Abstract
Free full text

Two-Year Survival Comparing Web-Based Symptom Monitoring vs Routine Surveillance Following Treatment for Lung Cancer
Associated Data
This study reports 2-year survival outcomes among patients with advanced nonprogressive stage IIA to IV lung cancer randomized to symptom monitoring during chemotherapy via web-based patient-reported outcomes vs standard scheduled imaging after treatment to detect symptomatic recurrence.
Symptom monitoring during chemotherapy via web-based patient-reported outcomes (PROs) was previously demonstrated to lengthen survival in a single-center study.1 A multicenter randomized clinical trial compared web-based monitoring vs standard scheduled imaging to detect symptomatic recurrence in patients with lung cancer following initial treatment. A planned interim analysis (9-month follow-up) found a significant survival benefit (19-month survival in the PRO group vs 12 months in the control group).2 We now present the final overall survival analysis.
Methods
As described previously,2 in this randomized trial, patients with advanced nonprogressive stage IIA (TXN1) to IV lung cancer were randomly assigned within 3 months of previous treatment to receive either web-based symptom monitoring via the Sentinel PRO system (Hyperion) or standard follow-up with scheduled imaging every 3 to 6 months (the trial protocol is available in Supplement 1). Eligible patients were recruited at 5 centers in France from June 2014 to January 2016. Nonprogressive patients treated for metastatic disease by tyrosine kinase inhibitors, maintenance antiangiogenic or chemotherapy, or immunotherapy were eligible. Allocation was generated centrally and concealed from investigators and participants. The study was reviewed and approved by the ethics review board from the University Hospital at Angers (France). All participants provided written informed consent.
In the PRO group, patients were invited to complete weekly self-reports of 13 common symptoms online between visits. The PRO system automatically triggered an alert email to the treating oncologist when patient-reported symptoms matched predefined criteria for severity and worsening. In both groups, additional imaging could be performed at the treating oncologist’s discretion.
The primary outcome was overall survival after 2 years of follow-up, which was selected based on prior pilot research.3 Survival data were gathered from patient follow-up by blinded investigators. After a preplanned interim analysis in January 2016 in which a significant survival improvement was observed, the data and safety monitoring committee mandated cessation of recruitment and crossover of control patients to the intervention. Sixty percent of the prespecified sample size was enrolled when recruitment was halted. The final date of follow-up was December 29, 2017.
Overall survival was estimated via the Kaplan-Meier method and compared between groups with a log-rank test (SAS version 9.3; SAS Institute). A 2-sided P<.05 was considered significant. Analyses were performed on an intention-to-treat basis and with censoring at crossover.4
Results
One hundred thirty-three patients were enrolled of whom 12 were ineligible, leaving a study population of 121 (60 in the intervention and 61 in the control group) (Figure 1). Baseline demographic and disease characteristics were well balanced between groups.2 The median age was 65 years (range, 36-88 years), 67% were men, 32% had stage III cancer, 63% had stage IV cancer, and 17% had small cell cancer. Following interim analysis, 10 of 34 living patients in the control group had not relapsed and therefore were eligible to cross over to the intervention. No participants were lost to follow-up.

At 9 months when recruitment was halted, 10 patients in the intervention group and 27 patients in the control group had died. Of the 34 living patients in the control group, 10 had not relapsed and crossed over to the intervention.
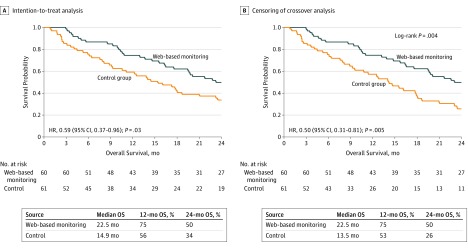
With 2 years of follow-up, 69 deaths were observed: 29 (47.5%) in the intervention group and 40 (66.7%) in the control group. The median overall survival was 22.5 months in the intervention group vs 14.9 months in the control group, without censoring for crossover (hazard ratio, 0.59 [95% CI, 0.37-0.96]; P =
= .03) (Figure 2A). Censoring crossover resulted in a median overall survival of 22.5 months in the intervention group vs 13.5 months in the control group (hazard ratio, 0.50 [95% CI, 0.31-0.81]; P
.03) (Figure 2A). Censoring crossover resulted in a median overall survival of 22.5 months in the intervention group vs 13.5 months in the control group (hazard ratio, 0.50 [95% CI, 0.31-0.81]; P =
= .005) (Figure 2B).
.005) (Figure 2B).
Discussion
Symptom monitoring via weekly web-based PROs following treatment for lung cancer was associated with increased survival compared with standard imaging surveillance. A potential mechanism of action is that symptoms suggesting adverse events or recurrence were detected earlier.
Limitations of this study include conduct only in France, early stopping of the trial and crossover of control patients, and inclusion of patients receiving maintenance therapy, who may have had increased interactions with care teams.
Notes
Section Editor: Jody W. Zylke, MD, Deputy Editor.
Notes
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jama.2018.18085
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jama/articlepdf/2721170/jama_denis_2019_ld_180062.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1001/jama.2018.18085
Article citations
Truth and dare: patients dare to tell the truth when using PROMs in clinical practice.
Qual Life Res, 03 Oct 2024
Cited by: 0 articles | PMID: 39363117
Sociodemographic Differences in Perspectives on Postpartum Symptom Reporting.
Appl Clin Inform, 15(4):692-699, 21 Aug 2024
Cited by: 0 articles | PMID: 39168155
Defining and Addressing Research Priorities in Cancer Cachexia through Transdisciplinary Collaboration.
Cancers (Basel), 16(13):2364, 27 Jun 2024
Cited by: 2 articles | PMID: 39001427
Review
Electronic symptom monitoring after lung cancer surgery: establishing a core set of patient-reported outcomes for surgical oncology care in a longitudinal cohort study.
Int J Surg, 20 Jun 2024
Cited by: 0 articles | PMID: 38896873 | PMCID: PMC11486944
Remote symptom monitoring with patient-reported outcome measures in outpatients with chronic kidney disease (PROKID): a multicentre randomised controlled non-inferiority study.
Clin Kidney J, 17(7):sfae176, 14 Jun 2024
Cited by: 0 articles | PMID: 39006159
Go to all (211) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Randomized Trial Comparing a Web-Mediated Follow-up With Routine Surveillance in Lung Cancer Patients.
J Natl Cancer Inst, 109(9), 01 Sep 2017
Cited by: 190 articles | PMID: 28423407
Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance.
J Thorac Cardiovasc Surg, 145(1):75-81; discussion 81-2, 03 Nov 2012
Cited by: 143 articles | PMID: 23127371
Detection of lung cancer relapse using self-reported symptoms transmitted via an internet web-application: pilot study of the sentinel follow-up.
Support Care Cancer, 22(6):1467-1473, 12 Jan 2014
Cited by: 39 articles | PMID: 24414998
[Follow-up of endometrial cancer].
Bull Cancer, 101(7-8):741-747, 01 Jul 2014
Cited by: 0 articles | PMID: 25025796
Review
 1
1