supplementary Figure 16
Supplementary Figure 17
Supplementary Figure 18
Supplementary Figure 19
Supplementary Figure 20
Supplementary Material 1
Supplementary Material 2
Supplementary Table S1
Supplementary Table S2
Supplementary Table S3
Supplementary Table S4
Supplementary Table S5
Notes
Author contributions. L. N. C., R. S., N. J., and F. N. designed and conducted the clinical trials. Z. M., S. G. S., L. N. C., R. S., N. J., F. N., and P. B. G. designed the study. Z. M., M. J., Y. H., Y. Z., Y. F., and P. B. G. developed the statistical methods and analysis plan, analyzed the data, and produced the figures and tables. Z. M., L. N. C., and P. B. G. wrote the manuscript, with all authors providing critical review. N. J., F. N., and P. B. G. made the decision to submit the manuscript for publication. All authors interpreted the data and approved the final article.
Acknowledgments. We thank the participants, investigators, and sponsors of the CYD14 and CYD15 trials.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health (NIH).
Financial support. Research reported in this publication was funded by Sanofi Pasteur and the National Institute of Allergy and Infectious Diseases, NIH, Department of Health and Human Services, under award number R37AI054165.
Potential conflicts of interest. L. N. C., R. S., N. J., and F. N. are employees of Sanofi Pasteur. Z. M., M. J., Y. H., Y. Z., Y. F., L. N. C., S. G. S., and P. B. G. received a contract from Sanofi Pasteur to conduct the statistical analysis work. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: Dengue Research Consortium Meeting, Sanofi Pasteur VaxDesign, Orlando, April 2017; P. B. Gilbert at the Harvard T.H. Chan School of Public Health Department of Immunology and Infectious Diseases, Boston, MA, April 2017; 38th Annual Conference of the International Society for Clinical Biostatistics, Vigo, Spain, July 2017; and 2017 Annual Meeting of the American Society of Tropical Medicine and Hygiene, Baltimore, MD, November 2017.
References
Articles from The Journal of Infectious Diseases are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/infdis/jix609
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/jid/article-pdf/217/5/742/24263243/jix609.pdf
Citations & impact
Impact metrics
Article citations
Dengue Fever Epidemics and the Prospect of Vaccines: A Systematic Review and Meta-Analysis Using Clinical Trials in Children.
Diseases, 12(2):32, 06 Feb 2024
Cited by: 0 articles | PMID: 38391779 | PMCID: PMC10887605
Review Free full text in Europe PMC
Household immunity and individual risk of infection with dengue virus in a prospective, longitudinal cohort study.
Nat Microbiol, 9(1):274-283, 18 Dec 2023
Cited by: 2 articles | PMID: 38110699
Four statistical frameworks for assessing an immune correlate of protection (surrogate endpoint) from a randomized, controlled, vaccine efficacy trial.
Vaccine, 42(9):2181-2190, 08 Mar 2024
Cited by: 1 article | PMID: 38458870
Review
A controlled effects approach to assessing immune correlates of protection.
Biostatistics, 24(4):850-865, 01 Oct 2023
Cited by: 3 articles | PMID: 37850938 | PMCID: PMC10583729
Homotypic antibodies target novel E glycoprotein domains after natural DENV 3 infection/vaccination.
Cell Host Microbe, 31(11):1850-1865.e5, 30 Oct 2023
Cited by: 0 articles | PMID: 37909048 | PMCID: PMC11221912
Go to all (57) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT01374516
- (1 citation) ClinicalTrials.gov - NCT01373281
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Microneutralization assay titer correlates analysis in two phase 3 trials of the CYD-TDV tetravalent dengue vaccine in Asia and Latin America.
PLoS One, 15(6):e0234236, 15 Jun 2020
Cited by: 7 articles | PMID: 32542024 | PMCID: PMC7295445
Secondary Analysis of the Efficacy and Safety Trial Data of the Tetravalent Dengue Vaccine in Children and Adolescents in Colombia.
Pediatr Infect Dis J, 39(4):e30-e36, 01 Apr 2020
Cited by: 3 articles | PMID: 32040014 | PMCID: PMC7182239
Analysis of Neutralizing Antibodies as a Correlate of Instantaneous Risk of Hospitalized Dengue in Placebo Recipients of Dengue Vaccine Efficacy Trials.
J Infect Dis, 225(2):332-340, 01 Jan 2022
Cited by: 2 articles | PMID: 34174082 | PMCID: PMC8915240
Tetravalent Dengue Vaccine: A Review in the Prevention of Dengue Disease.
Drugs, 76(13):1301-1312, 01 Sep 2016
Cited by: 27 articles | PMID: 27506852
Review
Funding
Funders who supported this work.
Department of Health and Human Services (1)
Grant ID: R37AI054165
NIAID NIH HHS (3)
Grant ID: R37 AI054165
Grant ID: UM1 AI068635
Grant ID: R01 AI122991





