Abstract
Free full text

Natural History of Cognitive Impairment in Critical Illness Survivors. A Systematic Review
Abstract
Long-term cognitive impairment is common among ICU survivors, but its natural history remains unclear. In this systematic review, we report the frequency of cognitive impairment in ICU survivors across various time points after ICU discharge that were extracted from 46 of the 3,350 screened records. Prior studies used a range of cognitive instruments, including subjective assessments (10 studies), single or screening cognitive test such as Mini-Mental State Examination or Trail Making Tests A and B (23 studies), and comprehensive cognitive batteries (26 studies). The mean prevalence of cognitive impairment was higher with objective rather than subjective assessments (54% [95% confidence interval (CI), 51–57%] vs. 35% [95% CI, 29–41%] at 3 months after ICU discharge) and when comprehensive cognitive batteries rather than Mini-Mental State Examination were used (ICU discharge: 61% [95% CI, 38–100%] vs. 36% [95% CI, 15–63%]; 12 months after ICU discharge: 43% [95% CI, 10–78%] vs. 18% [95% CI, 10–20%]). Patients with acute respiratory distress syndrome had higher prevalence of cognitive impairment than mixed ICU patients at ICU discharge (82% [95% CI, 78–86%] vs. 48% [95% CI, 44–52%]). Although some studies repeated tests at more than one time point, the time intervals between tests were arbitrary and dictated by operational limitations of individual studies or chosen cognitive instruments. In summary, the prevalence and temporal trajectory of ICU-related cognitive impairment varies depending on the type of cognitive instrument used and the etiology of critical illness. Future studies should use modern comprehensive batteries to better delineate the natural history of cognitive recovery across ICU patient subgroups and determine which acute illness and treatment factors are associated with better recovery trajectories.
ICU-related cognitive impairment is an important public health problem spanning the continuum of critical illness from acute admission (where it manifests as delirium) to long-term cognitive impairment (1). Delirium occurs in up to 80% of mechanically ventilated patients and is associated with increased mortality (2), cost of care ($4–$16 billion annually in the United States alone) (2), and development of long-term cognitive impairment (3). Long-term cognitive impairment is a common and severe complication of ICU admission (3), is comparable to sustaining moderate traumatic brain injury or developing mild Alzheimer’s disease (3), affects people of all ages (3), has protracted and incomplete recovery over a year after discharge (3), and carries an enormous cost to society by preventing previously able economically active people from returning to the workforce, thereby placing a huge burden on families and caregivers (1).
Although the burden of ICU-related cognitive impairment is well documented, its natural history remains poorly understood because of the wide range of cognitive instruments used and the variation in the timing and frequency of testing after ICU discharge.
In this article, we systematically review existing literature on ICU-related cognitive impairment and use these data to describe its natural history after ICU discharge. Partial results of this study were presented as a poster presentation at the CCCF meeting in Toronto, Ontario in November 2018 (4).
Methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement guideline for systematic reviews (5). The study was registered in the Prospective Register of Systematic Reviews register of the Center for Reviews and Dissemination (registration number: 72674).
Search Strategy
A professional clinical librarian conducted a literature search of Ovid Medline, EMBASE, and PsycINFO databases to identify potentially relevant, English-language articles. The search strategy and terms are shown in the online supplement. There were no date restrictions.
Eligibility Criteria
We included studies that reported the frequency of subjective or objective cognitive impairment in adult (≥18 yr of age) ICU survivors. We also included studies that reported average raw scores on the Mini-Mental State Examination (MMSE), because this was the most commonly used cognitive screening test.
We excluded studies that did not assess cognition directly (i.e., studies that used medical records or diagnostic codes to diagnose “dementia”). We also excluded abstracts, case reports, and studies that exclusively recruited patients admitted with acute neurological events (e.g., intracranial hemorrhage or traumatic brain injury) or anoxic brain injury after cardiac arrest. Where more than one study reported cognitive outcomes on an overlapping group of patients at the same time point, we included only the study with the greater sample size.
Study Selection
Two authors (K.H. and R.S.L.) independently screened the titles and abstracts of studies identified by the search for potentially eligible studies. The same two authors conducted full-text review in duplicate. Discrepancies were resolved by consensus.
Data Extraction and Outcomes
We extracted the following data from each study: primary objective, study design, sample size, patient population assessed, cognitive instrument(s) administered, timing of cognitive assessment relative to ICU discharge, the definition of cognitive impairment used by the authors, and the cognitive outcomes. We included the frequencies of both subjective cognitive impairments, as reported by patients or their caregivers, and objective impairments, as determined using various cognitive batteries. When cognition was assessed multiple times shortly after recovery from the critical illness and before hospital discharge, the highest prevalence was recorded.
We anticipated heterogeneity in study design, methodology, populations, and cognitive instruments among studies in this review. As a result, we grouped our results by the following predefined groups: subjective versus objective cognitive measures, acute respiratory distress syndrome (ARDS) versus mixed ICU populations (given that a number of studies focused on cognition in patients with ARDS), and MMSE screening test versus other more comprehensive cognitive batteries (given that MMSE is the most commonly used test).
To determine the weighted aggregate frequency of cognitive impairment at each time point, we divided the number of patients with cognitive impairment as defined by each study by the total number of patients from all studies at that time point and repeated this calculation across all reported time points. This analysis was done separately for subjective and objective cognitive outcomes, as well as for three predefined patient subgroups: ARDS, sepsis, and elderly ICU survivors.
Given that the MMSE was the most commonly used cognitive test, we compared frequency of cognitive impairment detected with MMSE versus other objective batteries. To summarize MMSE score at each time point, we calculated weighted scores taking the sample sizes of individual studies into account.
To visualize the natural history of ICU-related cognitive impairment, we used the above data to construct graphs depicting the frequency of cognitive impairment versus time from hospital discharge.
Assessment of Study Quality
Two investigators (K.H. and R.S.L.) independently assessed risk of bias for each study using the Newcastle-Ottawa Scale for cohort studies and the Cochrane risk-of-bias assessment tool for randomized controlled trials.
Results
Study Selection
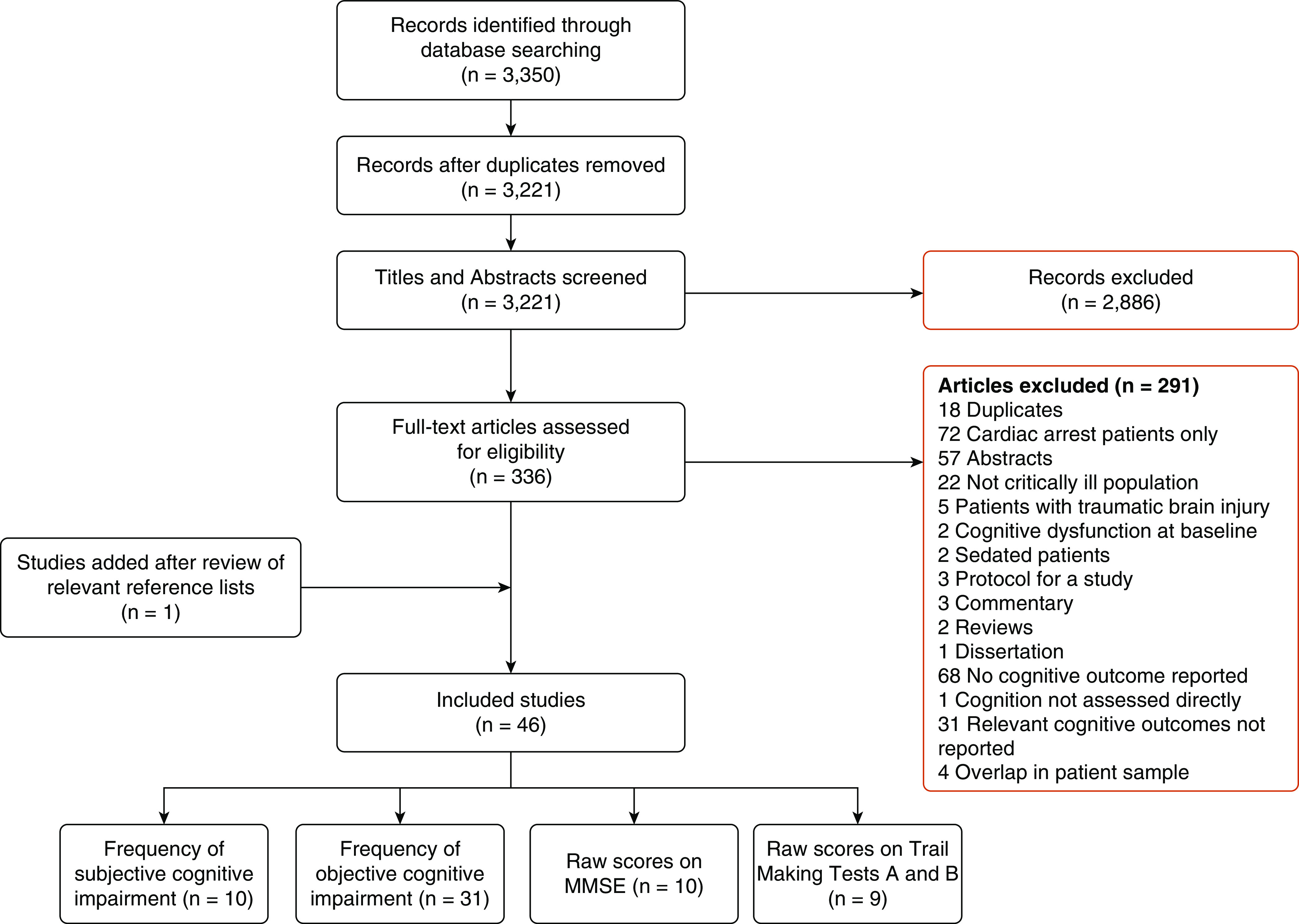
Our search yielded 3,350 records. After removal of 129 duplicates, 2,886 studies were excluded based on contents of title and abstract. After full-text review of 336 articles, 291 were excluded. Review of relevant references identified one relevant study, yielding 46 relevant studies that met the inclusion criteria and were included in this review. Among these, 10 studies reported the frequency of subjective and 31 studies of objective cognitive impairment. Ten studies reported average MMSE scores. A flow diagram of the search process is depicted in Figure 1.
Patient Characteristics in Included Studies
To better characterize patient mix from studies included in our review, we extracted admission diagnoses from Table 1 of each included study (where available). Patients with acute respiratory failure, including pneumonia and ARDS, composed 35% of our total sample, followed by post-operative patients (15%), cardiac patients (10%), or patient with sepsis or septic shock (8%). Admission diagnosis was unreported or listed as “other” in 20% of our sample.
Study Characteristics
Characteristics of the included studies are summarized in the online supplement. Most studies were prospective, longitudinal, and observational in design, ranging in sample sizes from 9 to 514 patients. Thirteen studies exclusively enrolled patients with ARDS (including those classified as having acute lung injury), one study exclusively enrolled patients with sepsis-related ARDS, and two studies exclusively enrolled older ICU survivors (≥65 yr of age and ≥80 yr of age). Although a variety of cognitive tests and batteries were used (online supplement), MMSE was the most common cognitive test, followed by Trail Making Test Parts A and B.
Pre-ICU Cognitive Impairment
Given that pre-ICU cognitive impairment can affect post-ICU cognitive results, 30 of 46 studies in our review explicitly state that they excluded patients with pre-ICU cognitive impairment. However, only 10 of 46 studies used a standardized questionnaire tool such as Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) to assess pre-ICU cognitive function.
Study Quality Assessment
The risk-of-bias assessments for observational studies and those for randomized controlled trials are summarized in the online supplement. Of the 39 observational studies included, 22 were rated as having good quality, 5 had fair quality, and 12 had poor quality. Of the five randomized controlled trials included, one had low risk of bias in all categories, three had at least one high-risk category, and one had two unknown categories.
Frequency of Subjective Cognitive Impairment in ICU Survivors
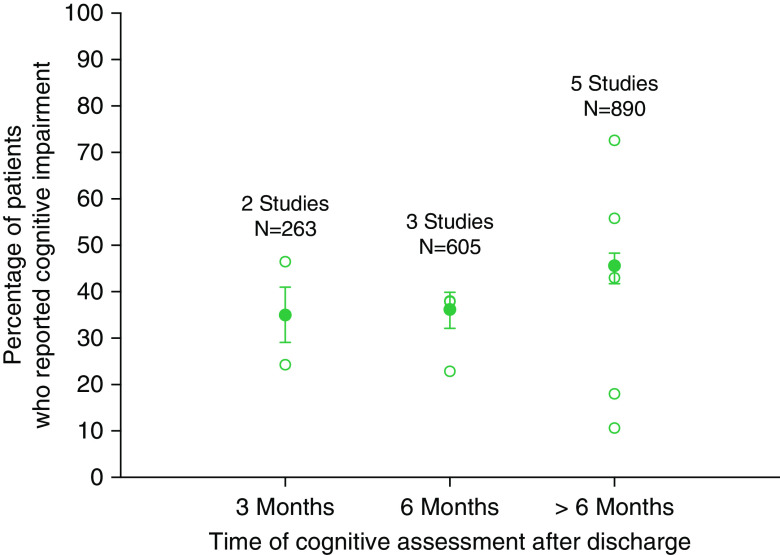
Ten studies reported frequency of cognitive impairment in ICU survivors using subjective measures at three time points after discharge: at 3 months (two studies), at 6 months (three studies), and at variable time points of >6 months (five studies). The aggregate frequency of subjectively reported cognitive impairment increased slightly, from 35% at 3 months to 45% at >6 months. (Figure 2).
Frequency of Objective Cognitive Impairment in ICU Survivors
Seven studies measured cognitive impairment using the MMSE and reported its frequency in hospital or at discharge (six studies), and at 3 (one study), 6 (three studies), and 12 (two studies) months after discharge. Twenty-five studies measured cognitive impairment in 25 distinct cohorts using other cognitive tests or batteries and reported its frequency in hospital or at discharge (10 studies), and at 2 (1 study), 3 (9 studies), 6 (3 studies), 9 (1 study), 12 (10 studies), 24 (1 study), and >24 (4 studies) months after discharge. Figure 3 demonstrates the frequency of cognitive impairment across various time points. Two cohorts of patients were reported by Pfoh and colleagues (6), and these are represented separately in Figure 3.
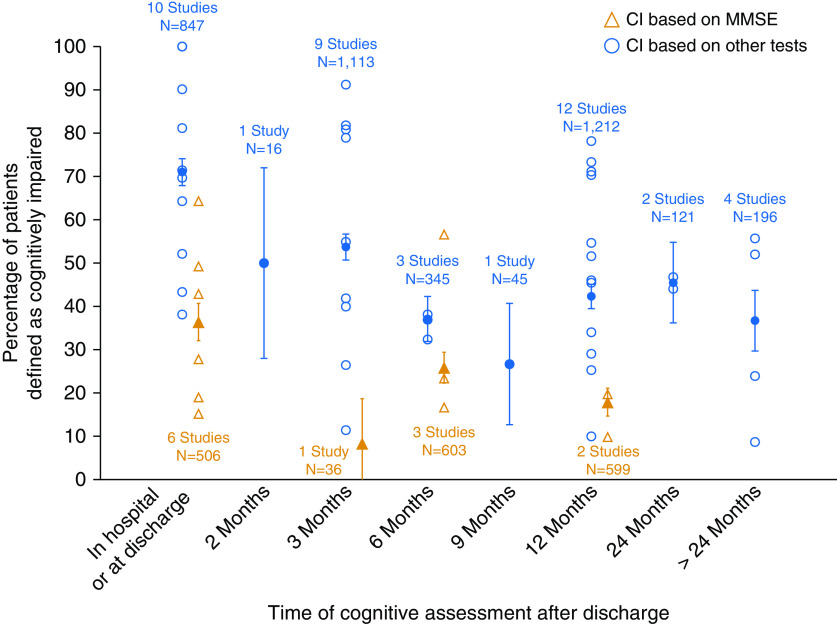
Frequency of cognitive impairment based on Mini-Mental State Examination (MMSE) only versus other measures. Open symbols represent frequency reported by individual studies. Solid symbols represent the aggregate frequency calculated based on all studies for that time point. Bars represent 95% confidence interval (CI).
Visual comparison of subjective (Figure 2) and objective (Figure 3) results suggests that the reported frequency of cognitive impairment was higher in studies that used objective measurements. For example, at 3 months, aggregate frequency of subjective cognitive impairment, weighted by study sample size, was 35% (95% confidence interval [CI], 29.1–41.0%; based on two studies), and that of objective cognitive impairment was 53.7% (95% CI, 50.7–56.7%; based on nine studies).
Using visual inspection of Figure 3, we also found that the prevalence of cognitive impairment detected with MMSE was lower than that detected with non-MMSE cognitive batteries across all time points. For example, at discharge the aggregate frequency of cognitive impairment, weighted by study sample size, was 36% (6 studies; range, 15–63%) based on the MMSE and 61% (10 studies; range, 38–100%) when other tests or batteries were used, and at 12 months they were 18% (2 studies: 10% and 20%) and 43% (12 studies; range, 10–78%), respectively.
Two data points were not included in the aggregate scores because they reported cognitive outcomes across multiple time points. de Azevedo and colleagues reported cognitive outcomes in the mixed population of ICU survivors at 3–18 (average, 11) months after discharge and found that half of their sample was classified as cognitively impaired on the basis of a comprehensive battery of tests (7). Similarly, Mikkelsen and colleagues (8) found that among 27 ARDS survivors, more than half were classified as cognitively impaired at variable time points up to 24 months after discharge (8). A similar proportion of these patients remained cognitively impaired at 3 years or longer follow-up (the latter data are included in Figure 3).
Cognitive Impairment on the Basis of MMSE
In studies where MMSE mean scores were reported, there appears to be a ceiling effect, with the degree of cognitive impairment classified as mild in the early course of recovery and no cognitive impairment after 12 months (Figure 4).
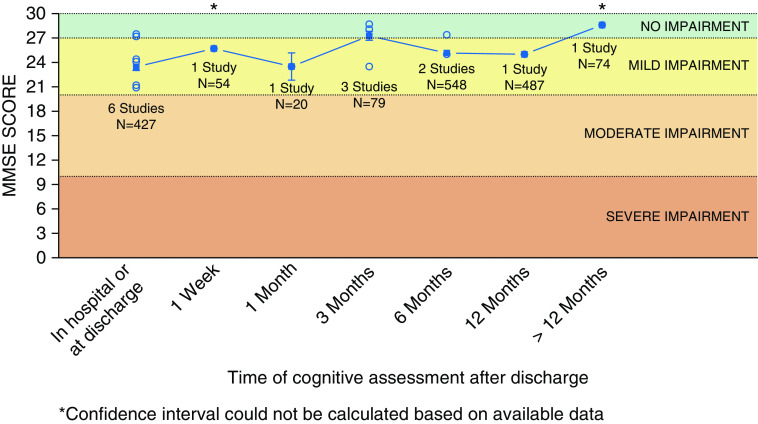
Aggregate raw scores on the Mini-Mental State Examination (MMSE) over time. Open circles represent mean or median MMSE scores as reported by individual studies. Solid squares represent the weighted average (accounting for sample size) calculated based on all studies for that time point. Line represents trend over time based on the weighted average. Bars represent 95% confidence interval.
Frequency of Cognitive Impairment in Subgroups of ICU Survivors
Twelve studies assessed objective cognitive function in patients with ARDS exclusively (including one study of sepsis-associated ARDS), whereas 13 studies recruited mixed populations of ICU patients admitted with any diagnosis, including ARDS (Figure 5). At hospital discharge and up to 3 months after discharge, patients with ARDS had higher prevalence of cognitive impairment than mixed ICU patients (approximately 80% vs. 50%), but this difference disappeared at 6 months. Cognitive impairment beyond 12 months has not been assessed in the mixed ICU group.
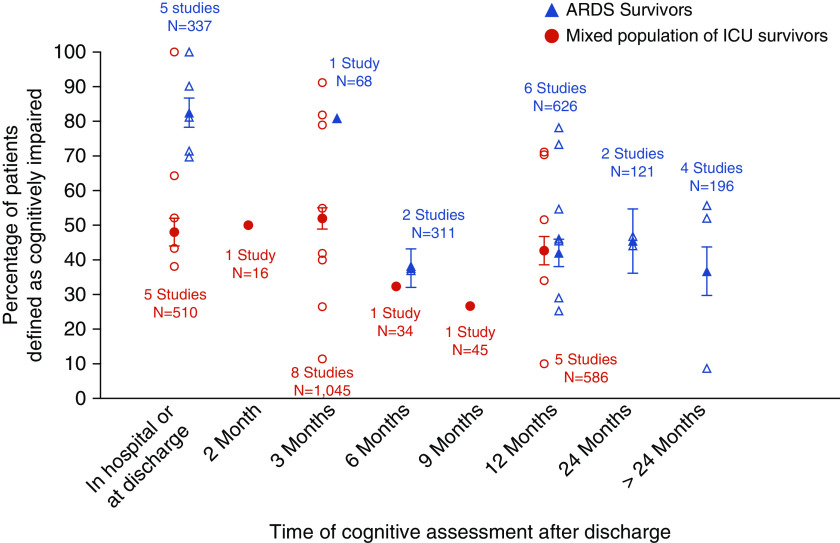
Frequency of cognitive impairment in acute respiratory distress syndrome (ARDS) versus mixed population of ICU survivors. Open symbols represent frequency reported by individual studies. Solid symbols represent the aggregate frequency calculated based on all studies for that time point. Bars represent 95% confidence interval.
Although our search identified a number of studies in patients with sepsis (9, 10), these studies were excluded from our review as they 1) did not explicitly test cognitive function, 2) did not report the frequency of cognitive impairment, 3) included patients admitted to non-ICU wards, or 4) focused exclusively on delirium (e.g., used delirium scores such as Confusion Assessment Method for the ICU to define cognitive impairment). Only one study assessed objective cognitive function in ICU survivors ≥65 years of age (11). Using the MMSE, this study found that only 15% (17 of 112) of patients were cognitively impaired at discharge and only 10% (11 of 112) at 12 months. In contrast, de Rooij and colleagues (12) found that relatives reported cognitive impairment in 73% of ICU survivors ≥80 years of age (12). These divergent findings may be explained in part by selection bias due to mortality, which was significantly higher in the first study (11) relative to the study by de Rooij and colleagues (12).
Discussion
Cognitive function is one of the best predictors of life quality, including academic and work success, levels of happiness, and even life expectancy (13–16). Given the expected increase in the number of ICU survivors due to the projected increase in the incidence of critical illness (17) and reduction in ICU mortality, ICU-related cognitive impairment is an important public health problem that requires urgent and innovative solutions.
Our systematic review identified 46 studies that examined cognition in ICU survivors at different time points ranging from ICU discharge and up to 13 years after ICU discharge. Collectively, our results suggest that cognitive impairment in the ICU survivors is common, ranging in mean prevalence from 35% (95% CI, 29–41%) to 81% (95% CI, 71–91%) at 3 months after ICU discharge, depending on whether subjective or objective cognitive assessments are used, respectively. It also highlights that not all objective measures are equivalent and that screening tests such as MMSE may underestimate the prevalence of cognitive impairment compared with comprehensive cognitive batteries (mean prevalence of 36% vs. 61% at ICU discharge, respectively). Certain ICU populations, such as patients with ARDS, have a higher prevalence of cognitive impairment compared with mixed ICU populations, at least earlier after ICU discharge (mean prevalence of 82% vs. 48% at ICU discharge, respectively), highlighting that underlying pathophysiologic mechanisms responsible for ICU-related cognitive impairment may differ between ICU populations. Finally, ICU-related cognitive impairment appears to persist in substantial number of ICU survivors even at 1 and 2 years after ICU discharge (mean prevalence of 42% and 46%, respectively)
Subjective versus Objective Cognitive Assessment
Our results suggest that early after discharge the prevalence of cognitive impairment appears to be higher when objective rather than subjective measures of cognition were used. After discharge, the frequency of objective impairment appeared to decline, whereas subjective prevalence increased. We speculate that this disconnect between objective and subjective data may be due to objective cognitive recovery with time combined with increased self-awareness of survivors regarding their new cognitive limitations. If true, this implies that some patients may lack insight into their cognitive deficits despite objective cognitive impairment at the time of discharge, either because of attribution of their cognitive deficits to the overall illness or poor self-appraisal (18, 19). Given that cognitive function is an important determinant of functional recovery (20, 21), failure to detect cognitive impairment at the time of discharge may result in missed therapeutic opportunity for some patients who may benefit from targeted cognitive interventions. For example, Zhao and colleagues showed that a cognitive intervention targeting different cognitive domains reduces cognitive deterioration at 3 months after ICU discharge (22). On the other hand, coping skills training did not improve psychological distress symptoms in ICU survivors compared with an education program, although subgroup analysis suggested benefit in patients with higher baseline of distress (23, 24). Given the paucity of interventions to enhance long-term survival after ICU discharge (25), additional interventional trials are needed. Some are already underway, including the VIOLET-BUD (Vitamin D to Improve Outcomes by Leveraging Early Treatment: Long-Term Brain Outcomes in Vitamin D Deficient Patients) trial, an ancillary study to a double-blinded placebo-controlled randomized trial, which aims to determine whether a single high dose of vitamin D3 improves 12-month cognition as assessed by the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) administered remotely by a neuropsychologist (ClinicalTrials.gov: NCT03733418). Furthermore, multidisciplinary longitudinal care for ICU survivors that incorporated physical rehabilitation can help integrate care and align treatment of post-ICU multimorbidities (26), such as cognitive impairment, with patient and family goals and values, as well as promote knowledge transfer and education (27).
Temporal Profile of Cognitive Impairment
Cognitive assessments occurred at several discrete time points, most commonly at discharge and at 3, 6, and 12 months. No rationale for the choice of these time intervals was provided in reviewed studies, and we speculate that these intervals reflect the operational and resourcing framework of previous studies (28).
In studies using MMSE, a screening tool for dementia, the prevalence of cognitive impairment was lower than in studies using comprehensive cognitive batteries (Figure 3), with most patients scoring in the mild to no impairment range on MMSE (Figure 4). Given that critical illness involves a range of pathophysiologic processes (e.g., ischemia, inflammation, cytotoxins, etc.) that result in both gray and subcortical white matter injury (29), a simple screening tool like MMSE may not detect multidomain cognitive deficits in ICU survivors. As a result, MMSE will miss cognitive impairment when one is present, as reflected by its poor sensitivity (37–60%) in the ICU survivor population (30). Although psychometric properties of other cognitive batteries have been assessed in non-ICU populations, they may not apply in the ICU survivors, as these patients are exposed to a mixture of injury mechanisms that lead to both cortical and subcortical white matter injury and are prone to coexisting psychiatric complications, including depression and post-traumatic stress disorder (1), which may affect performance on cognitive tests. Although clinicians’ familiarity with MMSE and Trail Making tests made them the most popular choice in reviewed studies, assessment of true prevalence of cognitive impairment will require more comprehensive batteries that tap into multiple cognitive domains and have clear neuroanatomical correlates corresponding to the patterns of injury seen in ICU survivors.
Pre-ICU Cognitive Impairment
Given that ICU admissions are unpredictable, most ICU studies lack comprehensive assessment of baseline cognitive function. It is therefore unclear how much of the observed cognitive impairment can be explained by pre-ICU cognitive impairment. In some cases, pre-ICU cognitive function can be estimated by means of questionnaire-based tools (e.g., IQCODE) (9), but this only provides crude measure of cognition and may miss subclinical cognitive impairment. In the future, population studies can use comprehensive web-based batteries (e.g., Cambridge Brain Sciences) (3, 31) to establish baseline cognitive function and then retest those who survive ICU admission. At the minimum, future work should incorporate tools such as IQCODE to screen for pre-ICU cognitive impairment.
Comparison of Cognitive Impairment Rates with Hospitalized Patients
Because our review excluded studies in non-ICU patients, we cannot directly compare the frequency of cognitive impairment in ICU versus non-ICU hospitalized patients. However, prior studies have reported cognitive impairment in 26% and 10% of noncardiac surgical patients aged 60 years at 1 week and 3 months after surgery, respectively (32). In another study, hospitalization for community-acquired pneumonia was associated with moderate to severe impairment in multiple cognitive domains in one-third of older patients (age ≥
≥ 65 yr) and in 20% of younger patients (33). However, 20% of the patients in that cohort had an ICU admission, which makes it hard to determine how much of observed impairment was due to the non-ICU hospitalization. Patients admitted to the ICU generally have higher illness severity and are therefore at risk for acute brain dysfunction. This is reflected in higher delirium rates in the ICU (up to 80%) (2) compared with non-ICU hospitalized patients (14–29%) (34). Furthermore, Iwashyna and colleagues showed that the prevalence of moderate to severe cognitive impairment increases in elderly patients who survive sepsis but not those who have nonsepsis general hospitalization (9). Future studies should examine if ICU-specific factors (e.g., illness severity or mechanical ventilation and its duration) contribute to cognitive impairment above that sustained as the result of the acute hospitalization.
65 yr) and in 20% of younger patients (33). However, 20% of the patients in that cohort had an ICU admission, which makes it hard to determine how much of observed impairment was due to the non-ICU hospitalization. Patients admitted to the ICU generally have higher illness severity and are therefore at risk for acute brain dysfunction. This is reflected in higher delirium rates in the ICU (up to 80%) (2) compared with non-ICU hospitalized patients (14–29%) (34). Furthermore, Iwashyna and colleagues showed that the prevalence of moderate to severe cognitive impairment increases in elderly patients who survive sepsis but not those who have nonsepsis general hospitalization (9). Future studies should examine if ICU-specific factors (e.g., illness severity or mechanical ventilation and its duration) contribute to cognitive impairment above that sustained as the result of the acute hospitalization.
Patient Subgroups
A subset of studies suggests that the prevalence of cognitive impairment may be higher in patients with ARDS compared with the mixed ICU population, at least up to 6 months after hospital or ICU discharge (Figure 4). The reason for this difference is unclear, but it may be related to altered brain oxygen delivery due to hypoxemia, which has been shown to be associated with cognitive impairment in patients with ARDS (35, 36). However, these conclusions warrant further evaluation, including direct comparison of temporal changes in cognitive function between patients with ARDS and general ICU patients. In our search we identified several studies examining cognitive outcomes in sepsis, but these studies were excluded for reasons stated in Results. The largest study by Iwashyna and colleagues (9) used data from the Health and Retirement Study and linked Medicare claims to determine changes in cognition as a result of sepsis admission. Cognitive function was assessed using tests of memory, serial 7 subtractions, naming, and orientation, as well as IQCODE in patients unable to be interviewed themselves. Survivors of sepsis had a 10.6 percentage point increase in the prevalence of moderate to severe cognitive impairment, whereas patients who had nonsepsis general hospitalization had no change in the prevalence of cognitive impairment. In a recent systematic review, Calsavara and colleagues summarized studies of postsepsis cognitive impairment and associated risk factors (10). They found that prevalence of cognitive impairment varied from 12.5% to 21% and was associated with depressive symptoms, central nervous system infection, length of hospitalization due to infection, and temporal proximity to the last period of infection. The authors noted high heterogeneity of studies and called for a common definition of cognitive impairment and appropriate neuropsychological tests as the next important steps in assessing cognitive function postsepsis. Since different disease states may cause brain injury in different ways (e.g., ischemic vs. inflammatory insults, toxin accumulation due to renal and/or hepatic failure, etc.), future studies should compare the natural history of cognitive impairment (and recovery) in different ICU phenotypes (e.g., ARDS, sepsis, etc.) using common neurocognitive tests and definitions of cognitive impairment.
Significance and Future Directions
Our review shows that the prevalence and temporal profiles of cognitive impairment in ICU survivors differ depending on whether subjective or objective measures are used. This observation is important, as it highlights the potential disconnect between patients’ subjective experience and objectively detected brain injury. Future prospective studies should determine the prevalence of this phenomenon and whether this “silent” cognitive impairment affects quality of life and cognitive and functional recovery of ICU survivors.
We also showed that prevalence of cognitive impairment varied depending on the type of cognitive instrument used. Although clinicians may be more familiar with some instruments, such as MMSE, these tools may not be optimal for detecting and monitoring cognitive impairment in ICU survivors, given heterogeneity of pathophysiologic mechanisms, variable neuroanatomical injury patterns, and the multidomain nature of the cognitive impairment. On the other hand, comprehensive cognitive batteries may be too cumbersome, labor intensive, and therefore impractical for routine use in clinical settings, which may explain the paucity of studies that measured cognition at more than one time point. Future studies should determine the best tools for ICU cognitive research that balance comprehensiveness, diagnostic utility, and ease of administration.
Heterogeneity in the type of cognitive instruments, definition of cognitive impairment, and domains assessed by included tests prevented domain-specific meta-analysis of cognitive impairment. The only consistent tests that were administered by multiple studies and at multiple time points were the Trail Making Tests A and B, which assess processing speed and visual attention and executive functioning, respectively. As seen from Figure 6B, executive functioning, as measured by the Trail Making Test B, was generally not impaired across different time points. However, these results should be interpreted with caution, because other comprehensive batteries (e.g., RBANS) indicate impairment in executive function (3). As a result, temporal evolution of domain-specific cognitive impairment in ICU survivors remains unclear, and future studies should aim to standardize the use of comprehensive batteries that assess multiple cognitive domains with a clear definition of cognitive impairment.
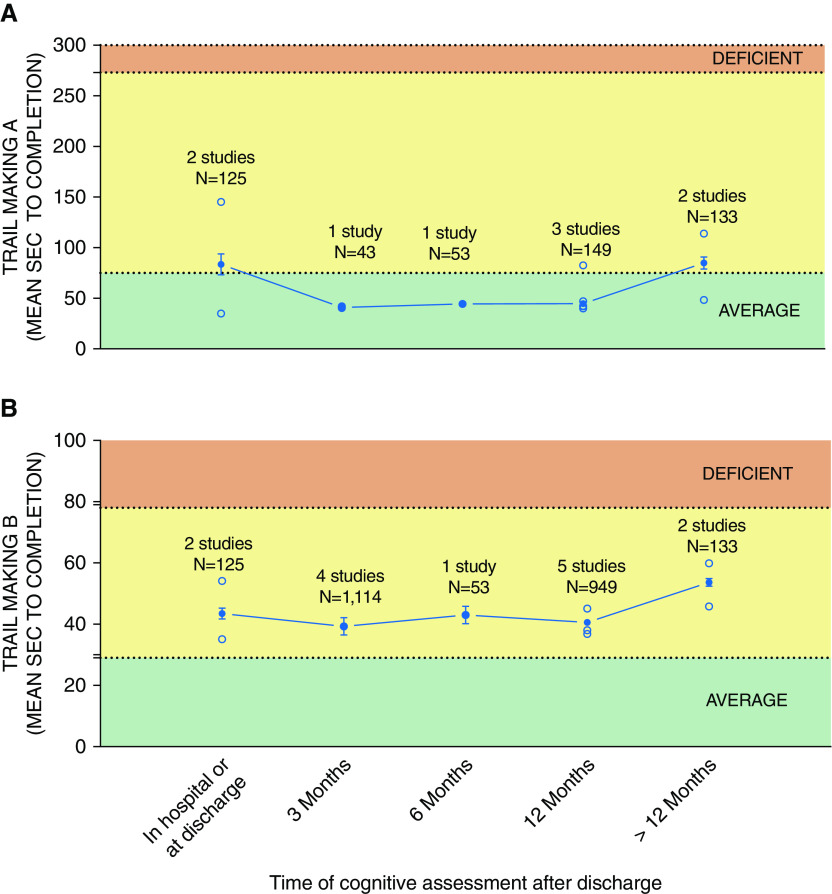
Aggregate time to test completion for (A) Trail Making Test A and (B) Trail Making Test B. Solid circles represent the weighted average (accounting for sample size) calculated based on all studies for that time point. Line represents trend over time based on the weighted average. Bars represent 95% confidence interval. Note that across multiple time points from ICU discharge, both tests failed to detect cognitive impairment in their respective domains (cognitive processing speed for Test A and executive function for Test B). Open symbols represent frequency reported by individual studies.
Furthermore, although some studies have high follow-up rates (37, 38), others have had challenges in retaining ICU survivors in follow-up studies, where 60% to 70% of patients are lost to follow-up at 3 and 12 months after discharge, respectively (28). Two systematic reviews identified numerous strategies that may reduce participant attrition in studies requiring in-person follow-up and found that systematic methods of participant contact and scheduling were the most commonly reported strategies (39), and the number of strategies used was positively correlated with retention rates (40). Similarly, Abshire and colleagues conducted surveys and in-depth semistructured interviews of a convenience sample of principle investigators to identify strategies that may increase participant retention rates, which included study reminders, study visit characteristics, emphasizing study benefits, and contact and scheduling strategies (41). In addition, web-based (31) or at-home methods of cognitive testing should be explored as the means to reduce the selection bias of only including patients who are able or willing to attend in person.
Although ICU survivors are a heterogeneous patient population, we identified a paucity of data on cognitive outcomes in specific ICU patient subgroups, with the exception of ARDS survivors. Given that the cause, prevalence, and temporal trajectory of cognitive impairment may differ between patient subgroups, future studies should delineate the cognitive outcomes in other ICU subgroups (e.g., sepsis and septic shock, non-head trauma, and the elderly). Although a recent study failed to show association between frailty and worse global cognition in ICU survivors (42), the high prevalence of frailty in younger and older patients in that study warrants further research to determine whether frailty modifies domain-specific cognitive recovery in ICU survivors (43).
Conclusions
Cognitive impairment in the ICU survivors appears to be common, severe, and persistent. However, its prevalence and temporal trajectory differ depending on the cognitive instruments used and the etiology of critical illness. The use of screening tests (e.g., MMSE) may underestimate the true prevalence and extent of cognitive impairment that is detected by more comprehensive test batteries (e.g., RBANS). However, repeating comprehensive batteries at arbitrary time intervals dictated by operational limitations of individual studies risks missing temporal nuances of cognitive recovery in individual patients. Future studies should focus on developing ICU-specific cognitive batteries that will allow comprehensive cognitive assessment at appropriate time intervals across different etiologies of critical illness. Such batteries will be critical to establishing the natural history of cognitive impairment (and recovery) in individual patients and determining which acute illness and treatment factors are associated with improved cognitive outcomes.
Acknowledgment
The authors thank Ms. Alla Iansavitchene for providing her expertise as a clinical librarian at London Health Sciences Centre.
Footnotes
Author Contributions: K.H., F.P., C.W.M., A.M.O., and M.S. were responsible for study design, data interpretation, and manuscript preparation and editing. K.H., R.S.L., and J.L.C. were responsible for data acquisition and interpretation. All authors had an opportunity to review the manuscript and approved its final submitted version.
CME will be available for this article at www.atsjournals.org.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201904-0816CI on February 20, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
References
Articles from American Journal of Respiratory and Critical Care Medicine are provided here courtesy of American Thoracic Society
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1164/rccm.201904-0816ci
Article citations
Altered functional brain connectivity, efficiency, and information flow associated with brain fog after mild to moderate COVID-19 infection.
Sci Rep, 14(1):22094, 27 Sep 2024
Cited by: 0 articles | PMID: 39333726 | PMCID: PMC11437042
Characterization of postintensive care syndrome in a prospective cohort of survivors of COVID-19 critical illness: a 12-month follow-up study.
Can J Anaesth, 71(9):1282-1301, 09 Sep 2024
Cited by: 0 articles | PMID: 39251486 | PMCID: PMC11408405
Long term cognitive dysfunction among critical care survivors: associated factors and quality of life-a multicenter cohort study.
Ann Intensive Care, 14(1):116, 29 Jul 2024
Cited by: 0 articles | PMID: 39073625 | PMCID: PMC11286902
Intensive care unit follow-up clinic activities: a scoping review.
J Anesth, 38(4):542-555, 23 Apr 2024
Cited by: 0 articles | PMID: 38652320
Review
Objective and subjective cognitive status after intensive care unit treatment for COVID-19.
Brain Behav Immun Health, 38:100786, 06 May 2024
Cited by: 2 articles | PMID: 38770194 | PMCID: PMC11103414
Go to all (51) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT03733418
Lay summaries
Plain language description
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Clinical phenotypes of delirium during critical illness and severity of subsequent long-term cognitive impairment: a prospective cohort study.
Lancet Respir Med, 6(3):213-222, 01 Mar 2018
Cited by: 183 articles | PMID: 29508705 | PMCID: PMC6709878
Severity of delirium in the ICU is associated with short term cognitive impairment. A prospective cohort study.
Intensive Crit Care Nurs, 31(4):250-257, 21 May 2015
Cited by: 15 articles | PMID: 26003476
Lack of clinically relevant correlation between subjective and objective cognitive function in ICU survivors: a prospective 12-month follow-up study.
Crit Care, 23(1):253, 12 Jul 2019
Cited by: 16 articles | PMID: 31300016 | PMCID: PMC6625117
Society of Critical Care Medicine's International Consensus Conference on Prediction and Identification of Long-Term Impairments After Critical Illness.
Crit Care Med, 48(11):1670-1679, 01 Nov 2020
Cited by: 133 articles | PMID: 32947467
Review

 1,3,4,5
1,3,4,5