Abstract
Background
The incidence and severity of coronavirus disease 2019 (COVID-19) among HIV-positive persons receiving antiretroviral therapy (ART) have not been characterized in large populations.Objective
To describe the incidence and severity of COVID-19 by nucleos(t)ide reverse transcriptase inhibitor (NRTI) use among HIV-positive persons receiving ART.Design
Cohort study.Setting
HIV clinics in 60 Spanish hospitals between 1 February and 15 April 2020.Participants
77 590 HIV-positive persons receiving ART.Measurements
Estimated risks (cumulative incidences) per 10 000 persons and 95% CIs for polymerase chain reaction-confirmed COVID-19 diagnosis, hospitalization, intensive care unit (ICU) admission, and death. Risk and 95% CIs for COVID-19 diagnosis and hospital admission by use of the NRTIs tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC), tenofovir alafenamide (TAF)/FTC, abacavir (ABC)/lamivudine (3TC), and others were estimated through Poisson regression models.Results
Of 77 590 HIV-positive persons receiving ART, 236 were diagnosed with COVID-19, 151 were hospitalized, 15 were admitted to the ICU, and 20 died. The risks for COVID-19 diagnosis and hospitalization were greater in men and persons older than 70 years. The risk for COVID-19 hospitalization was 20.3 (95% CI, 15.2 to 26.7) among patients receiving TAF/FTC, 10.5 (CI, 5.6 to 17.9) among those receiving TDF/FTC, 23.4 (CI, 17.2 to 31.1) among those receiving ABC/3TC, and 20.0 (CI, 14.2 to 27.3) for those receiving other regimens. The corresponding risks for COVID-19 diagnosis were 39.1 (CI, 31.8 to 47.6), 16.9 (CI, 10.5 to 25.9), 28.3 (CI, 21.5 to 36.7), and 29.7 (CI, 22.6 to 38.4), respectively. No patient receiving TDF/FTC was admitted to the ICU or died.Limitation
Residual confounding by comorbid conditions cannot be completely excluded.Conclusion
HIV-positive patients receiving TDF/FTC have a lower risk for COVID-19 and related hospitalization than those receiving other therapies. These findings warrant further investigation in HIV preexposure prophylaxis studies and randomized trials in persons without HIV.Primary funding source
Instituto de Salud Carlos III and National Institutes of Health.Free full text

Incidence and Severity of COVID-19 in HIV-Positive Persons Receiving Antiretroviral Therapy
Abstract
Visual Abstract.
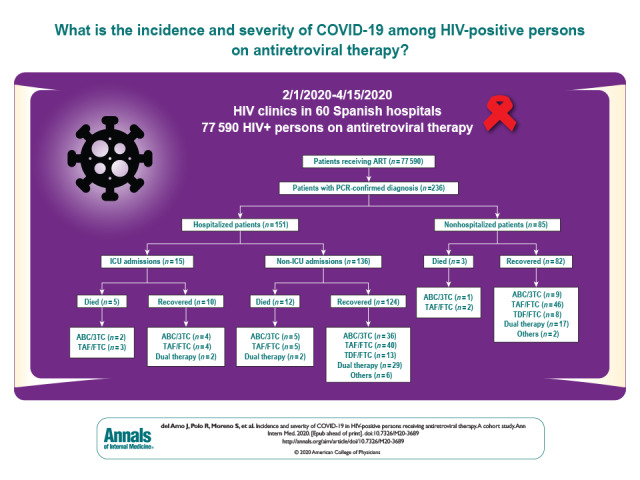
This study describes the incidence and severity of COVID-19 among 77 590 HIV-positive patients receiving antiretroviral therapy (ART). These findings warrant further investigation of HIV ART in HIV preexposure prophylaxis studies and randomized trials among persons without HIV.
Abstract
Background:
The incidence and severity of coronavirus disease 2019 (COVID-19) among HIV-positive persons receiving antiretroviral therapy (ART) have not been characterized in large populations.
Objective:
To describe the incidence and severity of COVID-19 by nucleos(t)ide reverse transcriptase inhibitor (NRTI) use among HIV-positive persons receiving ART.
Design:
Cohort study.
Setting:
HIV clinics in 60 Spanish hospitals between 1 February and 15 April 2020.
Participants:
77 590 HIV-positive persons receiving ART.
Measurements:
Estimated risks (cumulative incidences) per 10 000 persons and 95% CIs for polymerase chain reaction–confirmed COVID-19 diagnosis, hospitalization, intensive care unit (ICU) admission, and death. Risk and 95% CIs for COVID-19 diagnosis and hospital admission by use of the NRTIs tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC), tenofovir alafenamide (TAF)/FTC, abacavir (ABC)/lamivudine (3TC), and others were estimated through Poisson regression models.
Results:
Of 77 590 HIV-positive persons receiving ART, 236 were diagnosed with COVID-19, 151 were hospitalized, 15 were admitted to the ICU, and 20 died. The risks for COVID-19 diagnosis and hospitalization were greater in men and persons older than 70 years. The risk for COVID-19 hospitalization was 20.3 (95% CI, 15.2 to 26.7) among patients receiving TAF/FTC, 10.5 (CI, 5.6 to 17.9) among those receiving TDF/FTC, 23.4 (CI, 17.2 to 31.1) among those receiving ABC/3TC, and 20.0 (CI, 14.2 to 27.3) for those receiving other regimens. The corresponding risks for COVID-19 diagnosis were 39.1 (CI, 31.8 to 47.6), 16.9 (CI, 10.5 to 25.9), 28.3 (CI, 21.5 to 36.7), and 29.7 (CI, 22.6 to 38.4), respectively. No patient receiving TDF/FTC was admitted to the ICU or died.
Limitation:
Residual confounding by comorbid conditions cannot be completely excluded.
Conclusion:
HIV-positive patients receiving TDF/FTC have a lower risk for COVID-19 and related hospitalization than those receiving other therapies. These findings warrant further investigation in HIV preexposure prophylaxis studies and randomized trials in persons without HIV.
Primary Funding Source:
Instituto de Salud Carlos III and National Institutes of Health.
The recent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic (1, 2) has collided with the preexisting HIV pandemic. By 22 May 2020, 234 824 coronavirus disease 2019 (COVID-19) cases and 28 628 COVID-related deaths had been reported in Spain (3), the country with the highest HIV prevalence in Europe (4).
The relation between HIV and SARS-CoV-2, however, is unclear (5–7). Despite the higher mortality due to COVID-19 reported among some persons with immunosuppression (8), HIV infection was not identified as an important comorbid condition in hospitalized patients with COVID-19 in New York City (9) or Madrid (10). One possibility is that HIV-positive persons do not develop the intense immunologic response that often complicates the clinical course of COVID-19 (10). Yet, 8 HIV-positive patients with COVID-19 in Wuhan, China, had preserved CD4 cell counts (7).
In fact, COVID-19 might be expected to be more severe in HIV-positive persons, because risk factors for severity—older age, male sex, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, and kidney disease (9–12)—are common in this population. In Spain, more than 75% of HIV-positive persons are men (13, 14), and among those older than 50 years who are receiving antiretroviral therapy (ART), 24% have relevant comorbid conditions (CoRIS: AIDS Research Network Cohort. Personal communication, 18 March 2020). HIV-positive persons have a greater prevalence of geriatric syndromes, such as frailty, at younger ages (15, 16).
The lack of increased risk for serious COVID-19 among HIV-positive persons might be the result of their use of ART. Antiretroviral therapy was proposed as a protective factor against SARS in 2003, but the small number of cases did not permit conclusions to be drawn (17). Studies of molecular docking and extension reactions with RNA-dependent RNA polymerase (RNAdRNAp) suggest that nucleos(t)ide reverse transcriptase inhibitors (NRTIs), such as tenofovir disoproxil fumarate (TDF), tenofovir alafenamide (TAF), abacavir (ABC), and lamivudine (3TC), may be effective against SARS-CoV-2 by inhibiting RNAdRNAp (18–24).
More than 90% of known HIV-positive persons in Spain are receiving ART (25), most often 2 NRTIs plus an integrase inhibitor, a protease inhibitor, or a nonnucleoside reverse transcriptase inhibitor (NNRTI) (13). Here, we describe the incidence, clinical severity, and mortality of COVID-19, by type of NRTI regimen, among 77 590 HIV-positive patients receiving ART in Spain.
Methods
Data Sources
Between 1 February and 15 April 2020, the HIV clinics of 60 Spanish hospitals identified all polymerase chain reaction (PCR)-confirmed COVID-19 diagnoses among HIV-positive patients receiving ART. For each confirmed case, the clinics ascertained age, sex, and ART regimen at the time of the COVID-19 diagnosis. Regimens were classified according to both the NRTI backbone (TDF/FTC, TAF/FTC, ABC/3TC, or other drugs) and the third drug (integrase inhibitor, protease inhibitor, or NNRTI).
In addition, HIV clinics provided the total number of HIV-positive patients receiving ART who were being followed in their units. We obtained the age and sex distribution, as well as the distribution of ART regimens, of all HIV-positive persons from the 2019 National HIV Hospital Survey (13). To examine the possibility of recent changes in ART prescription not captured by the 2019 National HIV Hospital Survey, we compared this information with that provided by hospital pharmacies. The distribution was similar among the 36 hospital pharmacies that reported this information, and virtually identical for all hospitals in Madrid.
We accessed the national COVID-19 Health Information System to obtain the age and sex distribution of confirmed COVID-19 diagnoses in the general population. The age and sex distribution of the population of Spain was obtained from the National Statistics Institute.
Outcome Definitions
The diagnosis of laboratory-confirmed COVID-19 required positive results from a PCR test following the Ministry of Health protocols (26). Clinical severity was graded as diagnosis, hospital admission, intensive care unit (ICU) admission, and death. Length of hospitalization was calculated in days from the date of hospital admission to the date of discharge.
Statistical Analysis
For each patient, follow-up started on 1 February and ended on 15 April 2020. We therefore calculated the 75-day risk (cumulative incidence) and 95% CI for COVID-19 diagnosis, hospital admission, ICU admission, and death, overall and by age group, sex, and NRTI regimen. We used multivariable Poisson regression models to estimate risks and 95% CIs for COVID-19 diagnoses and hospital admissions by NRTI regimen.
We restricted the analyses to persons living in Madrid, the region with the highest number of COVID-19 diagnoses and largest between-hospital variability in ART regimens (and, therefore, where the choice of regimen is more likely to be determined by local hospital preferences). Finally, we compared age- and sex-standardized risks in HIV-positive patients receiving ART with those in the general population aged 20 to 79 years.
Analyses were conducted with Stata, version 15.0 (StataCorp).
Institutional Review Board Approval
This study was approved by the institutional review board at University Hospital Ramón y Cajal, Madrid, Spain.
Role of the Funding Source
The funding sources had no role in the design, conduct, or analysis of the study or in the decision to submit the manuscript for publication.
Results
Between 1 February and 15 April, 236 PCR-confirmed diagnoses of COVID-19 were made among 77 590 HIV-positive persons receiving ART in Spain. Of these, 151 (64%) were hospitalized, with 15 (6%) admitted to the ICU, and 20 (8%) died (Figure).
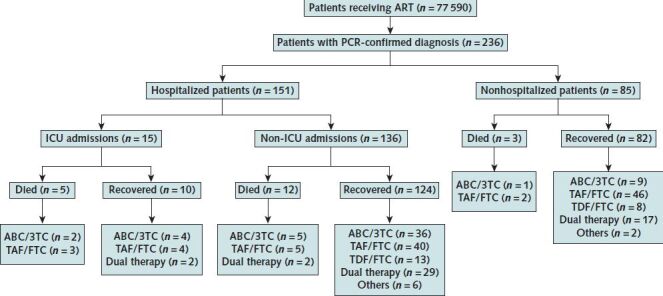
3TC = lamivudine; ABC = abacavir; ART = antiretroviral therapy; FTC = emtricitabine; ICU = intensive care unit; PCR = polymerase chain reaction; TAF = tenofovir alafenamide; TDF = tenofovir disoproxil fumarate.
Table 1 shows the distribution of age group, sex, and ART in the patients with COVID-19 compared with all 77 590 HIV-positive persons. The most common NRTI backbone used among HIV-positive patients was TAF/FTC (33%), followed by ABC/3TC (26%) and TDF/FTC (16%). In contrast, only 9% of the patients with COVID-19 received TDF/FTC. The proportion of persons receiving other regimens (only 3TC in dual therapies or NNRTI in patients treated with protease inhibitor monotherapy) was similar among those with and without COVID-19. Most patients received regimens based on integrase inhibitors (50%), followed by NNRTIs (21%) and protease inhibitors (19%), as the third drug.
Table 1. Characteristics of PCR-Confirmed COVID-19 Diagnoses, Hospital Admissions, ICU Admissions, and Deaths Among 77 590 HIV-Positive Persons Receiving ART, 1 February to 15 April 2020, Spain
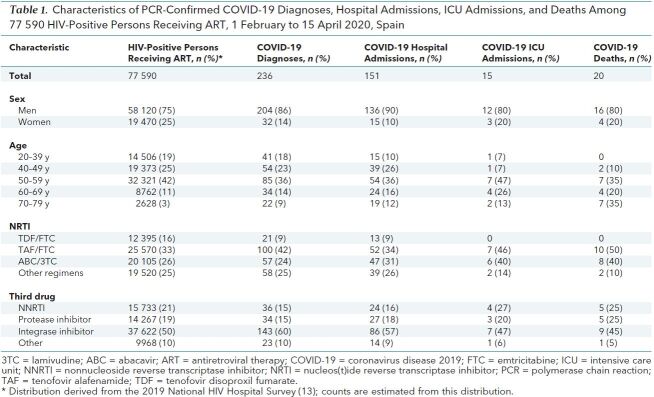
Table 2 shows the risks per 10 000 persons for COVID-19 diagnosis (30.4), hospital admission (19.5), ICU admission (1.9), and death (2.6). After standardization to the age and sex distribution of Spain, the risk per 10 000 among the 77 590 HIV-positive persons was 30.0 for COVID-19 diagnosis and 3.7 for death. For comparison, in the Spanish general population aged 20 to 79 years during the same period, the risk for COVID-19 diagnosis was 41.7 per 10 000 (33.0 per 10 000 after health care workers were excluded) and the risk for death was 2.1 per 10 000.
Table 2. Risk per 10 000 Persons for PCR-Confirmed COVID-19 Diagnosis, Hospital Admission, ICU Admission, and Death Among 77 590 HIV-Positive Persons Receiving ART, 1 February to 15 April 2020, Spain
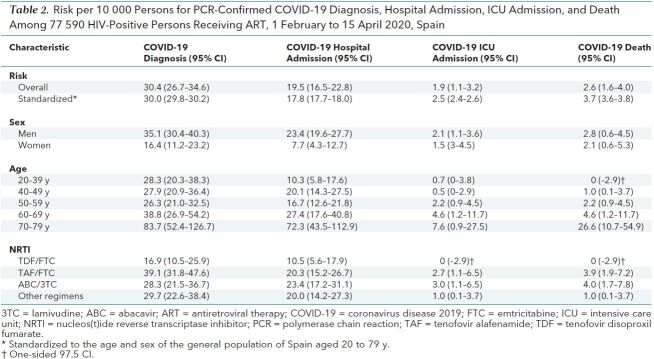
Table 2 also shows the risks among HIV-positive persons stratified by their baseline characteristics. The risks for COVID-19 diagnosis and hospitalization were greater in men than women and increased notably in those older than 70 years. After stratification by NRTI regimen, persons receiving TDF/FTC had the lowest risk for COVID-19 diagnosis (16.9 per 10 000) and hospitalization (10.5 per 10 000).
The median duration of hospitalization for 116 discharged patients was 7 days (interquartile range [IQR], 4 to 10 days) and increased by age: 4 days (IQR, 3 to 8 days) in the 20- to 39-year age group, 6.5 days (IQR, 4 to 9 days) in the 40- to 49-year group, 6 days (IQR, 5 to 10 days) in the 50- to 59-year group, 8 days (IQR, 6 to 11 years) in the 60- to 69-year group, and 9 days (IQR, 9 to 11 years) in the 70- to 79-year group.
The risks for diagnosis and hospitalization were 57% lower and 48% lower, respectively, in persons receiving TDF/FTC and those receiving TAF/FTC. These estimates did not materially change after the analysis was restricted to persons younger than 60 years and to those living in the Madrid region. The risks for diagnosis and hospitalization were 37% versus 20% lower, respectively, in 3 hospitals that predominantly used tenofovir as TDF/FTC versus 27 that predominantly used TAF/FTC.
Discussion
The risks for PCR-confirmed COVID-19 diagnosis, hospitalization, ICU admission, and death among HIV-positive persons receiving ART in Spain were greater in men and those older than 70 years. The risk for hospitalization varied by NRTI regimen and was lower in patients receiving TDF/FTC versus those receiving other regimens.
The observed age and sex patterns are consistent with those reported for HIV-negative persons (9–12). Risks for COVID-19 diagnosis in persons receiving ART were notably higher only in those aged 70 to 79 years. However, risk for hospitalization, ICU admission, and death, as well as duration of hospitalization, increased with age, consistent with the higher burden of comorbid conditions in older persons (9–12).
The lower risk for COVID-19 diagnosis among persons receiving TDF/FTC might be the result of less intensive testing for SARS-CoV-2 infection in this group compared with those receiving other ART regimens. However, no data—or even circumstantial evidence after consultation with treating physicians—exist to support such differential testing. Also, differential testing cannot explain the lower risk for hospitalization associated with TDF/FTC use. It is theoretically possible that persons who received TDF/FTC in February 2020 in Spain are a highly select group from which those most susceptible to SARS-CoV-2 infection have been removed. We cannot think of any mechanisms that might explain such an extreme form of depletion of susceptible persons.
Alternatively, another explanation for these findings is that TDF/FTC prevents serious COVID-19 in HIV-positive persons. Molecular docking (18–23) and other in vitro studies (24) suggest that NRTIs, such as TDF, TAF, ABC, and 3TC, might be effective against SARS-CoV-2 infection by inhibiting RNAdRNAp. This also might explain the 32% lower risk for COVID-19 diagnosis in persons receiving ABC/3TC compared with those receiving TAF/FTC. Tenofovir diphosphate (TFV-DP) is the common active triphosphate form of TAF or TDF and, because of its smaller size, has been proposed to fit better in the active site of SARS-CoV-2 RNAdRNAp (24). Compared with TAF, TDF produces higher blood levels of TFV-DP, and lower intracellular concentrations (27). In addition to its inhibition of the reverse transcriptase of HIV to ensure antiviral activity, tenofovir has been described as having various immunomodulatory effects in several animal and human cell lines (28–30). Interleukin (IL)-6, interferon-γ, IL-10, and monocyte chemoattractant protein-1 are increased in patients with severe COVID-19 (31), and tenofovir has been shown to diminish the production of the inflammatory cytokines IL-8, IL-10, and monocyte chemoattractant protein-1 in monocytes and peripheral blood mononuclear cells. This alters the cytokine balance toward IL-12 and thus promotes a T-helper type 1 response by inducing production of interferon-γ by T and natural killer cells (32). Whether higher extracellular levels of tenofovir correlate with an increased immunomodulatory action is unknown. In contrast to TDF (33), phosphorylated tenofovir concentrations in genital tract and rectal tissue achieved with TAF were almost unquantifiable in healthy seronegative volunteers (34). Penetration of antiretroviral drugs into the lung and other tissues has been shown for tenofovir in animal models (35) and for tenofovir, FTC, 3TC, and efavirenz in humans (36). Furthermore, TDF/FTC recently was shown to reduce SARS-CoV-2 titers in nasal washes from ferret infection models (37).
The patients receiving ART included in this study represent 65% of all persons receiving ART in Spain (25), the vast majority of whom do not have overt immunosuppression and 95% of whom have achieved HIV viral suppression (13, 25). In line with the greater all-cause mortality of HIV-positive persons compared with the general Spanish population (38), we found greater age- and sex-standardized mortality from COVID-19 in HIV-positive persons (3.7 per 10 000 compared) than in the general population (2.1 per 10 000). This comparison, however, is not straightforward, because biological age in persons with long-standing HIV infection is estimated to be 5 to 10 years greater than chronologic age (39–42), as the result of chronic immune activation by persistent gut microbial translocation, sustained chronic antigen stimulation, and co-infection by other pathogens (39, 42).
Comparisons with the general population also must be done with caution because of the inevitable reporting delays in the midst of a public health emergency (43). Although reporting delays are not expected for the HIV-positive persons in our study (clinicians were individually prompted to provide data from hospital records), the risk for COVID-19 diagnosis was lower in the HIV-positive population (30.0 per 10 000) than in the general population (41.7 per 10 000), even after removal of health care workers who were heavily tested (33.0 per 10 000). This risk was noticeably lower among HIV-positive persons receiving TDF/FTC (16.9 per 10 000). That this lower incidence of COVID-19 diagnosis can be explained by less social interaction or lower diagnostic intensity is unlikely. In the context of outbreaks in Spain, only clinically symptomatic persons seeking health care receive a PCR-confirmed diagnosis and, if anything, HIV-positive patients are expected to be investigated more intensely than the general population.
In summary, we took advantage of the overlap between 2 ongoing pandemics (HIV and SARS-CoV-2) in Spain. Our results suggest that the risk for COVID-19 diagnosis is not higher in HIV-positive persons than in the general population, and that HIV-positive patients receiving TDF/FTC had a lower risk for COVID-19 and related hospitalization than other HIV-positive persons. These findings warrant further investigation in studies of HIV preexposure prophylaxis and in randomized trials for the treatment and prevention of COVID-19 (44) in persons without HIV.
Appendix: Members of The Spanish HIV/COVID-19 Collaboration
Writing Committee (Members of The Spanish HIV/COVID-19 Collaboration Who Authored This Work)
Julia del Amo, MD, PhD; Rosa Polo, MD, PhD; Santiago Moreno, MD, PhD; Asunción Díaz, MD, PhD; Esteban Martínez, MD, PhD; José Ramón Arribas, MD, PhD; Inma Jarrín, PhD; Miguel A. Hernán, MD, DrPH
Collaborators (Members of The HIV/COVID-19 Collaboration Who Contributed to This Work but Did Not Author It)
Data Support
E.V. Martínez and M.J. Sierra (Coordinating Center for Emergencies, Ministry of Health); M. Sastre and R. Amillategui (National Center for Epidemiology, Instituto de Salud Carlos III)
COVID-19 Grupo de Estudio del SIDA-SEIMC (GeSIDA)*
J.L. Blanco (Hospital Clinic, Barcelona), J.R. Blanco (Hospital San Pedro, Logroño), J. Casado (Hospital Ramón y Cajal, Madrid), J. Olalla (Hospital Costa del Sol, Málaga)
National Plan on AIDS*
M. Cobos, G. Fagúndez, J. Gómez-Castellá, R. González, C. Iniesta, S. Martinez, R. Tuesta
Participating Hospitals and Investigators
Andalucía
Hospital Clínico San Cecilio de Granada: D. Vinuesa, J. Hernandez-Quero
Hospital Universitario Reina Sofía/Instituto Maimónides de Investigación Biomédica de Córdoba Granada: A. Rivero, M. Gallo
Hospital Costa del Sol, Málaga: A. Del Arco, J.M. García de Lomas
Hospital Virgen Macarena de Sevilla: A. Dominguez, M.J. Ríos
Hospital Universitario Virgen del Rocío/Instituto de Biomedicina de Sevilla/Consejo Superior de Investigaciones Científicas/Universidad de Sevilla: L.F. López-Cortés, N. Espinosa
Hospital Universitario Nuestra Señora de Valme, Sevilla: J. Macías, J.A. Pineda
Hospital Virgen de la Victoria, Málaga: J. Santos
Hospital de Jerez: J.A. Terrón
Hospital Universitario Virgen de las Nieves, Córdoba: C. Hidalgo
Aragón
Hospital Universitario Miguel Servet, Zaragoza: G. Samperiz, H. Navarro
Asturias
Hospital Universitario Central de Asturias, Oviedo: V. Asensi, M. Rivas
Baleares
Hospital Son Espases, Mallorca: A. Campins, M.A. Ribas
Canarias
Hospital Universitario de Santa Cruz de Tenerife Canarias: R. Pelazas, M.R. Alemán
Hospital Universitario Insular de Gran Canaria: J.L. Pérez-Arellano
Hospital Insular de Lanzarote: J.F. Lluch
Castilla La Mancha
Hospital General Virgen Luz de Cuenca: P. Geijo
Complejo Hospitalario Universitario de Albacete: E. Martínez-Alfaro, F. Mateos
Castilla y León
Hospital General de Segovia: E. Ferreira, P. Bachiller
Hospital Río Hortega de Valladolid: J. Gómez-Barquero, J. Abadía
H Clínico Universitario de Valladolid: C. Dueñas, L. Rodríguez
Complejo Asistencial de Ávila: M.A. Garcinuño, C. Grande
Complejo Asistencial Universitario de Palencia: J. Sánchez-Navarro
Hospital Universitario de Burgos: L. Buzón
Cataluña
Hospital Universitario Clinic de Barcelona: E. de Lazzari, A. Inciarte
Hospital Universitario Vall d'Hebron de Barcelona: J. García-Pérez, A. Curran
Hospital Universitario Mutua Terrassa: D. Dalmau, M. Cairó
Hospital Universitari Sagrat Cor–Grupo Quironsalud, Barcelona: M.R. Coll, D. de Mendoza
Hospital del Mar, Barcelona: H. Knobel, A. Guelar
Hospital Universitario Parc Tauli, Sabadell: G. Navarro Rubio, M. Navarro Vilasaró
Hospital Universitario de Bellvitge, Hospitalet, Barcelona: A. Imaz, D. Podzamczer
Hospital Universitario Joan XXIII, Institut d'Investigació Sanitária Pere Virgili, Tarragona: J. Peraire, A. Rull
Parc Sanitari Sant Joan de Déu, Sant Boi (Barcelona): V. Diaz-Brito, M. Sanmarti
Hospital Universitario Germans Trias i Pujol: E. Negredo
Hospital Sant Pau: P. Domingo; E. Molas
Galicia
Complejo Hospitalario Universitario de Ferrol: A. Mariño, N. Valcarce
Hospital Universitario Álvaro Cunqueiro, Vigo: A. Ocampo, G. Pousada
Hospital Clínico Universitario de Santiago de Compostela: E. Losada, A. Antela
Hospital Universitario de A Coruña: A. Mena
La Rioja
Hospital San Pedro, Logroño: J.A. Oteo
Madrid
Hospital Universitario Fundación Alcorcón: J.E. Losa, M. Velasco
Hospital Clínico San Carlos: J. Vergas, V. Estrada
Hospital Universitario 12 de Octubre: F. Pulido, M. de Lagarde
Hospital Universitario Infanta Sofía: I. Suárez-García, P. Ruiz-Seco
Hospital Universitario Fundación Jiménez Díaz: A. Cabello, M. Górgolas
Hospital Universitario La Paz: R. Montejano, R. Micán
Hospital Universitario de La Princesa, Madrid: J. Sanz-Sanz, I. de los Santos
Hospital Universitario Ramón y Cajal, Madrid: M.J. Pérez-Elías, J. Pérez-Molina
Hospital Universitario Quironsalud Madrid: P. Guisado
Hospital Universitario Príncipe de Asturias, Alcalá de Henares, Madrid: J. Sanz-Moreno, A. Arranz
Hospital Universitario Infanta Leonor: P. Ryan, G. Cuevas
Hospital Universitario Puerta de Hierro Majadahonda: A. Díaz de Santiago, A.A. Moreno
Hospital Universitario Severo Ochoa: M. Cervero
Hospital Universitario Gregorio Marañón: J.C. López Bernaldo de Quiros, F. Parras
Murcia
Hospital Universitario Virgen de la Arrixaca: C. Galera, H. Albendin
Hospital Universitario Reina Sofía: E. Bernal, A. Muñoz-Pérez
Hospital Virgen del Castillo Murcia: A. Soria
Navarra
Complejo Hospitalario de Navarra, Pamplona: M. Rivero-Marcotegui
País Vasco
Hospital Universitario Donostia; Instituto de Investigación BioDonostia, San Sebastián: M. Ibarguren, J.A. Iribarren
Hospital Universitario Araba, Vitoria: J. Portu, Z. Ortiz de Zárate
Valencia
Hospital General de Elche y Universidad Miguel Hernández, Alicante: M. Masiá, C. Robledano
Hospital General Universitario de Alicante: X. Portilla, M. Carreres
Hospital Universitario Dr. Peset y Universidad de Valencia: A. Artero, M. Madrazo
Hospital Universitario y Politécnico La Fe: M. Tasias, M. Montero
Hospital Clínico Universitario de Valencia: M.J. Galindo
Hospital Arnau de Vilanova, Valencia: J. Flores
* In alphabetical order.
Footnotes
This article was published at Annals.org on 26 June 2020.
* For members of The Spanish HIV/COVID-19 Collaboration, see the Appendix.
References
Full text links
Read article at publisher's site: https://doi.org/10.7326/m20-3689
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7394316
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.7326/m20-3689
Article citations
Effect of Tenofovir Alafenamide Fumarate on the outcomes of hospitalized COVID-19 patients: a prospective, block-balanced, open-label, randomized controlled trial.
BMC Pharmacol Toxicol, 25(1):78, 17 Oct 2024
Cited by: 0 articles | PMID: 39420385 | PMCID: PMC11484439
Genetic variants regulating the immune response improve the prediction of COVID-19 severity provided by clinical variables.
Sci Rep, 14(1):20728, 05 Sep 2024
Cited by: 0 articles | PMID: 39237611 | PMCID: PMC11377536
SARS-CoV-2 Variants and Clinical Outcomes of Special Populations: A Scoping Review of the Literature.
Viruses, 16(8):1222, 30 Jul 2024
Cited by: 0 articles | PMID: 39205196 | PMCID: PMC11359867
Review Free full text in Europe PMC
Deciphering Factors Linked With Reduced Severe Acute Respiratory Syndrome Coronavirus 2 Susceptibility in the Swiss HIV Cohort Study.
J Infect Dis, 230(2):e292-e304, 01 Aug 2024
Cited by: 0 articles | PMID: 38227786 | PMCID: PMC11326820
SARS-CoV-2 seropositivity in African women living with HIV and their infants.
BMC Infect Dis, 24(1):693, 11 Jul 2024
Cited by: 0 articles | PMID: 38992577 | PMCID: PMC11241888
Go to all (184) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Tenofovir disoproxil fumarate/emtricitabine and severity of coronavirus disease 2019 in people with HIV infection.
AIDS, 36(15):2171-2179, 23 Aug 2022
Cited by: 12 articles | PMID: 36382436 | PMCID: PMC9673178
Tenofovir disoproxil fumarate and coronavirus disease 2019 outcomes in men with HIV.
AIDS, 36(12):1689-1696, 15 Jul 2022
Cited by: 14 articles | PMID: 35848570 | PMCID: PMC9444875
SWIFT: prospective 48-week study to evaluate efficacy and safety of switching to emtricitabine/tenofovir from lamivudine/abacavir in virologically suppressed HIV-1 infected patients on a boosted protease inhibitor containing antiretroviral regimen.
Clin Infect Dis, 56(11):1637-1645, 29 Jan 2013
Cited by: 32 articles | PMID: 23362296 | PMCID: PMC3641864
Effects of nucleoside reverse transcriptase inhibitor backbone on the efficacy of first-line boosted highly active antiretroviral therapy based on protease inhibitors: meta-regression analysis of 12 clinical trials in 5168 patients.
HIV Med, 10(9):527-535, 01 Oct 2009
Cited by: 27 articles | PMID: 19785663
Review
Funding
Funders who supported this work.
NIAID NIH HHS (2)
Grant ID: R01 AI102634
Grant ID: R37 AI102634





