Abstract
Free full text

Germ Cell–Specific Retinoic Acid Receptor α Functions in Germ Cell Organization, Meiotic Integrity, and Spermatogonia
Abstract
Retinoic acid receptor α (RARA), a retinoic acid–dependent transcription factor, is expressed in both somatic and germ cells of the testis. Rara-null male mice with global Rara mutations displayed severely degenerated testis and infertility phenotypes. To elucidate the specific responsibility of germ cell RARA in spermatogenesis, Rara was deleted in germ cells, generating germ cell–specific Rara conditional knockout (cKO) mice. These Rara cKO animals exhibited phenotypes of quantitatively reduced epididymal sperm counts and disorganized germ cell layers in the seminiferous tubules, which worsened with aging. Abnormal tubules lacked lumen, contained vacuoles, and showed massive germ cell sloughing, all characteristics similar to those observed in Rara-null tubules. Spermatocyte chromosomal spreads revealed a novel role for germ cell RARA in modulating the integrity of synaptonemal complexes and meiotic progression. Furthermore, the initiation of spermatogenesis from spermatogonial stem cells was decreased in Rara cKO testes following busulfan treatment, supporting a role of germ cell RARA in spermatogonial proliferation. Collectively, the evidence in this study indicates that RARA produced in male germ cells has a broad spectrum of functions throughout spermatogenesis, which includes the maintenance of seminiferous epithelium organization, the integrity of the meiotic genome, and spermatogonial proliferation and differentiation. The results further suggest that germ cell RARA has dual functions: intrinsically in germ cells, balancing proliferation and differentiation of spermatogonia, and controlling genome integrity during meiosis; and extrinsically in the crosstalks with Sertoli cells, controlling the cell junctional physiology for coordinating proper spatial and temporal development of germ cells during spermatogenesis.
Spermatogenesis is a kinetically and spatially fine-tuned and orchestrated process of producing spermatozoa in the seminiferous tubules of the testis. In mammals, spermatogenesis relies on the pool of spermatogonial stem cells (SSCs) that reside within specialized microenvironments in the tubules, called niches. In the niches, SSCs undergo asynchronous divisions to produce more SSCs, which remain in the niche, and transit-amplifying progenitor spermatogonia, which are the source for spermatogonial population expansion. A single round of spermatogenesis is comprised of three continuous processes: mitosis of progenitor spermatogonia and differentiation of spermatogonia; meiotic divisions of spermatocytes; and spermiogenesis, the maturation process of haploid spermatids to spermatozoa (1).
Fat-soluble vitamin A has been shown to be necessary for a healthy testis (2–4). Without vitamin A in the diet, animals are infertile, and testes are devoid of most germ cells, with only type A spermatogonia remaining in mice (5, 6). The biologically active signaling molecule of vitamin A is its acid form, retinoic acid (RA), and the need for RA in the testis was successfully demonstrated with repeated injection of RA, which restored spermatogenesis in the testis after vitamin A depletion (7). It is known that RA triggers a coupled spermatogonial differentiation and meiotic initiation process, as well as the spermiogenesis process in the testis (4, 5, 8–11).
RA receptors (RARs), of which there are three subtypes (α, β, and γ), serve as RA ligand–dependent transcription factors regulating the expression of RA-responsive target genes (12). RARγ (RARG) has been demonstrated to play a role in the transition from progenitor spermatogonia to differentiated spermatogonia (13). RARβ was found to be dispensable for spermatogenesis (14, 15). Regarded as indispensable for spermatogenesis is RARα (RARA). Animals with Rara inactivated in all cells (Rara-null) showed a male infertility phenotype (16), with the observed phenotype being similar to that seen in the vitamin A–deficient testes. Closer examination of testes from Rara-null mice at various stages of animal development revealed degeneration and apoptosis of early meiotic germ cells, abnormalities in spermiogenesis, and the loss of proper germ cell organizations in the seminiferous tubules (17–19).
Cellular localization studies showed the expression of Rara transcripts (10, 20) and RARA proteins (10, 21–24) in both Sertoli cells and germ cells in the testis. In germ cells, RARA is not only present in gonocytes, stem cells, and progenitor spermatogonia, but it is also present in preleptotene and early meiotic spermatocytes, as well as in elongating spermatids (10, 21–24). The highest expression of Rara mRNA was at stages VIII and IX in stage-synchronized rats (25). Perhaps not coincidentally, these are the stages of the spermatogenic cycle where the concentration of RA ligand is at its highest (26), when progenitor spermatogonia are transitioning to differentiated spermatogonia, differentiated spermatogonia are entering the beginning phase of meiosis, and spermatids are detaching from Sertoli cells by a process called spermiation. These results with the phenotypes observed in Rara-null mice (17–19) suggested an important role of RARA in spermatogonia, early meiotic spermatocytes, and later in haploid spermatids.
Distinct functional roles of a gene in either the Sertoli cell or germ cell can be investigated using the SSC transplantation technique that involves transplantation of donor germ cells into germ cell–depleted recipient testes (27). In successful transplantation, the SSCs of donor germ cells become the source of progenitor spermatogonia and regenerate spermatogenesis in the recipient testis after a recovery period. Previously, it was shown that Rara-null Sertoli cells could support the initiation of spermatogenesis from the wild-type (WT) donor germ cells, with most of WT SSCs advancing to spermatocytes and few to round and elongated spermatids in half of the seminiferous tubules, although the germ cell organization was not normal (19). The result suggested that WT SSCs could migrate to the niche provided by Rara-null Sertoli cells without apparent problems and start spermatogenesis. However, the germ cell organization morphology of the recovered tubules was similar to the tubule morphology of Sertoli cell–specific Rara conditional knockout (cKO) mouse testes, where there is a loss of round spermatids (15). Contrastingly, SSC transplantations showed that donor Rara-null germ cells were virtually incapable of colonizing a WT recipient testis (19). These results together suggested that Sertoli cell RARA was partly responsible for proper germ cell organization in the seminiferous tubules and maintenance of advanced germ cells, whereas germ cell RARA was vital in the SSC ability to colonize the niche environment after spermatogonial transplantation.
To test the hypothesis that RARA has distinct and pivotal functions in germ cells, we constructed germ cell–specific Rara cKO mice. Using germ cell–specific Rara cKO mice, we found that RARA has multiple, diverse roles throughout spermatogenesis: in the maintenance of organized germ cell layers in the seminiferous tubules, in the preservation of the integrity of meiotic genome and progression, and in the initiation of spermatogenesis from SSCs after busulfan treatment. Also, equally integral to the clockwork of spermatogenesis, germ cell RARA was shown to crosstalk with Sertoli cells to control the integrity of the blood–testis barrier (BTB). Collectively, the results suggest a scenario where RARA in germ cells intrinsically regulates germ cell proliferation, differentiation, and meiotic activities, and extrinsically influence Sertoli cells, finely coordinating proper temporal development and spatial arrangement of germ cells in the murine testis.
Materials and Methods
Animals
B6.C-Tg (CMV-cre)1Cgn/J (Jax no. 006054; RRID: IMSR_JAX:006054) (28), Tg(Stra8-icre)1Reb/J (Stra8-iCre) (Jax no. 008208; RRID: IMSR_JAX:008208) (29), and B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J (R26R-EYFP) (Jax no. 006148; RRID: IMSR_JAX:006148) (30) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C57BL/6J-Rarafl/fl (Rarafl/fl) mice were generated and used in a two-step breeding scheme to obtain germ cell–specific deletion of both Rarafl alleles (see “Results” and Fig. 1). Mice were housed in the Washington State University animal facility on a 14-hour light: 10-hour dark cycle, with water and standard rodent chow (LabDiet® JL rat and mouse/auto 6F 5K67) ad libitum. Ethical care and maintenance of animals were carried out in accordance with protocols following National Institutes of Health guidelines approved by the Institutional Animal Care and Use Committee of Washington State University.
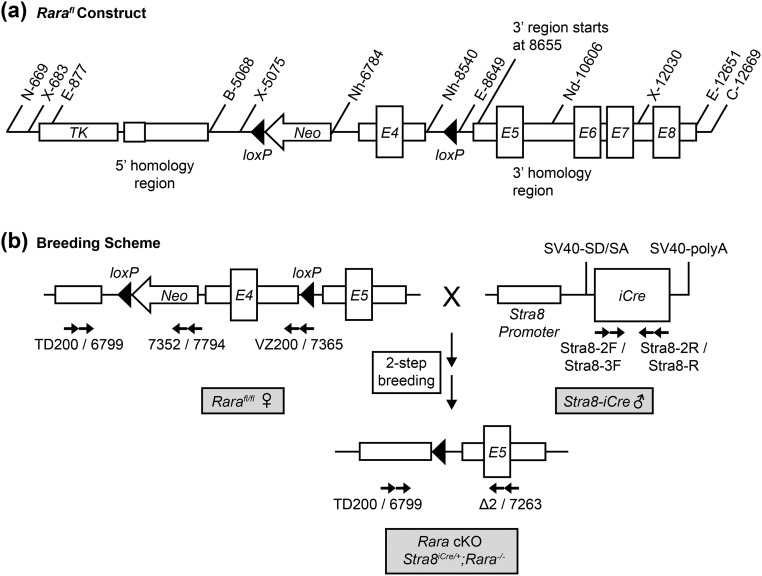
Floxed Rara construct and breeding scheme for germ cell–specific excision of exon 4, coding for a portion of the B domain on RARA. (a) Rara targeting vector with black triangles indicating locations of loxP sites flanking the region containing exon 4 (E4, “floxed” region). Numeric notations indicate base pairs with internal reference to gene coding sequence, and alphanumeric notations indicate locations of restriction enzyme activities. (b) Two-step breeding scheme to obtain homozygous Rara germ cell–specific knockout (Rara cKO: Stra8iCre/+;Rara−/−) animals using Rara floxed females (left) and Stra8-iCre males (right). Numeric and alphanumeric notations with arrows beneath each construct indicate primers (Table 1) and primer directions used in genotyping. B, BamHI; C, ClaI; E, EcoRI; N, NotI; Nd, NdeI; Neo, neomycin; Nh, NheI; polyA, polyadenylation; SD/SA, splice donor/splice acceptor; SV40, simian virus 40; TK, thymidine kinase; X, XhoI.
Isolation of tail DNA and sperm head DNA
To obtain tail DNA for genotyping, tail snips of 0.5 cm were cut and digested using Viagen DirectPCR lysis reagent (Viagen Biotechnology, Los Angeles, CA) with proteinase K (Life Technologies, Carlsbad, CA) at 55°C for 4 to 6 hours or digested with MyTaq extract-PCR fast digestion kit (Bioline, Luckenwalde, Germany), following the manufacturers’ protocols. Crude DNA lysates were obtained after the digestion and deactivation of proteinase K at 85°C for 45 minutes.
For isolation of sperm head DNA, fresh epididymal sperm from caudal epididymides were collected and suspended in nucleus isolation medium (NIM) containing 121.6 mM KCl, 7.8 mM Na2HPO4, 1.4 mM KH2PO4, 0.1% polyvinyl alcohol, and 10 mM EDTA. Sperm heads and tails were detached by sonication (Branson Sonifier 250; Emerson, St. Louis, MO) and centrifuged to collect the pellets. The sperm pellet was then resuspended in one part fresh NIM buffer layered over two parts 70% sucrose and centrifuged for 1 hour to separate sperm heads from tails. Separation of heads from tails was confirmed under an inverted phase-contrast Olympus IMT-2 microscope (Olympus, Center Valley, PA).
The sperm head pellet was suspended in NIM buffer, containing 0.1 M dithiothreitol and DNA extraction buffer [1 M Tris-HCl (pH 8), 0.5 M EDTA, and 10% SDS], and incubated for 20 minutes at 65°C to release nuclear content. Proteins were digested with proteinase K for 1 hour at 55°C, precipitated on ice for 15 minutes in protein precipitation solution (Promega Corporation, Madison, WI), and centrifuged at 4°C for 20 minutes. Equal volumes of ice-cold isopropanol and Glycoblue (Invitrogen, Carlsbad, CA) were added and mixed by inversion, incubated at −20°C for 2 hours, and centrifuged at 13,500 rpm at 4°C for 30 minutes to precipitate DNA. The DNA pellet was washed with 70% cold ethanol twice, dried, and resuspended with distilled water for use in genotyping.
Genotyping
For genotyping, the typical PCR reaction mix was 25 μL, composed of 1× GoTaq PCR buffer (Promega Corporation), 0.25 mM 2′-deoxynucleoside 5′-triphosphate (Invitrogen), 0.5 μM forward and reverse primers (Invitrogen), 0.25 U of GoTaq (Promega Corporation), and 1 μL of tail or sperm head DNA solution. Forward and reverse primer pairs for genotyping the Stra8-iCre transgene and the Rara+, Rarafl, and Rara− alleles are listed in Table 1. Rara-specific primer positions are illustrated on the Rara gene, shown in Fig. 1(b).
Table 1.
Primer Sequences Used in Genotyping
| Allele Detected | Primer Name | Primer Sequence (5′→3′) | PCR Fragment |
|---|---|---|---|
| WT Rara (Rara+) | TD200 | TTAACTCCTCCTGAACTTTGGGAAG | 554 bp |
| VZ200 | TCCGACTTGCGACTCCCTCTACTCA | ||
| 6799 | GTGCCCTTCCCTCCATCTTCCTTA | 589 bp | |
| 7365 | GTCCTCCTCCGACTTGCGACTCC | ||
| Floxed Rara (Rarafl) | 6799 | GTGCCCTTCCCTCCATCTTCCTTA | 575 bp |
| 7352 | CATCGCCTTCTATCGCCTTCTT | ||
| 6799 | GTGCCCTTCCCTCCATCTTCCTTA | 1017 bp | |
| 7794 | ACCTTGCTCCTGCCGAGAAAGT | ||
| Deleted Rara (Rara−) | 6799 | GTGCCCTTCCCTCCATCTTCCTTA | 487 bp |
| 7263 | GCCCTGCGACACTCACACTCCTT | ||
| 6799 | GTGCCCTTCCCTCCATCTTCCTTA | 406 bp | |
| Δ2 | CGGGTCACCTTGTTGATGATGCA | ||
| Stra8-iCre | Stra8-2F | GTGCAAGCTGAACAACAGGA | 865 bp |
| Stra8-R | GTCCCCATCCTCGAGCAGCCTC | ||
| Stra8-3F | TGCCCAAGAAGAAGAGGAAA | 403 bp | |
| Stra8-2R | CCAGCATCCACATTCTCCTT |
Testicular tissue collection
Male mice at postnatal day (P)1 to P365 were humanely euthanized and testes were collected, fixed in 4% paraformaldehyde (PFA) or Bouin solution for 30 minutes to 6 hours, dependent on the size of testis, embedded in paraffin wax, cut into 4-μm-thick sections, and mounted on HistoBond slides (VWR, Radnor, PA).
Germ cell enrichment, RNA isolation, and reverse transcription and real-time quantitative PCR
Germ cells were enriched from testes using a two-step digestion process, as described (31). Briefly, testes were removed from P4 and P8 animals, detunicated, and tubules were placed in a digestion media of Hanks balanced salt solution (Sigma-Aldrich, St. Louis, MO) and trypsin (Gibco, Thermo Fisher Scientific, Waltham, MA). After shearing of tubules via pipette mixing, cells were incubated at 37°C, centrifuged, resuspended in a second digestion media composed of trypsin (Gibco), Hanks balanced salt solution, collagenase, DNase, and hyaluronidase (Sigma-Aldrich), and incubated for 15 to 20 minutes at 37°C. After fetal bovine serum (Sigma-Aldrich) was added to cells and incubated for another 5 minutes at 37°C to stop enzyme activities, cells were passed through a 40-μm mesh sieve screen (Corning, Corning, NY) and centrifuged. Enriched cells were then used for RNA isolation using TRIzol reagent (Invitrogen), following the manufacturer’s protocol.
cDNA was synthesized (reverse transcribed) from 500 ng of total RNA isolated from germ cells using an iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). A reaction mixture of 25 μL contained 12.5 μL of 2× Power SYBR Green PCR master mix (Applied Biosystems Technology, Foster City, CA), 500 nM forward and reverse primers, 5 μL of diluted cDNA (1:20 dilution), and distilled water. Real-time quantitative PCR (qPCR) was performed as follows: 1 cycle at 50°C for 2 minutes, 1 cycle at 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, and 1 cycle at 60°C for 1 minute (Applied Biosystems 7500 Fast real-time PCR system). Forward and reverse primers for real-time qPCR were designed using Primer Express version 2.0 (Applied Biosystems Technology) and are listed in Table 2. The real-time RT-qPCR was conducted on at least three animal samples with each sample in a technical duplicate or triplicate. The abundance of mRNA was evaluated using the 2−ΔCT method (32). Cycle threshold values for Rara and the two housekeeping genes for ribosomal S2 protein (Rps2) and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) were determined using the 7500 software version 2.0.1 (Applied Biosystems Technology). Cycle threshold values for Rara and Stra8 were normalized to those of either Rps2 or Gapdh values in each sample, and the abundance of Rara and Stra8 were calculated relative to the level in the reference sample (P8 WT value for each target).
Table 2.
Primers Used for Real-Time qPCR
| Gene Name | Forward Primer (5′→3′) | Reverse Primer (5′→3′) |
|---|---|---|
| Rara | ATCGAGACCCAGAGCAGCA | TGTCCCGGTGACACGTATACA |
| Stra8 | TGGTAGGGCTCTTCAACAACCT | CCTATTCAGTACCTGCCACTTTGAG |
| Gata4 | TCTGGAGGCGAGATGGGAC | TCTGGCAGTTGGCACAGGA |
| Hsd3b1 | TGTGCCAGCCTTCATCTTCTG | GTGGCCATTCAGGACGATCTT |
Immunohistochemistry and immunofluorescence of tissue sections
Testicular sections fixed in 4% PFA were deparaffinized, and antigens were heat retrieved via microwaving slide sections in 0.01 M sodium citrate solution. Antibody incubations were conducted in a humidified chamber. For immunohistochemistry (IHC), tissue sections were blocked with 10% normal goat serum (Vector Laboratories, Burlingame, CA) at room temperature and incubated with a primary antibody made in rabbit at 4°C overnight, followed by biotinylated goat anti-rabbit IgG secondary antibody (Vector Laboratories) at room temperature. Sections were further washed in PBS, treated with streptavidin-peroxidase (Vector Laboratories) and DAB-Plus substrate kit (Invitrogen), counterstained with hematoxylin, and sealed with HistoMount (Invitrogen).
For immunofluorescence (IF) using mouse monoclonal antibody, the Mouse on Mouse basic kit (Vector Laboratories) was used following the manufacturer’s protocol. Briefly, tissue sections were first blocked with blocking reagent from the kit, incubated with primary antibody overnight at 4°C, washed in PBS, and then incubated with secondary antibody supplied in the kit. For IF using rabbit polyclonal antibody, tissue sections were treated with 0.2% Triton X-100, blocked with 10% normal goat serum, incubated with primary antibody overnight at 4°C, washed in PBS, incubated with biotinylated anti-rabbit IgG secondary antibody (Vector Laboratories), followed by streptavidin-conjugated Alexa Fluor 488 or 555 (Life Technologies), and mounted with VectaShield reagent (Vector Laboratories). If needed, 4′,6-diamidino-2-phenylindole (DAPI) (1:10,000; Life Technologies) was included with streptavidin-conjugated Alexa Fluor to counterstain DNA. IHC and IF sections were visualized using a Leica DMRB microscope (Leica, Wetzlar, Germany). Digital images were obtained using a Leica DFC 310 FX digital camera.
Sperm count and fertility analysis
Sperm were collected from the caudal epididymis of adult mice at P75, P120, P180, and P365. Specifically, epididymides were cut into pieces and placed in PBS at 37°C for 5 minutes and gently pipette mixed to facilitate sperm release. Fresh sperm were counted on a hemocytometer under an inverted phase-contrast Olympus IMT-2 microscope. For fertility analysis, Rara cKO males at P120, P180, and P300 were mated to C57BL/6J WT females aged between P50 and P126. Breeding pairs were kept together for 1 week, and vaginal plugs were confirmed before separation. Number and sex of live pups before P5 were recorded for multiple litters per male breeder age.
Biotin permeability assay
The integrity of Sertoli cell tight junctions was examined, as previously described with some modifications (33). Male mice at P60 to P75 were humanely euthanized, and testes from WT and Rara cKO mice were collected. Then, 10 mg/mL water-soluble EZ-Link Sulfo-NHS-LC-Biotin reagent (Thermo Fisher Scientific, Rockford, IL) in PBS with 1 mM calcium chloride (PBS-C) was injected into testes, followed by submersion of testes in biotin solution, and incubation at room temperature for 30 minutes. Negative controls were injected with and submerged in PBS-C. Testes were then fixed in 4% PFA solution overnight at 4°C, embedded in paraffin wax, cut into 4-μm-thick sections, and mounted on HistoBond slides (VWR). The slides were further incubated with streptavidin-conjugated Alexa Fluor 555 (Life Technologies) and mounted with VectaShield reagent (Vector Laboratories). Sections were visualized using a Leica DMRB microscope. Digital images were obtained using a Leica DFC 310 FX digital camera.
Spermatocyte chromosomal spreads and immunostaining
Chromosomes of spermatocytes from P15, P17, P19, and P21 pups were spread on glass slides, as previously described (34). Briefly, seminiferous tubules were isolated and submerged in a defined water-based hypotonic buffer for the swelling and release of spermatocytes from tubules. Spermatocyte suspension was transferred to a precleaned glass slide coated with 1.1% PFA (pH 9.2) containing Triton X-100. Spermatocytes were dispersed across the slide by manual rocking. Slides were dried overnight in a humid chamber, cleaned with 0.04% Photo-Flo (Kodak, Rochester, NY), and air-dried before staining.
For immunostaining, chromosomal spreads were blocked in 10% normal donkey serum solution (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBS containing 3% BSA (Sigma-Aldrich) and 0.05% Triton X-100 for 60 to 90 minutes at room temperature. Antibody incubations were conducted at 37°C in a humidified chamber with plastic or glass coverslips. When the rabbit polyclonal primary anti- synaptonemal complex protein 3 (SYCP3) antibody purchased from Abcam (Cambridge, MA) (35) was used at 1:100 dilution, the secondary antibody used was rhodamine donkey anti-rabbit (1:50; Jackson ImmunoResearch Laboratories). When the mouse monoclonal primary anti-γH2AX antibody purchased from MilliporeSigma (Burlington, MA) (36) was used at 1:750 dilution, the secondary antibody used was FITC donkey anti-mouse (1:50; Jackson ImmunoResearch Laboratories). After antibody incubations, spreads were washed in PBS, counterstained with DAPI, rinsed with distilled water, and sealed with VectaShield (Vector Laboratories) and rubber cement. Imaging for meiotic stage progression and meiotic defects was performed using Leica DM6B and Zeiss Axiovert 200M microscopes (Zeiss, Oberkochen, Germany).
TUNEL assay
Testis sections fixed in 4% PFA from P15, P17, and P19 were deparaffinized in preparation for the TUNEL apoptotic assay. The TUNEL assay labels DNA ends indicating DNA breaks, a signature of cells undergoing apoptosis (37). Apoptotic cells were detected according to the manufacturer’s instructions (Promega Corporation). As a positive control, tissue sections were pretreated with deoxyribonuclease I (Promega Corporation). Conversely, tissue sections were incubated without terminal deoxynucleotidyl transferase recombinant enzyme for negative control. Sections were visualized using a Leitz DMRB microscope, and digital images were obtained using a Leica DFC 310 FX digital camera.
Busulfan treatment and restoration of spermatogenesis in the seminiferous tubules
Animals aged 31 to 43 days were injected with 35 mg/kg body weight with busulfan (Sigma-Aldrich) and left to recover for either 4 or 8 weeks (38, 39). Testes were collected after recovery periods, fixed in 4% PFA, mounted in paraffin wax, cut into 4-μm-thick sections, and mounted on HistoBond slides (VWR). To examine the recovery of spermatogenesis in the seminiferous tubules, testicular sections from mice recovered for 4 weeks or 8 weeks were deparaffinized and stained with hematoxylin and eosin. Sections were examined using a Leica DMRB microscope, and digital images were obtained using a Leica DFC 310 FX digital camera.
Statistical analysis
For experiments requiring quantification of seminiferous tubules, only round-shaped tubules were counted. Statistical significance was determined by pairwise comparisons of the means using the Student t method from JMP, versions 11 to 13 software (SAS Institute, Cary, NC). A P value of <0.05 was considered significant. The P values between 0.05 and 0.1 are noted.
Results
Generation of the Rarafl/fl mouse strain
The Rara gene was isolated from a mouse bacterial artificial chromosome (BAC) clone (Invitrogen). Nucleotide numbers in the Rara genomic sequence are those from the Ensembl gene ID ENSMUSG00000037992. The genomic location of Rara is 98608821 to 98646063. The Rara DNA fragment containing exon 4 (nucleotides 98637229 to 98638047) was amplified by PCR using the mouse BAC clone 331 as a template, which was then ligated into the NheI site of pGkNeo(A) to produce the construct pGkE4Neo(A). The 3′-homologous region of Rara (nucleotides 98639103 to 98643084) was amplified by PCR using the mouse BAC clone 331 as a template and then ligated into the EcoRI site of pGkE4Neo(A) to produce pGk3′E4Neo(A). The 5′-homologous region of Rara (nucleotides 98635270 to 98637228) was amplified by PCR using the mouse BAC clone 331 as a template and then ligated into the BamHI and HindIII sites of pTK-KS(+)(SE) to produce pTK-KS(+)5′. After digestion with BamHI and NotI, the DNA restriction fragment containing the 5′- homologous region of Rara along with thymidine kinase was ligated into the NotI-BamHI site of pGk3′E4Neo(A)5′ to produce the Rara gene–targeting construct pGk3′E4Neo(A)5′TK. Consequently, exon 4 of Rara was placed in a sequence flanked by two loxP sites, termed as “floxed” Rara (Rarafl) [Fig. 1(a)]. The resulting Rarafl construct was verified by DNA sequencing.
Recombinant embryonic stem cells containing the Rarafl construct were injected into blastocysts, which were transferred into pseudopregnant females. After selection, backcrossing and genotyping, Rarafl/fl mice in the C57BL/6J genetic background were generated (C57BL/6J-Rarafl/fl). Exon 4, coding for a portion of the B domain of the protein RARA, is common to the two main RARA isoforms (α1 and α2) and is necessary for the transcriptional activation and action of RARA (40). Thus, the excision of exon 4 by improved causes recombination with cyclization (iCRE) recombinase, which creates an in-frame deletion of 3489 bp, is predicted to disrupt RARA function. Rarafl/fl mice were mated to B6.C-Tg (CMV-cre)1Cgn/J mice (The Jackson Laboratory) to verify the deletion.
Germ cell–specific deletion of Rara
It was known that Stra8-iCre transgenic mice express the iCre transgene as early as P3 in male germ cells (41). Previously, the morphology of the seminiferous tubules from global heterozygous Rara mutant mice was examined and found that histological abnormalities were minor, compared with the dramatic disruption in germ cell organization observed in Rara-null seminiferous tubules, with both Rara alleles deleted (19). Thus, to achieve an in-frame excision of both Rarafl alleles in germ cells, a two-step breeding scheme was conducted to generate germ cell–specific Rara cKO pups with Stra8iCre/+;Rara−/− germ cell genotype [Fig. 1(b)]. In the first step of the breeding scheme, Rarafl/fl females (C57BL/6J genetic background) were mated to Stra8-iCre males (FVB genetic background) to generate Stra8iCre/+;Rarafl/+ F1 generation heterozygotes. In the second step, F1 males were bred to Rarafl/fl females to generate F2 pups. DNA from tail snips of the F1 and F2 generations was used to genotype, and only F2 pups with Stra8iCre/+;Rarafl/− tail genotype, which is homozygous knockout in germ cells and heterozygous in somatic cells, were used for Rara cKO sample collection [Fig. 1(b)]. WT control animals were generated in a similar way as the two-step breeding scheme except that C57BL/6J-Rara+/+ females were used in place of Rarafl/fl females. In this way, the F2 generation WT males have the same strain genetic composition (75% C57BL/6J and 25% FVB) as do the Rara cKO mice, but they have not been exposed to the Rarafl gene. Animal genotypes were confirmed using PCR, as described in the “Materials and Methods” section. Locations of primer pairs are shown in Fig. 1(b) and primer sequences are listed in Table 1.
Expression of Stra8-iCre using R26R-EYFP mice
The earliest expression of Stra8-iCre during mouse development was investigated using R26R-EYFP mice (The Jackson Laboratory), which have a loxP-flanked STOP sequence following the enhanced yellow fluorescent protein (Eyfp) gene inserted into the Gt(ROSA)26Sor locus [Fig. 2(a)]. To determine the expression of iCRE between P0 and P7 in detail, R26R-EYFP females were mated to Stra8-iCre males to generate Eyfp;Stra8-iCre F1 (Eyfp F1) pups that express EYFP in the Stra8-iCre–expressing germ cells. In Eyfp F1 mice, iCRE recombinase in Stra8-iCre–expressing germ cells recombines the two loxP sites, deleting the STOP sequence and allowing the expression of EYFP [Fig. 2(a)].
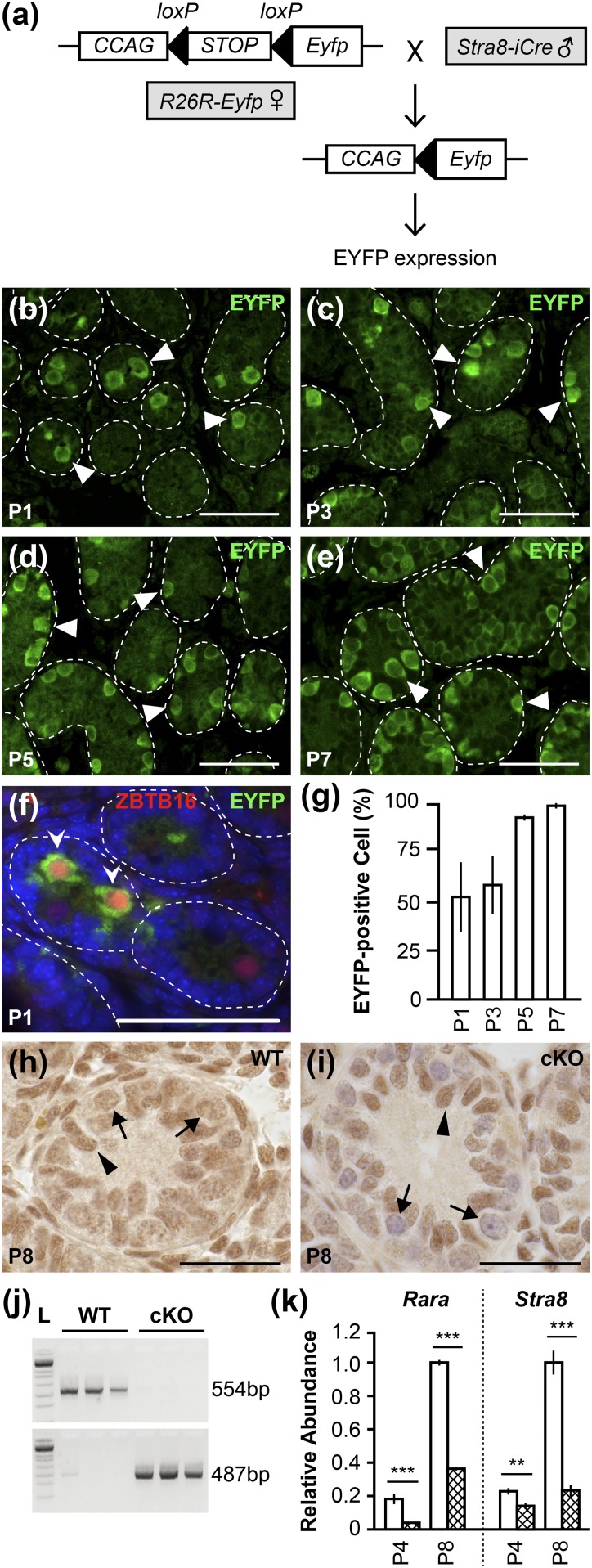
Verification of Rara inactivation in germ cells. (a) Mating between the R26R-Eyfp females carrying an iCRE-activity reporter construct and Stra8-iCre males leads to EYFP expression in germ cells. CCAG, chicken β-actin promoter. (b–e) Green fluorescent cells are germ cells with iCRE activity (white arrowheads) in testis cross-sections from animals at P1, P3, P5, and P7. (f) Gonocytes with iCRE activity are EYFP- and ZBTB16-positive germ cells (white pointed arrowheads) in P1 testis cross-sections. Scale bars, 50 µm. (g) Percentage of tubules containing at least one EYFP-positive cell in animals at P1, P3, P5, and P7. n = 3 to 4 for P1, P3, and P7; n = 2 for P5. Error bars are SD. (h and i) IHC for RARA on testicular sections from WT and Rara cKO mice at P8. Black arrowheads indicate Sertoli cells; black arrows indicate germ cells. n = 3. Scale bars, 50 µm. (j) PCR products obtained using DNA template isolated from epididymal sperm heads in WT and cKO animals at P75. A 487-bp fragment represents the deleted form of Rara allele, and a 554-bp fragment represents the WT Rara allele. n = 3 to 5. (k) Real-time RT-qPCR analysis on RNA collected from enriched germ cell populations from WT (open bar) and Rara cKO (cross-hatched bar) animals at P4 and P8 for Rara (***P < 0.001; WT and cKO n = 3) and Stra8 (**P < 0.01, ***P < 0.001; WT n = 6, cKO n = 4). Error bars are SEM. L, DNA ladder.
Fresh testicular tubules were isolated from mice at P0, P1, P2, P3, P4, and P5 and examined for EYFP expression under fluorescent light. iCRE recombinase was active as early as P0 with 20% of F1 pups showing EYFP signal, and from P1 to P5 with 100% of F1 pups showing EYFP expression in the tubules (n = 5 to 7; data not shown). To further investigate the expression of EYFP in germ cells, testicular sections were immunostained with rabbit primary polyclonal anti-GFP antibody purchased from Abcam (42) at 1:500 dilution, which detects the protein product of Eyfp, and then were scored for tubules containing one or more EYFP-positive cells [Fig. 2(b)–2(e), arrowheads). A mean total number of 50 tubules per animal were scored. At P1 and P3, 52.2% and 58.2% of tubules had at least one EYFP-positive germ cell, respectively [Fig. 2(g)]. At P5 and P7, virtually all tubules contained EYFP-positive germ cells (92.6% and 98.6%, respectively). The earliest germ cells expressing EYFP were gonocytes, as evidenced by the positive coimmunolocalization of EYFP and zinc finger and BTB domain–containing protein 16 (ZBTB16) in gonocytes from P1 animals [Fig. 2(f), pointed arrowheads] using anti-GFP antibody (42) and mouse monoclonal anti-ZBTB16 antibody (43) at 1:500 dilution (MilliporeSigma). ZBTB16 is expressed in gonocytes, SSCs, and progenitor spermatogonia (44).
Efficiency of Stra8-iCre expression in germ cells
IHC assays were conducted on testicular sections from P8 animals using rabbit polyclonal anti-RARA antibody purchased from Santa Cruz Biotechnology (Dallas, TX) (45) at 1:400 dilution. RARA immunostaining in WT testis cross-sections [Fig. 2(h)] was uniform for both germ cells (arrows) and Sertoli cells (arrowheads), indicating that they both express RARA. In contrast, there was a markedly reduced level of RARA immunostaining in germ cells (arrows) of Rara cKO testis cross-sections [Fig. 2(i)] compared with Sertoli cells (arrowheads).
To further characterize the efficiency of Stra8-iCre expression in germ cells, without any potential contribution of expression from somatic cells, epididymal sperm were collected from adult WT and Rara cKO animals at P75 and DNA was isolated from the sperm heads. Epididymal sperm preparation is virtually free of somatic cells. The WT Rara allele was identified with the TD200/VZ200 primer set (Table 1), generating a 554-bp fragment, and the deleted form of the Rara allele was detected with the 6799/7263 primer set (Table 1), producing a 487-bp fragment. Genotyping of epididymal sperm DNA from Rara cKO animals showed that whereas the WT Rara allele was absent, the DNA fragment that is indicative of the deleted form of the Rara allele was present with 100% efficiency [Fig. 2(j)].
To determine the amount of Rara mRNAs in the enriched population of germ cells, germ cells were isolated from WT and Rara cKO testes. Enriched germ cells from Rara cKO testes had significantly reduced levels of Rara mRNA in animals at P4 (P < 0.001) and P8 (P < 0.0001) vs WT controls [Fig. 2(k)]. To control for the level of somatic cell contamination in the enriched germ cell population, real-time RT-qPCR analyses were conducted to evaluate mRNA levels of the genes Gata4 (a marker for the Sertoli cell) and Hsd3β1 (a marker for the Leydig cell). There were no statistically significant differences in Gata4 or Hsd3β1 mRNA levels between WT and cKO samples (data not shown), indicating similar somatic cell contamination in both samples. These experiments collectively show that Rara is inactivated (exon 4 deleted) in germ cells of Rara cKO mice. Additionally, the expression of Stra8, an RA-responsive gene (46), was determined using real-time RT-qPCR to verify that RARA signaling in the animal model was impaired. Indeed, there were 1.6-fold (P < 0.01) and 4.3-fold (P < 0.0001) declines in the level of Stra8 mRNA in Rara cKO mice compare with the WT controls at P4 and P8, respectively [Fig. 2(k)].
Morphology of seminiferous tubules in germ cell–specific Rara cKO mice was disrupted throughout development
Bouin-fixed testicular sections of WT and Rara cKO animals throughout development were deparaffinized and stained with hematoxylin and eosin (Fig. 3). The WT control seminiferous tubules exhibited the typical, healthy germ cell associations in the seminiferous epithelium showing strictly organized and defined germ cell layers [Fig. 3(a), 3(c), 3(e), 3(g), 3(i), 3(k), 3(m), 3(o), and 3(q)]. In contrast, abnormalities were varied and abundant in the tubules of Rara cKO mice throughout development [Fig. 3(b), 3(d), 3(f), 3(h), 3(j), 3(l), 3(n), 3(p), and 3(r)]. Morphological abnormalities were first evident at P15 [Fig. 3(b)] and almost all testes in Rara cKO animals contained a mix of normal tubules with an assortment of abnormal ones after P15. Germ cell sloughing, virtually blocking the lumen [Fig. 3(f), 3(j), and 3(l)], was the most common phenotype, and other tubule abnormalities included the presence of vacuoles of varying sizes and multinucleated cells as well as missing germ cell layers [Fig. 3(j), 3(n), 3(p), and 3(r)]. The degeneration of tubules was progressively more severe with aging, and by P180 and P365, most tubules were highly vacuolated with layers of germ cells missing [Fig. 3(n), 3(p), 3(r)], and in one animal (out of seven examined) at P365, all tubules were Sertoli cells only. Consistently, the epididymides of WT animals [Fig. 3(s)] contained only spermatozoa, whereas epididymides of Rara cKO mice [Fig. 3(t)] displayed immature germ cells, both round spermatids and pachytene spermatocytes, as shown in the inset.
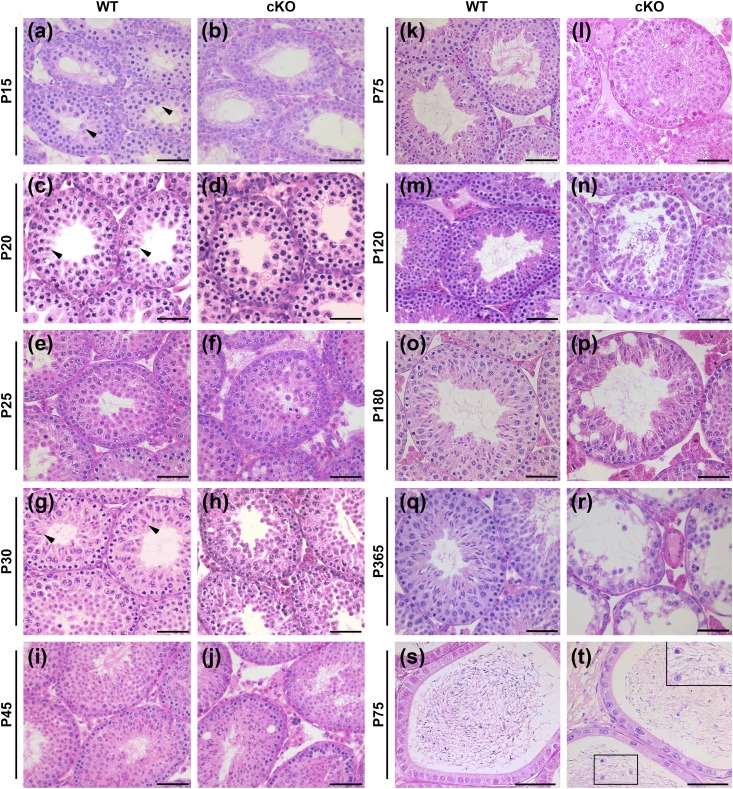
Histological analyses show increased morphological abnormalities in testicular and epididymal cross-sections of Rara cKO animals. Hematoxylin and eosin–stained testis cross-sections of (a, c, e, g, i, k, m, o, and q) WT and (b, d, f, h, j, l, n, p, and r) Rara cKO animals at various ages from P15 to P365. (a, c, and g) Black arrowheads in WT animals show most advanced cell types at P15, P20, and P30, which are pachytene spermatocytes, round spermatids, and elongated spermatids, respectively. (s and t) Hematoxylin and eosin–stained epididymis cross-sections of (s) WT and (t) Rara cKO mice at P75. Insets in (t) show immature cells at a higher magnification. Seminiferous tubules in Rara cKO animals showed (f, j, and l) sloughing cells blocking the lumen, vacuolization, and (n, p, and r) missing germ cell layers. Scale bars, 50 µm in (a)–(r). Scale bars, 100 µm in (s) and (t).
A mean total of 150 tubules per animal were scored for abnormalities that included germ cell sloughing, vacuolization, multinucleated cells, tubules lacking lumen, and missing germ cell layers. From P25 onward, Rara cKO animals had a significantly increased number of abnormal tubules (P < 0.05 or P < 0.01), an average 40% of the total tubules, vs WT controls [Fig. 4(a)]. Additionally, a mean total of 150 tubules per animal were scored for germ cell sloughing specifically. Relative to WT, Rara cKO testes had a significantly higher level of sloughing germ cells at P45 (P < 0.05) and P75 (P < 0.01) [Fig. 4(b)]. Nearly all abnormal tubules at P75 had the massive cell sloughing phenotype, which consisted of 33% of the seminiferous tubules from Rara cKO mice at P75. Although the mean level of sloughing was higher in P120, the P value was not significant due to a large animal-to-animal variation. By P180, massive germ cell sloughing had subsided.
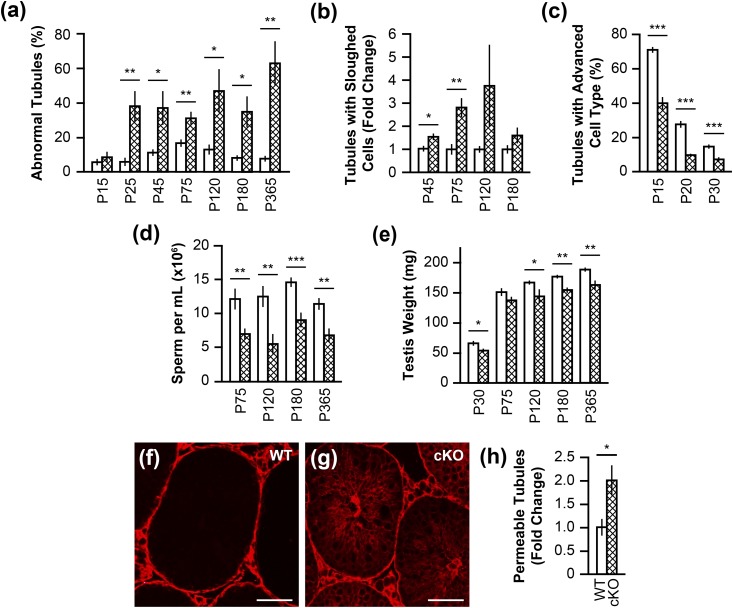
Quantitation of increased seminiferous tubule abnormalities, delayed progression of germ cell development, decreased sperm count and testis weights, and compromised BTB in Rara cKO testes. (a) Percentage of tubules with abnormalities was present in Rara cKO mice (cross-hatched bar) compared with WT mice (open bar) at P15 and onward, up to P365 (*P < 0.05, **P < 0.01). n = 5 to 10. Error bars are SEM. (b) Tubules containing sloughed germ cells were quantified in mice at P45, P75, P120, and P180, presented as fold change relative to the WT level (*P < 0.05, **P < 0.01). n = 4 to 5. Error bars are SEM. (c) Percentage of tubules containing the most advanced cell types in the testis of Rara cKO mice compared with WT mice at P15, P20, and P30 (***P < 0.001). n = 3. Error bars are SEM. (d) Sperm counts, shown in millions per milliliter, in mice at P75, P120, P180, and P365 (**P < 0.01, ***P < 0.001). n = 5 to 14. Error bars are SEM. (e) Testis weights, measured in milligrams (*P < 0.05, **P < 0.01). n = 9 to 29. Error bars are SEM. (f–h) Tubules were scored for the presence of biotin in the adluminal compartment (red), presented as fold change relative to the WT level (*P < 0.05). n = 3 to 4. Error bars are SEM. Scale bars, 50 µm.
Reduced number of spermatocytes and spermatids in Rara cKO animals
To characterize the relative efficiency of spermatogenesis in the testis, seminiferous tubules containing the most advanced germ cell types at P15, P20, and P30 were counted. Large pachytene spermatocytes [Fig. 3(a), arrowheads], round spermatids [Fig. 3(c), arrowheads], and step 8 to 10 elongating spermatids [Fig. 3(g), arrowheads] were reported to be the most advanced cell types in P15, P20, and P30 animals, respectively (47). A mean total number of 200 tubules per animal were scored. In testes of P15 animals, 39.8% of Rara cKO tubules contained large pachytene spermatocytes compared with 70.8% in WT testes [Fig. 4(c)]. The decline in the tubules with the most advanced germ cell types was persistent at P20 and P30 in Rara cKO animals. There were only 9.6% of tubules containing round spermatids at P20, and 7.1% tubules containing step 8 to 10 elongating spermatids at P30 in Rara cKO animals, whereas in the WT animals, 27.5% consisted of round spermatids at P20 and 14.6% consisted of step 8 to 10 elongating spermatids at P30 [Fig. 4(c)]. At each age observed, germ cell development in Rara cKO animals was significantly impeded compared with the WT animals (P < 0.001).
A reduction in sperm count of germ cell–specific Rara cKO males did not affect fertility
To gain an overall view of spermatogenesis competence in germ cell–specific Rara cKO animals, testis weights were recorded, and epididymal sperm were isolated and counted. There was on average a significant reduction of 43% for the epididymal sperm counts in Rara cKO males at P75, P120, and P365 (P < 0.01), and at P180 (P < 0.001), relative to WT controls [Fig. 4(d)]. These data were reflected by significant decreases in testis weight of Rara cKO animals compared with WT at P30 and P120 (P < 0.05), as well as at P180 and P365 (P < 0.01) [Fig. 4(e)]. However, the 43% reduction in sperm yield was not enough to affect fertility in germ cell–specific Rara cKO males, as there were no differences in the number of pups sired between Rara cKO and WT males at ages P120, P180, and P300 (Table 3). This was not surprising because as little as 10% of control sperm production was enough to restore fertility (48) when other parameters were standard. Previously, an infertility phenotype was observed in global Rara-null mice, where sperm counts dropped to 1.7% of the WT counterpart (19). These findings together highlight the fact that both germ cell RARA and Sertoli cell RARA must be abrogated to have an infertility phenotype.
Table 3.
Fertility Analysis
| Age | Genotype | Breeding Males | Pups per Litter | P Value |
|---|---|---|---|---|
| P120 | WT | 17 | 7.8 | 0.5297 |
| Rara cKO | 18 | 7.3 | ||
| P180 | WT | 13 | 7.7 | 0.8612 |
| Rara cKO | 16 | 7.6 | ||
| P300 | WT | 4 | 7.8 | 0.1755 |
| Rara cKO | 5 | 8.8 |
The P value calculated between WT and cKO animals is based on the number of pups per litter for each age.
BTB is perturbed in Rara cKO animals
The disorganization of germ cells in Rara cKO testes suggested possible disruption of the integrity of the BTB. BTB is composed of inter–Sertoli cell tight junctions, ectoplasmic specializations, desmosome-like junctions, and gap junctions between Sertoli cells and germ cells, and together form an impermeable barrier. The BTB separates the tubule into the basal compartment containing spermatogonia and the adluminal compartment containing spermatocytes and spermatids toward the center of the tubule in the lumen (1). A biotin permeability assay was used to assess the integrity of BTB. Testes were collected from WT and germ cell–specific Rara cKO adult mice (P60 to P75) and were injected and incubated with a solution containing biotin. Biotin was prohibited from entering the adluminal compartment of seminiferous tubules when the BTB was intact, as shown in Fig. 4(f). For each animal, the percentage of tubules with biotin in the adluminal compartment was calculated, based on a mean total of 150 tubules per animal scored. In the Rara cKO testes, there was a twofold increase in tubules with biotin leakage to the adluminal compartment compared with WT (P < 0.05) [Fig. 4(g) and 4(h)]. The results suggest that more of the BTB was breached in the Rara cKO testes.
Deletion of Rara in germ cells interferes with the progression of spermatocytes through meiosis
A sizable decrease in the number of pachytene spermatocytes in Rara cKO animals compared with WT animals [Fig. 4(c)] suggests an alteration in the progression of meiosis. Spermatocytes from the P10 to P20 ages include the four stages of meiotic prophase during the first wave of spermatogenesis: leptotene, zygotene, pachytene, and diplotene (49). These stages carry out the stepwise chromosome pairing, synapsis, and genetic recombination that occur during meiotic prophase.
The status of meiotic progression was assessed using two methods. In the first immunological method, testis cross-sections from animals at P11, P15, and P20 were incubated with an anti-γH2AX antibody (36), counterstained with DAPI, and processed for IF detection. Anti-γH2AX antibody (36) immunostains the phosphorylated histone 2AX, localized to DNA double-stranded breaks that occur at the beginning of meiosis. Positive immunostaining for γH2AX in leptotene and zygotene spermatocytes was diffused throughout the cell, whereas in pachytene spermatocytes, immunostaining was limited to the sex chromosomes and is seen as punctate stains (50). At P11, the percentage of tubules with leptotene and zygotene spermatocytes was higher in the WT animals, albeit not statistically significant [Fig. 5(a), 5(b), and 5(e)]. By P15 (P < 0.01) and P20 (P < 0.05), the balance shifted, and the percentage of tubules with leptotene and zygotene spermatocytes was significantly higher in the Rara cKO animals compared with the WT animals [Fig. 5(c)–5(e)]. A mean total of 200 tubules per animal were scored.
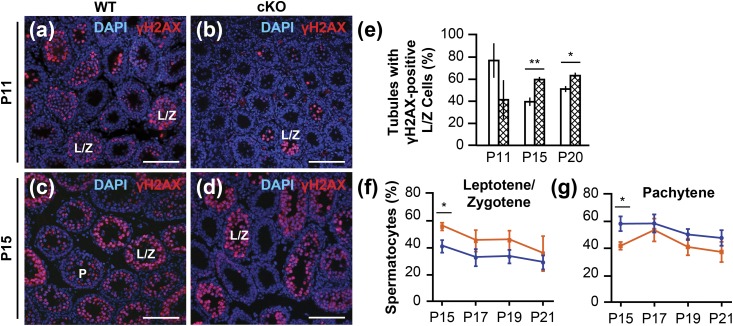
Meiotic progression delayed in Rara cKO mice. (a–d) Testicular cross-sections of WT and Rara cKO mice at P11 and P15; γH2AX-positive cells are in red. L/Z indicates tubules containing leptotene and zygotene spermatocytes; P indicates tubules containing pachytene spermatocytes. Scale bars, 100 µm. (e) Quantification of the percentage of tubules with γH2AX-positive leptotene and zygotene spermatocytes for WT and Rara cKO animals at P11, P15, and P20 ages (*P < 0.05, **P < 0.01). n = 3 to 8. Error bars are SEM. (f and g) From chromosomal spreads, the percentages of meiotic prophase cells in leptotene/zygotene and pachytene stages are shown for WT (blue circles) and Rara cKO (orange squares) mice at P15, P17, P19, and P21 ages (*P < 0.05). n = 3 to 5. Error bars are SEM.
In the second method, meiotic stage progression was analyzed from chromosomal spreads of spermatocytes prepared from P15, P17, P19, and P21 animals and immunostained with anti-SYCP3 antibody (35). Homologous chromosomes are paired, or synapsed, along a proteinaceous complex called the synaptonemal complex (SC) in spermatocytes, and the protein SYCP3 is located in the lateral element of SCs (51). Based on the immunostaining pattern of SYCP3 on SCs, cells were classified into one of three meiotic prophase groups: leptotene/zygotene, pachytene, and diplotene stages. The percentage of cells in each meiotic prophase stage was calculated from the total number of 100 spermatocytes per animal that were randomly selected. Rara cKO animals had a significant increase of leptotene and zygotene spermatocytes at P15 (P < 0.05) and had a significantly lower percentage of pachytene spermatocytes at P15 (P < 0.05) compared with WT controls [Fig. 5(f) and 5(g)]. The trend of accumulation of leptotene and zygotene spermatocytes, as well as the decline of pachytene spermatocytes in Rara cKO animals compared with WT controls, continued through P17, P19, and P21. There was a low percentage, ~5%, of spermatocytes in diplotene stages that did not differ between WT and cKO animals at each age examined (data not shown). The results from both immunological staining and chromosomal spread methods attest that the meiotic progression was indeed disrupted in the Rara cKO testes.
Deletion of Rara in germ cells induces SC defects in pachytene spermatocytes
Unexpectedly, examination of WT and Rara cKO chromosomal spreads from P15, P17, P19, and P21 animals revealed increased SC defects in Rara cKO pachytene spermatocytes [Fig. 6(b) and 6(c)]. In pachytene spermatocytes, homologous chromosomes are completely synapsed along the SC. Using chromosomal spreads immunostained with anti-SYCP3 antibody (35) and anti-γH2AX antibody (36), 50 cells per animal were scored for the following SC defects in pachytene spermatocytes: fragmentation, forks, gaps, and partial asynapsis. Fragmentation was defined as segments of SC that measured at least one-third the length of the nearest intact SC, existing separately from the 19 autosomal SCs and the XY body. Forks were defined as asynapsis at the telomere end of the SC, with each separated segment less than one-third the length of the SC. Partial asynapsis was defined as separated segments equal to or greater than one-third the length of the SC. Gaps were defined as an absence of SYCP3 staining in the SC that was a distance of at least twice the width of the SC. The most common chromosomal defect observed was chromosomal fragmentation [Fig. 6(b) and (c), arrows; Fig. 6(f)], which was increased in Rara cKO animals at P15 (P = 0.0673), P17 (P = 0.0532), and P21 (P = 0.0869) compared with WT controls [Fig. 6(d), white bars]. Other defects included the persistence of γH2AX foci on autosomal chromosomes marking unpaired double-stranded breaks [Fig. 6(b) and 6(c), arrowheads; Fig. 6(g)], partial asynapsis, forks, and gaps in the SC of the pachytene spermatocytes. Accordingly, there was a significant decrease in perfect synapsis [Fig. 6(d), gray bars; Fig. 6(e)] in pachytene spermatocytes of Rara cKO animals at P15 and P17 (P < 0.05) compared with WT controls.
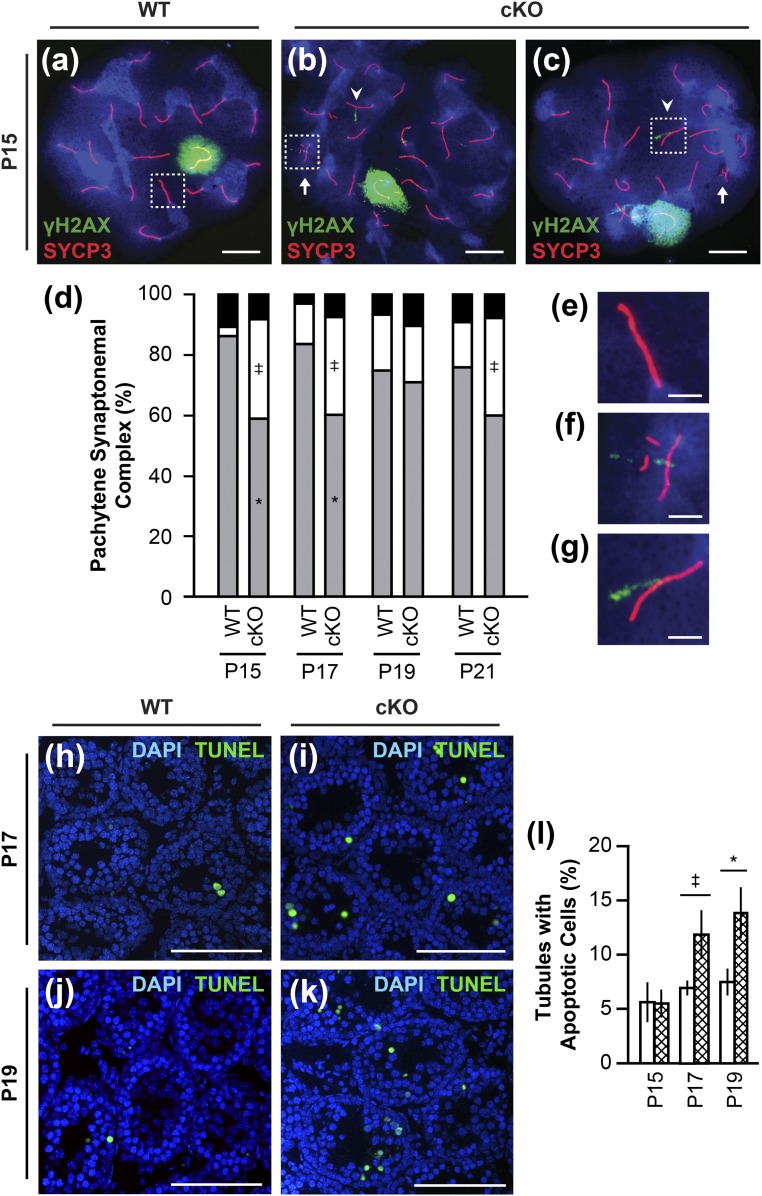
Defective synapsis and increased SC fragmentation in pachytene spermatocytes, followed by increased apoptosis in Rara cKO mice. (a–c and e–g) Chromosomal spreads of pachytene spermatocytes from WT and Rara cKO animals at P15. SYCP3 immunostain showing SC (red), γH2AX immunostain showing DNA double-stranded breaks (green), and DAPI DNA stain (blue) were used. White box in (a) outlines perfect synapsis enlarged in (e). White arrows in (b) and (c) indicate chromosomal fragmentation; a representative outlined by a white box in (b) is enlarged in (f). White arrowheads in (b) and (c) indicate autosomal γH2AX staining; a representative outlined by a white box in (c) is enlarged in (g). Scale bars, 10 μm in (a–c); 2.5 µm in (e–g). (d) From chromosomal spreads, the percentage of cells with perfect synapsis (gray), containing fragmented SC segments (white), and containing other defects (forks, gaps, and partial asynapsis) (black) are shown (‡P < 0.1, *P < 0.05). n = 3 to 5. (h–k) Apoptotic cells visualized by TUNEL assay (green) and DNA visualized by DAPI stain (blue) in seminiferous tubules from WT and Rara cKO mice at P17 and P19. Scale bars, 100µm. (l) Percentage of tubules containing three or more apoptotic cells (‡P < 0.1, *P < 0.05). n = 5 to 6. Error bars are SEM.
Germ cell apoptosis is enhanced in Rara cKO germ cells
The prevalence of chromosomal defects in Rara cKO spermatocytes suggested that these germ cells could be undergoing apoptosis. Apoptosis is a normal process during spermatogenesis, with the highest numbers of apoptotic cells in mice from P8 to P22 (52). TUNEL assays were performed on testicular sections from Rara cKO and WT mice at P15, P17, and P19 to detect apoptotic cells [Fig. 6(h)–6(k)]. The mean total number of 200 tubules was scored. The percentage of tubules containing three or more TUNEL-positive cells was determined. There was an increase in cells undergoing apoptosis in Rara cKO testes from animals at P17 (P = 0.0722) and at P19 (P < 0.05) compared with WT controls [Fig. 6(l)]. Many of the germ cells undergoing apoptosis were spermatocytes, from their DAPI-stained morphology and the location in the tubules. TUNEL-positive spermatocytes were increased in Rara cKO animals at P17 compared with WT controls (P = 0.0536; data not shown), supporting the notion that SC defects are monitored by the meiotic checkpoints, sending Rara cKO spermatocytes with SC defects to the apoptotic program.
Diminished capacity of spermatogonial progenitor regeneration in Rara cKO animals
The presence of Sertoli cell–only tubules in 1-year-old Rara cKO mice raised the question of whether Rara cKO animals may have problems with RA-responsive progenitor regeneration and long-term maintenance of spermatogenesis. To assess the regenerative capacity of SSCs, WT and Rara cKO animals were injected intraperitoneally with busulfan at 35 mg/kg body weight. Busulfan treatment ablates the actively proliferating and differentiating spermatogonia (53), but it still allows for the reinitiation of spermatogenesis from the small number of endogenous stem cells remaining in their niche (27, 39). After busulfan treatment and recovery periods, testicular sections were scored as either empty [Fig. 7(a), E] or recovered [Fig. 7(c), R] tubules. Empty tubules were identified as having virtually no germ cells other than very few lingering early spermatogonia. Recovered tubules were identified as containing spermatogonia and spermatocytes or spermatids. A mean total number of 300 tubules per animal were scored. At 4 weeks postinjection with busulfan, WT and Rara cKO testes showed comparable depletion of germ cells in seminiferous tubules [Fig. 7(a), 7(b), and 7(e)], indicating successful ablation of most endogenous germ cells (27, 54). At 8 weeks postinjection, WT testes displayed a recovery of spermatogenesis in 29.3% of tubules [Fig. 7(c) and 7(e)], similar to previously published data of spermatogenesis recovery 70 days postinjection with 30 mg/kg busulfan (38). The recovery of spermatogenesis in WT testes at the 8-week postinjection period was significantly increased from the 4-week period (P < 0.05). In contrast, Rara cKO testes showed recovery in only 5.5% of tubules at 8 weeks postinjection, which was different from the 4-week postinjection depleted levels, but not statistically significant. Comparing the Rara cKO testis recovery to the WT testis recovery at 8-week postinjection times, there was 5.3-fold fewer recovered tubules in Rara cKO compared with WT animals at P = 0.0834 [Fig. 7(e)].
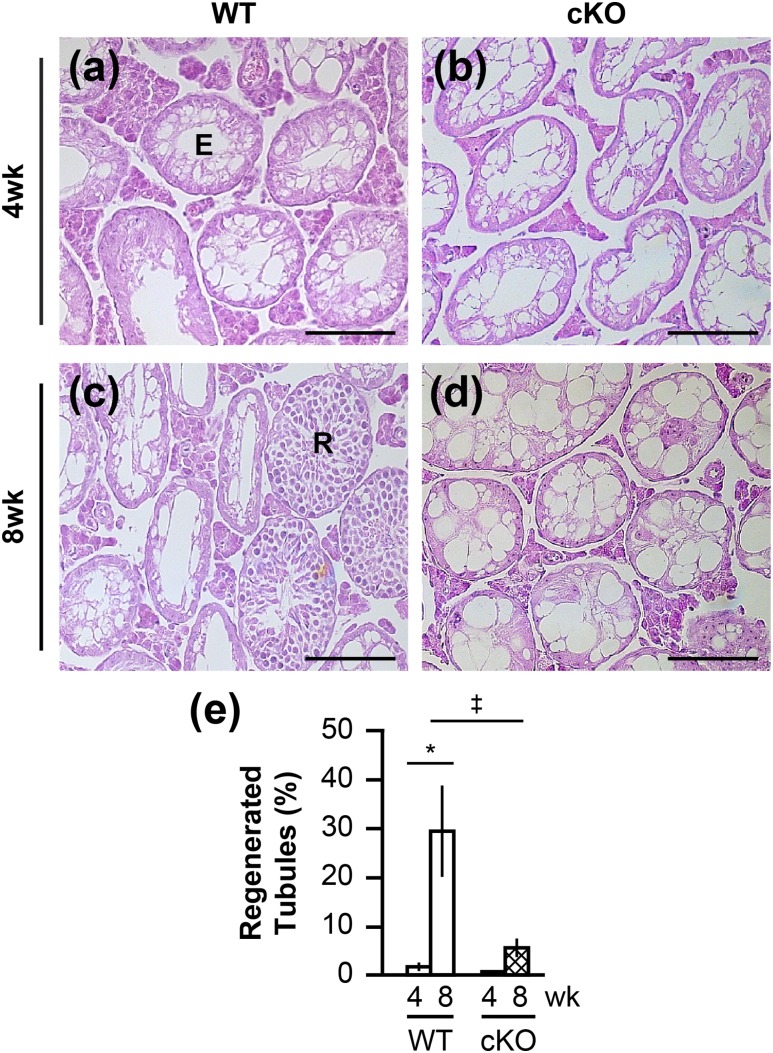
Decreased regenerative capacity of SSCs. (a–d) Hematoxylin and eosin–stained testis cross-sections of (a) WT and (b) Rara cKO animals injected with busulfan and allowed to recover for (a and b) 4 wk or (c and d) 8 wk. Scale bars, 100 µm. (e) Quantification of germ cell regeneration in WT and Rara cKO animals at 4- and 8-wk recovery times (‡P < 0.1, *P < 0.05). n = 6 to 11. Error bars are SEM. E, empty tubule; R, regenerated tubule.
Discussion
Germ cell RARA is critical for proper germ cell layer organization of the seminiferous epithelium and progression of spermatogenesis, just as RARA is in the Sertoli cell
For maintaining life-long and stepwise waves of spermatogenesis clockwork, RA is required as a pivotal regulator. However, which of the three subtypes of retinoid receptors (α, β, γ) and which cell types in the seminiferous epithelium (somatic cells, various developing germ cells) are the responsible mediators of RA action are still not clearly delineated. Previously, histological analyses of Rara-null mouse testes and Sertoli cell–specific Rara cKO testes revealed the phenotypes of germ cell disorganization, vacuolization of the seminiferous epithelium, enhancement of germ cell apoptosis, and impediment of germ cell development during the first wave of spermatogenesis (15, 19). At the time, with no counterpart results from the germ cell–specific Rara cKO mouse model available, it was interpreted that Rara-depleted Sertoli cells of Sertoli cell–specific Rara cKO mouse model were responsible for the mutant phenotypes observed in Rara-null mice (15).
Interestingly, using the germ cell–specific Rara cKO mouse model constructed in the current study, similar phenotypes were exhibited in the seminiferous tubules of Rara cKO testes, including disrupted germ cell layer organization, vacuole formation, indicative of missing germ cells, and massive sloughing of germ cells in the lumen (Figs. 3 and and4).4). The massive sloughing of germ cells stood as the most prominent organization phenotype, significant at P45 and P75. Germ cell sloughing was potentially caused by a premature detachment of germ cells from Sertoli cells. After massive sloughing, most tubules were highly vacuolated with layers of germ cells missing as animal aged to P180 and P365, and at P365, one animal was observed to have all Sertoli cell–only tubules (Fig. 3). Additionally, there was a significant obstruction in the progression of germ cell development, as manifested by the delayed appearance of the most advanced cell types at various ages of mice during the first wave of spermatogenesis (Fig. 4). Thus, the data from these studies unequivocally advocate that germ cell RARA is just as critical a molecular player as Sertoli cell RARA is in the development of germ cells in the testis. The logical corollary is that both Sertoli cell and germ cell RARAs are transcriptional regulators influential for the systematic organization of germ cell layers of the seminiferous epithelium, as well as the maintenance of normal chronological progression of germ cell development.
Germ cell RARA crosstalks with Sertoli cells
It was no surprise that spatial disturbance of germ cell arrangements and the chronological disturbance in germ cell development in the Rara mutant testis models could be originating from issues with germ cells crossing the BTB, which involves both inter–Sertoli cell interactions and germ–Sertoli cell interactions via various junctional molecules that make up the BTB (55). Normally, preleptotene spermatocytes transmigrate through the BTB by breaking the inter–Sertoli tight junctions ahead of them and reforming the junctions behind them, without leaving the BTB open at any time, forming a transient compartment [Fig. 8(b)]. The process involves germ cells breaking inter–Sertoli tight junction proteins by transiently interfacing with Sertoli cells via their own germ cell junctional molecules (56). Interestingly, the highest expression of Rara mRNA and RARA in germ cells was in preleptotene and leptotene spermatocytes (10, 20–23). We propose that RARA in preleptotene spermatocytes participates in this transmigration process [Fig. 8(b)]. Moreover, massive sloughing of germ cells also argues for problems with germ cell–Sertoli cell adhesive interactions in Rara cKO testes. Because it has been shown that RARs modulate transcription of genes associated with cell adhesion and cell–extracellular matrix interactions (57), it is possible that ablation of RARA in germ cells could disturb the regulation of some proteins involved in the germ and Sertoli cell interactions, which could then lead to compromised function of the BTB. Indeed, biotin leaked more readily from Rara cKO testes than from WT controls (Fig. 4). Reports of BTB breach in adult testis on a vitamin A–deficient condition as well as a restricted RA condition via metabolic inhibition are supporting our findings (58, 59). Collectively, these results strongly suggest that the germ cell RARA is the molecular player participating in the crosstalk between germ cells and Sertoli cells for regulating transmigration of preleptotene spermatocytes through the BTB [Fig. 8(b), double-end arrows]. Furthermore, it is also likely that the inefficiency in the crosstalk is responsible for the premature release of a massive number of germ cells in Rara cKO testis.
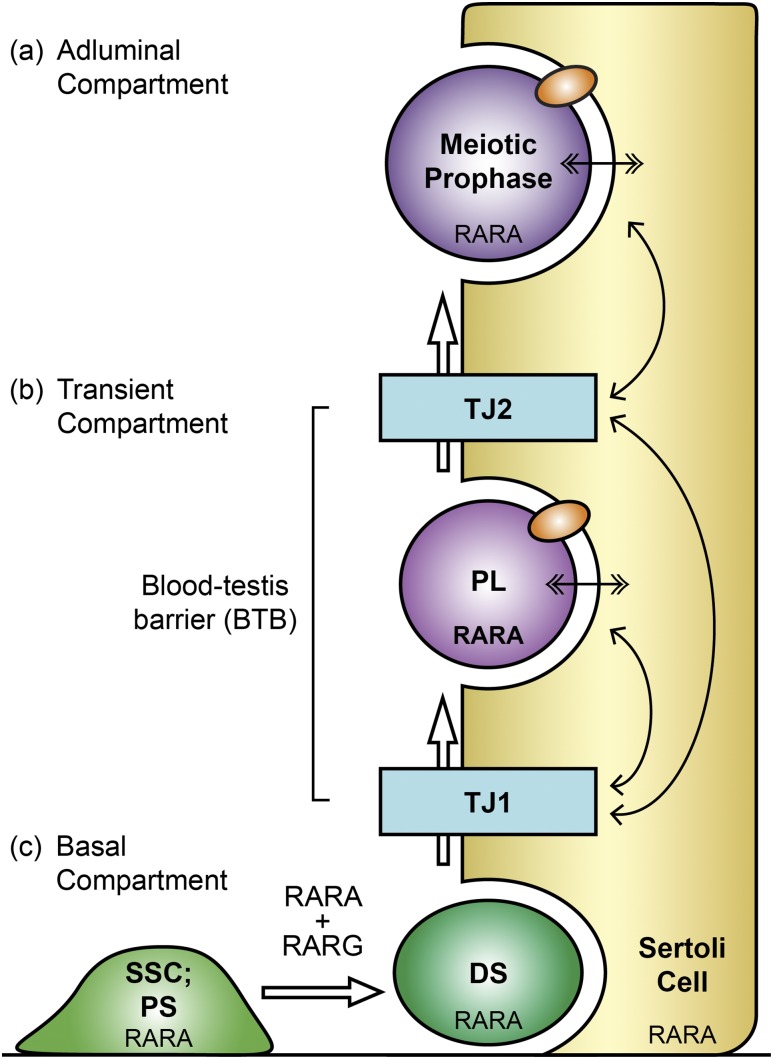
Schematic drawing showing three potential functions of germ cell RARA evident from the characterization of germ cell–specific Rara cKO mouse model. (a) Germ cell RARA has a direct role in meiotic prophase spermatocytes (dark purple), keeping SC integrity. (b) Preleptotene spermatocyte (PL; light purple) expresses RARA at the highest level in the testis. We propose that germ cell RARA in PL regulates crosstalk (double-ended arrows) with Sertoli cells (yellow) for PL to traverse BTB. PL breaks the inter–Sertoli tight junction (TJ1; blue) and enters into the transient compartment where the TJ2 (blue) ahead is still intact. In the transient compartment, germ cells interact with Sertoli cells via junctional molecules (orange ovals), which also mediate Sertoli–germ cell contact in the adluminal compartment. When TJ1 reforms behind PL, PL breaks TJ2 to enter into the adluminal compartment, past the BTB. (c) Germ cell RARA has a function in progenitor spermatogonia (PS, SSC; light green), similar to germ cell RARG, for the spermatogonial differentiation, producing differentiated spermatogonia (DS; dark green) and the initiation of meiosis. White arrows throughout indicate germ cell differentiation steps.
A novel role of RARA in SC integrity and normal progression in meiosis
In addition to being integral in germ cell differentiation, we show, to our knowledge for the first time, that RA via RARA supports meiotic progression by monitoring the SCs. Significantly impeded germ cell development during the first wave of spermatogenesis (Fig. 4) provided a sufficient purpose to examine the meiotic progression in detail between P11 and P20 ages. As suspected, a meiotic progression traffic jam was detected with an accumulation of leptotene/zygotene spermatocytes between P13 to P21, with a concomitant decrease in pachytene spermatocytes in the conditional mutant mice (Fig. 5). These results, obtained through two methods of immunological study of the testis cross-sections and chromosomal spreads, demonstrate that germ cell RARA may be a culpable player in the regulation of meiotic progression.
Moreover, unexpectedly, meiotic germ cells with a deletion of Rara in germ cells revealed prominent phenotypes of fragmented meiotic chromosomes and significant decreases in numbers of cells with perfect synapsis (Fig. 6). The evidence suggests that germ cell RARA is directly regulating the SC integrity in the meiotic prophase [Fig. 8(a)]. Our exciting finding couples well with a recent study showing a decreased RA ligand level associated with increased meiotic defects, including varying degrees of unsynapsed homologous chromosomes and a decrease in cells with perfect synapsis (59). However, to our knowledge, the current study is the first to demonstrate that it is the germ cell RARA that mediates the RA effects on SC integrity. These SC defects seen in pachytene spermatocytes undoubtedly disrupted meiotic progression in Rara cKO testes. Spermatocytes must pass several checkpoints to progress through meiosis properly, and a failure to pass through these checkpoints could cause spermatocytes to undergo apoptosis (60–62). Previously, meiotic prophase germ cells were revealed to undergo apoptosis in Rara-null mice (19). In the current study, Rara cKO spermatocytes experienced a higher rate of apoptosis, indicating that germ cell Rara ablation may contribute to more failures in passing the meiotic checkpoints. Similar results are observed in mice with a knockout of Hsp70-2 gene, which is required for desynapsis of SCs (52). Hsp70-2 knockout mice displayed an increased level of apoptosis at P17 in pachytene spermatocytes.
Germ cell RARA plays a role in the initiation of spermatogenesis from SSCs and progenitors
After busulfan treatment and recovery, there were about fivefold fewer recovered tubules in Rara cKO mice compared with the WT controls (Fig. 7). These results demonstrate that germ cell RARA has a role in the initiation of spermatogenesis from remaining SSCs and progenitor population. Supportive of this, RARA is expressed in the inhibitor of DNA binding 4-eGFP–positive spermatogonial population enriched in SSCs, as well as in the inhibitor of DNA binding 4-eGFP–negative spermatogonial population enriched in progenitor spermatogonia, known to be RA responsive (24). Moreover, the onset of RA signaling is known to start around P3 and P4 (63), around the time of SSC establishment in the germline niche, the proliferation of progenitor spermatogonia, and the beginning phase of spermatogonial differentiation (47). These results together strongly implicate the potential function of RARA in SSCs and progenitor spermatogonia. Because floxed Rara can be deleted as early as in gonocytes, the mutated Rara could also be poised to affect the early gonocyte population in germ cell–specific Rara cKO animals. Future studies are necessary to decipher the exact cellular location of RARA function in neonatal ages.
Intriguingly, the weak recovery of spermatogenesis from the endogenous stem cells in Rara cKO mice after busulfan treatment was still less severe than virtually no recovery observed when Rara-null germ cells were injected into the Rara+/+ recipient testes in spermatogonial transplantation experiments (19). Contrasting the two methods, it is clear that injected donor SSCs in the spermatogonial transplantation method have an additional step of transmigration through the BTB to establish in the germline niche located along the basal lamina near the blood supply (64, 65). In the busulfan method (39), the endogenous stem cells are already established in the germline niche to reinitiate spermatogenesis, eliminating a potential challenging complication rising from SSC transmigration through the BTB. These results support the notion that germ cell RARA regulates crosstalks with Sertoli cells in modulating the junctional integrity of the BTB [Fig. 8(b)] for the reverse transmigration of SSCs on the way to the germline niche. Future studies are necessary to investigate the junctional proteins, or any of their upstream regulators, by which germ cell RARA could crosstalk with Sertoli cells for regulating the BTB integrity.
Germ cell RARA has a broader spectrum of responsibility than does RARG in spermatogenesis
Retrospectively, it is not surprising that RARA has a broader role in germ cells than RARG, which had been known as the germ cell RAR critical for the transition of progenitor spermatogonia to differentiated spermatogonia (13). During the neonatal period, the expression of RARG is limited to germ cells in a relatively small window of germ cell development to SSCs, progenitor spermatogonia, and A1 differentiated spermatogonia, and not to more differentiated KIT-positive spermatogonia or meiotic cells (13, 22, 24). In comparison, expression of RARA in testicular germ cells is almost ubiquitous. RARA has precisely the same overlapping expression pattern as RARG during the neonatal period, but, also, RARA is present in gonocytes, SSCs, progenitor spermatogonia, differentiated spermatogonia, meiotic cells, and elongating spermatids (10, 21–24). Because both Rara and Rarg had to be ablated in spermatogonia by Ngn3 promoter–driven Cre to have a full effect on spermatogonial differentiation (13), it is logical to conclude that RARA and RARG share overlapping and disparate functions in SSCs and progenitor spermatogonia [Fig. 8(c)]. Furthermore, the current study shows that germ cell RARA has functions in meiotic prophase as a legitimate guardian of the genome integrity during meiosis [Fig. 8(a)]. Increased SC defects in Rara cKO meiotic germ cells implicate potential difficulties with the double-stranded break formation and resolution, as well as the assembly and maintenance of SCs in germ cells lacking RARA.
These results are intriguing considering a report that RA activates two molecular pathways for meiosis, a novel REC8 pathway and a STRA8-dependent pathway, which involve proteins associated with the integrity of SCs (66, 67). REC8 is one of the cohesin proteins that mediates sister chromatid cohesion, SC formation, and proper synapsis (68). The reduced distribution of REC8 has been shown to cause a local separation of sister chromatids and imperfect SC formation, similar to the phenotypes observed in Rara cKO spermatocytes. The STRA8 pathway may also be involved as a downstream mediator of RARA in meiotic prophase cells because it is known to mediate premeiotic DNA replication, double-stranded break formation, expression of recombinase DMC1, and SYCP3 expression (66, 67). In light of these results, future studies are necessary to investigate whether germ cell RARA acts as a transcriptional regulator of both molecular pathways, and whether RARA or RARG regulates one or both of these pathways.
In conclusion, the current study provides detailed characterization of multiple roles that germ cell RARA plays in various postnatal stages of male germ cell development. Unlike germ cell RARG that has a focused function in the spermatogonial differentiation, RARA plays overlapping functions with RARG, as well as acts during the meiotic prophase to keep genome integrity during the assembly of SCs for perfect synapsis of homologous autosomal chromosomes and the formation of chromosomal breaks and repair for the exchange of genetic recombination [Fig. 8(a) and 8(c)]. Additionally, germ cell RARA crosstalks with Sertoli cells to control the integrity of BTB, most likely regulating junctional molecules used in germ and Sertoli cell interactions [Fig. 8(b)]. Collectively, these results highlight the complexity and diversity of the functions germ cell RARA conducts throughout spermatogenesis, as well as demonstrate a need for follow-up studies to elucidate the varied transcriptional programs that RARA regulates in the germ cells.
Acknowledgments
We thank Dr. Hui Li for contributing to the production of the Rarafl construct and William D. Willis for contributing to the production of Rara floxed mice. We also thank Kylie Nelson, Jennifer Onken, Sophie Ascaso, and Ciera Sitton for technical assistance. We are grateful to Dr. Timothy Doyle for critical reading of the manuscript.
Financial Support: This work was supported by National Institutes of Health Grant T32GM008336 (to N.R.P.) from the National Institute of General Medical Sciences, National Institutes of Health Grant T32GM083864 (to S.M.L.) from the National Institute of General Medical Sciences, and by National Institutes of Health Grant HD44569 (to K.K.) from the National Institute of Child Health and Human Development.
This work was supported by National Institutes of Health Grant T32GM008336 (to N.R.P.) from the National Institute of General Medical Sciences, National Institutes of Health Grant T32GM083864 (to S.M.L.) from the National Institute of General Medical Sciences, and by National Institutes of Health Grant HD44569 (to K.K.) from the National Institute of Child Health and Human Development.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
| BAC | bacterial artificial chromosome |
| BTB | blood–testis barrier |
| cKO | conditional knockout |
| DAPI | 4′,6-diamidino-2-phenylindole |
| Eyfp | enhanced yellow fluorescent protein |
| iCRE | improved causes recombination with cyclization |
| IF | immunofluorescence |
| IHC | immunohistochemistry |
| NIM | nucleus isolation medium |
| P | postnatal day |
| PBS-C | PBS with 1 mM calcium chloride |
| PFA | paraformaldehyde |
| qPCR | quantitative PCR |
| RA | retinoic acid |
| RAR | retinoic acid receptor |
| RARA | retinoic acid receptor α |
| Rarafl/fl | C57BL/6J-Rarafl/fl |
| RARG | retinoic acid receptor γ |
| R26R-EYFP | B6.129X1-Gt(ROSA)26Sortm1(EYFP)Cos/J |
| SC | synaptonemal complex |
| SSC | spermatogonial stem cell |
| Stra8-iCre | Tg(Stra8-icre)1Reb/J |
| SYCP3 | synaptonemal complex protein 3 |
| WT | wild-type |
| ZBTB16 | zinc finger and broad complex, tramtrack, and bric-a-brac domain–containing protein 16 |
References
Articles from Endocrinology are provided here courtesy of The Endocrine Society
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
Editorial: Insights in developmental endocrinology: 2023.
Front Endocrinol (Lausanne), 15:1453023, 01 Aug 2024
Cited by: 1 article | PMID: 39149123 | PMCID: PMC11324551
Strategies for developing retinoic acid receptor alpha-selective antagonists as novel agents for male contraception.
Eur J Med Chem, 261:115821, 25 Sep 2023
Cited by: 2 articles | PMID: 37776573
Retinoic Acid Receptor Alpha Is Essential in Postnatal Sertoli Cells but Not in Germ Cells.
Cells, 11(5):891, 04 Mar 2022
Cited by: 2 articles | PMID: 35269513 | PMCID: PMC8909012
A systematic review of retinoic acid in the journey of spermatogonium to spermatozoa: From basic to clinical application.
F1000Res, 11:552, 20 May 2022
Cited by: 1 article | PMID: 35967975 | PMCID: PMC9345263
Review Free full text in Europe PMC
Differential RA responsiveness among subsets of mouse late progenitor spermatogonia.
Reproduction, 161(6):645-655, 05 May 2021
Cited by: 5 articles | PMID: 33835049 | PMCID: PMC8105290
Go to all (12) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Ensembl Genome Browser
- (1 citation) Ensembl - ENSMUSG00000037992
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Potential functions of retinoic acid receptor A in Sertoli cells and germ cells during spermatogenesis.
Ann N Y Acad Sci, 1120:114-130, 28 Sep 2007
Cited by: 27 articles | PMID: 17905941
Conditional ablation of DIS3L2 ribonuclease in pre-meiotic germ cells causes defective spermatogenesis and infertility in male mice.
Theranostics, 14(14):5621-5642, 03 Sep 2024
Cited by: 0 articles | PMID: 39310107 | PMCID: PMC11413780
Irradiation affects germ and somatic cells in prepubertal monkey testis xenografts.
Mol Hum Reprod, 23(3):141-154, 01 Mar 2017
Cited by: 16 articles | PMID: 28130393
Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes.
Microsc Res Tech, 73(4):241-278, 01 Apr 2010
Cited by: 120 articles | PMID: 19941293
Review
Funding
Funders who supported this work.
NICHD NIH HHS (1)
Grant ID: R01 HD044569
NIGMS NIH HHS (2)
Grant ID: T32 GM008336
Grant ID: T32 GM083864
National Institute of Child Health and Human Development (1)
Grant ID: HD44569
National Institute of General Medical Sciences (2)
Grant ID: T32GM083864
Grant ID: T32GM008336





