Abstract
Free full text

Advantages of the Parent Nucleoside GS-441524 over Remdesivir for Covid-19 Treatment
Abstract
While remdesivir has garnered much hope for its moderate anti-Covid-19 effects, its parent nucleoside, GS-441524, has been overlooked. Pharmacokinetic analysis of remdesivir evidences premature serum hydrolysis to GS-441524; GS-441524 is the predominant metabolite reaching the lungs. With its synthetic simplicity and in vivo efficacy in the veterinary setting, we contend that GS-441524 is superior to remdesivir for Covid-19 treatment.
While remdesivir has demonstrated efficacy against Covid-19, its broad translational applicability has been hampered by limited supply and distribution1 due to the difficulty of its synthesis2 and its obligatory intravenous (IV) administration requiring an inpatient setting. We recently described in a general audience publication3 the advantages that the parent nucleoside of remdesivir, GS-441524, has over remdesivir itself for the treatment of Covid-19. Fundamentally, our investigation into the metabolism of remdesivir evidences premature serum hydrolysis of its phosphate prodrug, followed by dephosphorylation.4−6 As a result, the major metabolite circulating in the bloodstream is the parent nucleoside, GS-441524, even though remdesivir (monophosphate nucleotide prodrug) was the species initially administered. Accounting for this broader pharmacokinetic (PK) rationale, we herein provide a detailed analysis of the literature that supports the use of GS-441524 over remdesivir for the treatment of Covid-19.
The Phosphate Prodrug on Remdesivir Is Not Intended for Lung-Specific Delivery
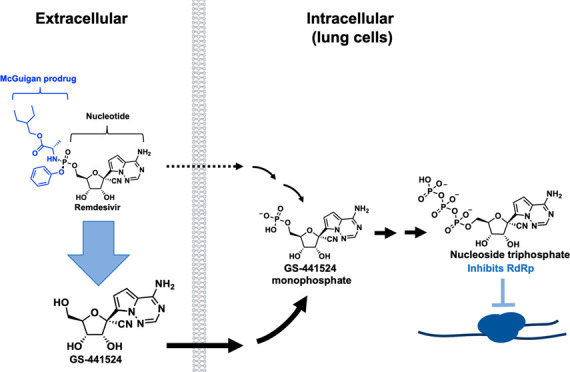
Remdesivir is a structural analogue of adenosine monophosphate (AMP) that interferes with the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp).7 The anionic phosphate moiety on remdesivir is masked by McGuigan prodrug moieties8 (phenol and l-alaninate ethylbutyl ester) to enhance cell permeability. In principle, these prodrug moieties would be removed intracellularly—first by esterases (cathepsin A/carboxylesterase 1) and then by phosphoramidases (HINT1-3)9 to release the monophosphorylated nucleotide. This would then be phosphorylated twice to give the active NTP7,9 (Figure Figure11a), which is substrate-competitive with ATP for incorporation by viral RdRp and inhibition of viral RNA synthesis.7 The McGuigan phosphate prodrug was partly developed to overcome the perceived rate-limiting first phosphorylation step toward the active triphosphorylated species. Bioactivation of the prodrug first involves carboxylesterases (CES1) and cathepsin A (CTSA), followed by phosphoramidases (histidine triad nucleotide binding proteins; HINTs; Figure Figure11a).9−11 Protein expression data from the Human Protein Atlas show that these enzymes (CES1, CTSA, HINT1, 2, 3) all have high expression in the liver, with minimal expression in type II pneumocytes in the lung12 (Figure Figure22). For the HINT family of phosphoramidases, there is some slight variation in each isoform’s tissue-specific expression (Figure Figure22b, c); however, all 3 isoforms show high expression in the GI tract, liver, and kidneys. From the pattern of bioactivation for McGuigan prodrugs, it follows that the most significant accumulation active NTP will be in cell types with high expression of CES1/CTSA/HINT1-3, such as the liver. Preferential bioactivation of McGuigan prodrugs such as remdesivir could explain the grade 3/4 adverse events related to liver and kidney damage in Covid-19 patients treated with remdesivir.13 Seeing that the enzymes involved in McGuigan prodrug hydrolysis are hardly expressed in the lungs undermines its utility in the context of a primarily respiratory disease such as Covid-19.
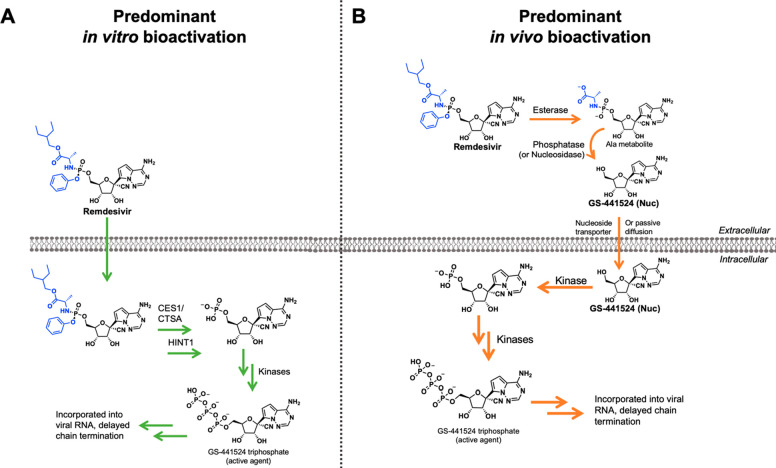
McGuigan prodrugs on remdesivir are prematurely hydrolyzed in serum. (A) The ideal bioactivation of remdesivir predominately occurs in vitro. (B) The presence of serum enzymes in vivo predominately results in premature hydrolysis of the phosphate prodrugs, followed by dephosphorylation to the nucleoside, GS-441524.
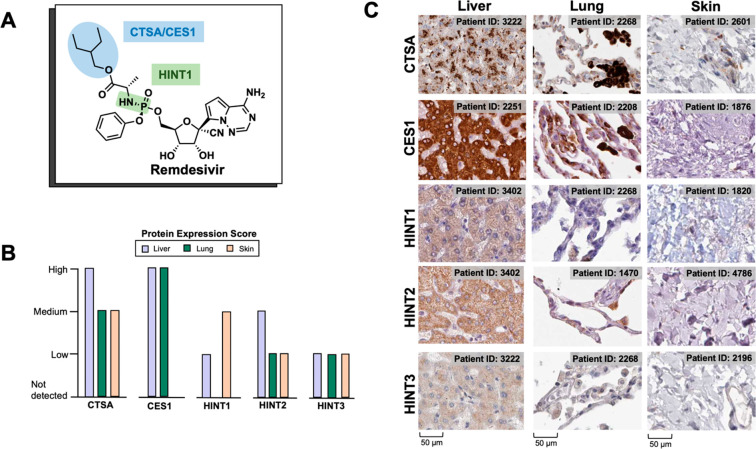
McGuigan prodrugs on remdesivir are preferentially bioactivated in the liver. (A) Labile prodrug moieties on remdesivir with corresponding bioactivation enzymes. (B) Relative tissue mRNA expression of initial prodrug bioactivating enzymes for RDV (CES1/CTSA/HINT1) adapted from the HPA data set on the Human Protein Atlas reported as median-centered protein-coding transcripts per million (pTPM). Overall, McGuigan prodrug bioactivating enzymes are more highly expressed in the liver than in the lungs. (C) Immunohistochemistry images from the Human Protein Atlas indicating expression for ProTide bioactivating enzymes. Brown regions indicate enzyme expression while blue regions indicate absent expression. For the lung, pneumocytes—cells frequently infected by Covid-19—are characterized by a threadlike appearance. Expression in the liver is generally higher compared to lung for all enzymes. For CTSA, darkly stained regions are associated with macrophages. IHC images for the skin are included to show lack of enzyme expression. Antibodies used: CTSA (CAB024930), CES1 (HPA046717), HINT1 (HPA044577).
GS-441524 Is the Predominant Metabolite in the Bloodstream When Remdesivir Is Administered IV
Hydrolytic enzymes are ubiquitous in serum.14 This is one physiological factor that, especially for prodrugs,15 prevents direct extrapolation of bioactivation mechanisms observed in vitro to the in vivo setting. For example, esterases and phosphatases are abundantly present in serum across species.16,17 Premature serum hydrolysis of the McGuigan prodrug on remdesivir is thus unsurprising (Figure Figure11b). Multiple studies have demonstrated that the nucleoside, GS-441524, is the predominant species in serum after remdesivir is administered (Figure Figure33b, c).4−6 All studies that have investigated the PK of remdesivir in nonhuman primates (NHP) have concluded that intact remdesivir exhibits a short plasma half-life of about 0.4 h in serum, with “persistence” of the downstream nucleoside, GS-441524 (Figure Figure33c).4,6 IV injection of remdesivir in NHP results in GS-441524 being present in serum at concentrations 1000-fold higher than remdesivir throughout a 7-day treatment course6 (Figure Figure33b). This recurring phenomenon can first be explained by the abundance of plasma esterases, as the phosphoramidases (HINT1) involved in removal of the l-alanine have a strictly intracellular presence (see Human Protein Atlas HINT1). Inadvertent biotransformation of remdesivir to GS-441524 can be explained by the following sequence: (1) esterase removal of the l-alaninate ester, (2) intramolecular cyclization, displacement of the phenolate, followed by reopening of the ring, (3) cleavage of the phosphate ester by serum phosphatases or nucleosidases (Figure Figure11b). The proposed serum bioactivation mechanism accounts for the general substrate constraints for each class of enzyme. For instance, CES1 is named as one of the enzymes involved in McGuigan prodrug hydrolysis. However, this does not preclude other esterases from acting on its l-alaninate ester. A study conducted by Sheahan and colleagues specifically investigated the PK of remdesivir in carboxylesterase 1c deficient mice (Ces1c–/–).5 Even in this Ces1c–/– model, the half-life of remdesivir was still short (t1/2 ~ 25 min), supporting the notion that other esterases are capable of performing the initial hydrolysis reaction. Thus, the abundance of hydrolytic enzymes in serum explains the persistent, multispecies observation that GS-441524 is the predominant metabolite when remdesivir is administered.4−6 For the fleeting duration of time that remdesivir is in the blood (prior to hydrolysis to GS-441524), the expression of bioactivating enzymes for McGuigan prodrugs suggests that the highest concentrations of NTP formation by remdesivir—rather than GS-441524—would occur in cell types with high expression of CES1/CTSA/HINT1. This largely favors the liver over the lungs (Figure Figure22). Differential expression of prodrug bioactivating enzymes likely explains the wide range of EC50 values with remdesivir in vitro.18−20
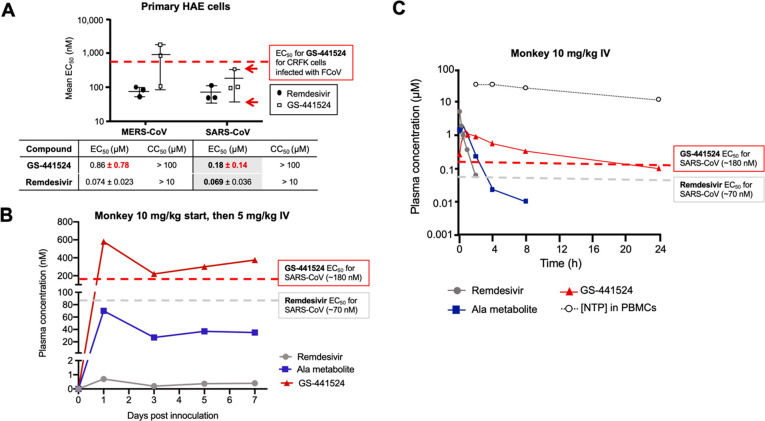
Unlike remdesivir, GS-441524 persists in serum at concentrations above the EC50 value required against SARS-CoV-infected primary HAE cells for long durations. (A) In vitro potency data replotted from Agostini et al. mBio, 2018.2 Primary HAE cells were infected with either MERS-CoV or SARS-CoV and treated with either GS-441524 (open squares) or remdesivir (closed circles). Mean EC50 of GS-441524 for SARS-CoV-infected HAE cells was found to be 0.18 ± 0.14 μM (note large standard deviations, red arrows). A study by Murphy et al. shows that GS-441524 has an EC50 value of 0.78 μM against FCoV-infected CRFK cells (red dashed line),22 which is higher than the EC50 value for GS-441524 against SARS-CoV-infected primary HAE cells. (B) Estimated metabolite concentrations for a PK experiment in a SARS-CoV-2 primate model replotted from Williamson et al. Nature, 2020.6 Primates were initially injected IV with 10 mg/kg of remdesivir 12 h postinoculation with SARS-CoV-2 and then 5 mg/kg of remdesivir every 24 h after. Throughout the experiment, GS-441524 is present in serum at concentrations ~1000-fold higher than remdesivir; the concentration of GS-441524 is consistently above the EC50 value in SARS-CoV-infected primary HAE cells (red dashed line) at all time points taken in the experiment. In contrast, the concentration of remdesivir in serum never exceeds that required to give the EC50 value against SARS-CoV-infected primary HAE cells (gray dashed line). (C) PK data replotted from Warren et al. Nature, 20163 following IV injection (10 mg/kg) of remdesivir in NHP. Dashed lines indicate the approximate EC50 values of GS-441524 (red) or remdesivir (gray) needed to reach EC50 in SARS-CoV primary HAE cells obtained in (A). Unlike remdesivir, the concentration of drug required to give the EC50 value against SARS-CoV primary HAE cells is maintained for significantly longer with GS-441524 than with remdesivir.
GS-441524 Is Exceptionally Effective and Well-Tolerated against Clinical Presentations of Feline Coronavirus
There are currently no studies that have compared the antiviral activities of remdesivir and GS-441524 in vivo, with most focusing exclusively on remdesivir. Where GS-441524 has been investigated in vivo is in the veterinary setting.21−23 Cats infected with feline coronavirus (FCoV) present with a serious disease known as feline infectious peritonitis (FIP). While long considered fatal in its severe manifestations,24 a study conducted by Pedersen and colleagues showed that GS-441524 is capable of treating cats suffering from FIP with a 96% cure rate.21 Pedersen noted the “impressive” safety profile of GS-441524, with no systemic signs of toxicity observed when administered subcutaneously at 4 mg/kg.21 In a more recent study, Pedersen and colleagues escalated the dose of GS-441524 (5–10 mg/kg) to treat neurological manifestations of FIP; this translates to about 350–700 mg in a 70 kg human, greatly exceeding the dose currently given to patients treated with remdesivir (200 mg loading, then 100 mg).13,25 Even at these higher doses, they found that GS-441524 treatment resulted in the long term resolution of neurological FIP with an excellent safety profile: minimal dose-related toxicities were observed.23
GS-441524 Shows Comparable Efficacy in Cell-Based Models of Primary Human Lung and Cat Cells Infected with Coronavirus
In vitro potency comparisons between GS-441524 and remdesivir are ultimately moot in the context of respiratory diseases such as SARS-CoV-2, if GS-441524 is the predominant species that reaches the lungs. To better gauge the efficacy of GS-441524 against SARS-CoV-2, it is helpful to first compare EC50 values between coronavirus infected human and cat cells, as the clinical efficacy of GS-441524 has already been well-established in cats.21 GS-441524 has an EC50 value of 0.78 μM in CRFK cells infected with FCoV (Figure Figure33a).26 At the time of publication, a study by Agostini and colleagues is the only report that has compared the antiviral activities of GS-441524 and remdesivir in primary human airway epithelial (HAE) cells, the most clinically relevant in vitro model of the lung, infected with either SARS-CoV or MERS-CoV.27 While the mean EC50 value of remdesivir is lower for both SARS-CoV and MERS-CoV-infected cells, close inspection of the data reveals large standard deviations between the EC50 values obtained from GS-441524 and remdesivir making these potency differences not statistically significant (Figure Figure33a).27 For instance, against SARS-CoV-infected HAE cells, GS-441524 has a reported EC50 of 0.18 (±0.14) μM, which is comparable, if not lower, than that required to exert antiviral activity against FCoV-infected cells in vitro. Most significantly, the EC50 concentration for GS-441524 against SARS-CoV-infected primary HAE cells is sustained in the plasma of NHP for nearly the entire duration of the single-dose, 24 h PK experiment conducted by Warren and colleagues (Figure Figure33c). In contrast, the EC50 concentration for remdesivir against SARS-CoV-infected primary HAE cells diminishes after ~2 h. The dominance of GS-441524 over remdesivir in serum was even more pronounced in Williamson’s 7-day PK study, in which GS-441524 was present in serum at concentrations 1,000-fold greater than remdesivir at every measured time point (Figure Figure33b).6 Coupled with the robust antiviral activity that GS-441524 has demonstrated against FIP, these data compel further investigations into the therapeutic and prophylactic utility of GS-441524 against SARS-CoV-2 in patients.
Concluding Remarks
SARS-CoV-2 is a respiratory virus that primarily affects the lungs.12 While remdesivir has shown some efficacy in patients with advanced Covid-19,13 its phosphate prodrug is fundamentally not designed for lung-specific delivery. Enzymes that activate the McGuigan prodrug are preferentially expressed in tissues such as the liver, which results in uneven distribution of active NTP formation via remdesivir that disfavors the lungs. Practically, the structural complexity of the McGuigan prodrug28 adds unnecessary synthetic difficulty that hampers mass production and impedes distribution.1 Above all else, premature hydrolysis of the McGuigan prodrug, followed by dephosphorylation in serum such that GS-441524 is the predominant metabolite4,5,29 compels studies investigating its utility in patients with Covid-19. In contrast to the prodrug activating enzymes that activate remdesivir, bioactivation of GS-441524 relies on expression of the kinase responsible for initial phosphorylation (likely adenosine kinase, ADK). According to the Human Protein Atlas, ADK is moderately expressed across all tissues, suggesting that administration of GS-441524 would result in even distribution across tissues. The remarkable safety profile of GS-441524, indicated by selectivity indices in vitro (EC50/CC50 ratio)2,19,30 and by clinical observations in cats,21−23 suggest that higher dosing and lung NTP loading could be achieved with GS-441524 without encountering serious adverse effects. GS-441524 is also a structurally simple molecule that is easier to synthesize compared to remdesivir,2 which would ease mass production and distribution. Especially amidst the documented premature serum hydrolysis of remdesivir to GS-441524,4,5,29 we see several advantages to using GS-441524 over remdesivir for patients with Covid-19. While GS-441524, is not included in the emergency use authorization of remdesivir, the exigence of the pandemic may allow ordinary regulatory hurdles to be overcome, especially as these two drugs yield the same active species. An investigational new drug (IND) waiver or an emergency IND could be filed. The FDA has previously made allowances for prodrugs and their corresponding active substances, as in the case of Lenflunomide/Teriflunomide. Further investigations into the anti-Covid-19 utility of GS-441524 are thus imperative.
Acknowledgments
We thank Niels Pedersen, Steve Kirsch, and David Piwnica-Worms for helpful discussions, Cong-Dat Pham for assistance with research, and Pat Skerrett for assistance with our general audience article published in STAT. This work was supported by the American Cancer Society (RSG-15-145-01-CDD).
Author Contributions
V.C.Y. and F.L.M. researched and analyzed the literature. V.C.Y. wrote the manuscript.
References
- Cohen E.; Azad A.The US Government’s Supply of Covid-19 Drug Remdesivir Runs out at the End of the Month. CNN. June 8, 2020. [Google Scholar]
- Siegel D.; Hui H. C.; Doerffler E.; Clarke M. O.; Chun K.; Zhang L.; Neville S.; Carra E.; Lew W.; Ross B.; et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1- f ][Triazin-4-Amino] Adenine C -Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60 (5), 1648–1661. 10.1021/acs.jmedchem.6b01594. [Abstract] [CrossRef] [Google Scholar]
- Yan V. C.; Muller F. L.Gilead Should Ditch Remdesivir and Focus on Its Simpler and Safer Ancestor. STAT. May 14, 2020. [Google Scholar]
- Warren T. K.; Jordan R.; Lo M. K.; Ray A. S.; Mackman R. L.; Soloveva V.; Siegel D.; Perron M.; Bannister R.; Hui H. C.; et al. Therapeutic Efficacy of the Small Molecule GS-5734 against Ebola Virus in Rhesus Monkeys. Nature 2016, 531, 381–385. 10.1038/nature17180. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Sheahan T. P.; Sims A. C.; Graham R. L.; Menachery V. D.; Gralinski L. E.; Case J. B.; Leist S. R.; Pyrc K.; Feng J. Y.; Trantcheva I.; et al.Broad-Spectrum Antiviral GS-5734 Inhibits Both Epidemic and Zoonotic Coronaviruses. Sci. Transl. Med. 2017, 9 ( (396), ), eaal3653.10.1126/scitranslmed.aal3653 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Williamson B. N.; Feldmann F.; Schwarz B.; Meade-White K.; Porter D. P.; Schulz J.; Van Doremalen N.; Leighton I.; Yinda C. K.; Pérez-Pérez L.; et al. Clinical Benefit of Remdesivir in Rhesus Macaques Infected with SARS-CoV-2. Nature 2020, 10.1038/s41586-020-2423-5. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Gordon C. J.; Tchesnokov E. P.; Feng J. Y.; Porter D. P.; Gotte M. The Antiviral Compound Remdesivir Potently Inhibits RNA-Dependent RNA Polymerase from Middle East Respiratory Syndrome Coronavirus. J. Biol. Chem. 2020, 295, 4773.10.1074/jbc.AC120.013056. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Alanazi A. S.; James E.; Mehellou Y. The ProTide Prodrug Technology: Where Next?. ACS Med. Chem. Lett. 2019, 10 (1), 2–5. 10.1021/acsmedchemlett.8b00586. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Murakami E.; Wang T.; Babusis D.; Lepist E.-I.; Sauer D.; Park Y.; Vela J. E.; Shih R.; Birkus G.; Stefanidis D.; et al. Metabolism and Pharmacokinetics of the Anti-Hepatitis C Virus Nucleotide Prodrug GS-6620 Downloaded From. Antimicrob. Agents Chemother. 2014, 58, 1943–1951. 10.1128/AAC.02350-13. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Bieganowski P.; Garrison P. N.; Hodawadekar S. C.; Faye G.; Barnes L. D.; Brenner C. Adenosine Monophosphoramidase Activity of Hint and Hnt1 Supports Function of Kin28, Ccl1, and Tfb3. J. Biol. Chem. 2002, 277 (13), 10852–10860. 10.1074/jbc.M111480200. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Chou T.-F.; Baraniak J.; Kaczmarek R.; Zhou X.; Cheng J.; Ghosh B.; Wagner C. R. Phosphoramidate Pronucleotides: A Comparison of the Phosphoramidase Substrate Specificity of Human and Escherichia Coli Histidine Triad Nucleotide Binding Proteins. Mol. Pharmaceutics 2007, 4 (2), 208–217. 10.1021/mp060070y. [Abstract] [CrossRef] [Google Scholar]
- Wichmann D.; Sperhake J.-P.; Lütgehetmann M.; Steurer S.; Edler C.; Heinemann A.; Heinrich F.; Mushumba H.; Kniep I.; Schröder A. S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020, M20–2003. 10.7326/M20-2003. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Beigel J. H.; Tomashek K. M.; Dodd L. E.; Mehta A. K.; Zingman B. S.; Kalil A. C.; Hohmann E.; Chu H. Y.; Luetkemeyer A.; Kline S.; et al. Remdesivir for the Treatment of Covid-19 — Preliminary Report. N. Engl. J. Med. 2020, 10.1056/NEJMoa2007764. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Cooke A. M.; Baron D. N.Section of Medicine with Section of Pathology-Serum Enzymes in Clinical Practice; 1963; Vol. 56. [Europe PMC free article] [Abstract]
- Testa B.; Mayer J. M.Hydrolysis in Drug and Prodrug Metabolism: Chemistry, Biochemistry, and Enzymology; VHCA, 2003. [Google Scholar]
- Bahar F. G.; Ohura K.; Ogihara T.; Imai T. Species Difference of Esterase Expression and Hydrolase Activity in Plasma. J. Pharm. Sci. 2012, 101 (10), 3979–3988. 10.1002/jps.23258. [Abstract] [CrossRef] [Google Scholar]
- Yong J. M. Origins of Serum Alkaline Phosphatase. J. Clin. Pathol. 1967, 20 (4), 647–653. 10.1136/jcp.20.4.647. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pruijssers A. J.; George A. S.; Schäfer A.; Leist S. R.; Gralinksi L. E.; Dinnon Iii K. H.; Yount B. L.; Agostini M. L.; Stevens L. J.; Chappell J. D.; et al. Remdesivir Potently Inhibits SARS-CoV-2 in Human Lung Cells and Chimeric SARS-CoV Expressing the SARS-CoV-2 RNA Polymerase in Mice. bioRxiv 2020, 10.1101/2020.04.27.064279. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Lo M. K.; Jordan R.; Arvey A.; Sudhamsu J.; Shrivastava-Ranjan P.; Hotard A. L.; Flint M.; McMullan L. K.; Siegel D.; Clarke M. O.; et al. GS-5734 and Its Parent Nucleoside Analog Inhibit Filo-, Pneumo-, and Paramyxoviruses. Sci. Rep. 2017, 7 (1), 43395.10.1038/srep43395. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Choy K.-T.; Wong A. Y.-L.; Kaewpreedee P.; Sia S. F.; Chen D.; Hui K. P. Y.; Chu D. K. W.; Chan M. C. W.; Cheung P. P.-H.; Huang X.; et al. Remdesivir, Lopinavir, Emetine, and Homoharringtonine Inhibit SARS-CoV-2 Replication in Vitro. Antiviral Res. 2020, 178, 104786.10.1016/j.antiviral.2020.104786. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Pedersen N. C.; Perron M.; Bannasch M.; Montgomery E.; Murakami E.; Liepnieks M.; Liu H. Efficacy and Safety of the Nucleoside Analog GS-441524 for Treatment of Cats with Naturally Occurring Feline Infectious Peritoniti. J. Feline Med. Surg. 2019, 21 (4), 271–281. 10.1177/1098612X19825701. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Murphy B. G.; Perron M.; Murakami E.; Bauer K.; Park Y.; Eckstrand C.; Liepnieks M.; Pedersen N. C. The Nucleoside Analog GS-441524 Strongly Inhibits Feline Infectious Peritonitis (FIP) Virus in Tissue Culture and Experimental Cat Infection Studies. Vet. Microbiol. 2018, 219, 226–233. 10.1016/j.vetmic.2018.04.026. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Dickinson P. J.; Bannasch M.; Thomasy S. M.; Murthy V. D.; Vernau K. M.; Liepnieks M.; Montgomery E.; Knickelbein K. E.; Murphy B.; Pedersen N. C. Antiviral Treatment Using the Adenosine Nucleoside Analogue GS −441524 in Cats with Clinically Diagnosed Neurological Feline Infectious Peritonitis. J. Vet. Intern. Med. 2020, 10.1111/jvim.15780. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Addie D.; Belák S.; Boucraut-Baralon C.; Egberink H.; Frymus T.; Gruffydd-Jones T.; Hartmann K.; Hosie M. J.; Lloret A.; Lutz H.; et al. Feline Infectious Peritonitis. ABCD Guidelines on Prevention and Management. J. Feline Med. Surg. 2009, 11 (7), 594–604. 10.1016/j.jfms.2009.05.008. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Wang Y.; Zhang D.; Du G.; Du R.; Zhao J.; Jin Y.; Fu S.; Gao L.; Cheng Z.; Lu Q.; et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020, 395 (10236), 1569–1578. 10.1016/S0140-6736(20)31022-9. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Murphy B. G.; Perron M.; Murakami E.; Bauer K.; Park Y.; Eckstrand C.; Liepnieks M.; Pedersen N. C. The Nucleoside Analog GS-441524 Strongly Inhibits Feline Infectiousperitonitis (FIP) Virus in Tissue Culture and Experimental Cat Infection Studies. Vet. Microbiol. 2018, 219, 226–233. 10.1016/j.vetmic.2018.04.026. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Agostini M. L.; Andres E. L.; Sims A. C.; Graham R. L.; Sheahan T. P.; Lu X.; Clinton Smith E.; Brett Case J.; Feng J. Y.; Jordan R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease Downloaded From. mBio 2018, 9 (2), ee00221–18. 10.1128/mBio.00221-18. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
- Jarvis L. M.. Scaling up Remdesivir amid the Coronavirus Crisis. C&EN. April 20, 2020. [Google Scholar]
- Williamson B. N.; Feldmann F.; Schwarz B.; Meade-White K.; Porter D. P.; Schulz J.; Doremalen N.; van Leighton I.; Yinda C. K.; Pérez-Pérez L.; et al. Clinical Benefit of Remdesivir in Rhesus Macaques Infected with SARS-CoV-2. bioRxiv 2020, 2020.04.15.043166. [Europe PMC free article] [Abstract] [Google Scholar]
- Cho A.; Saunders O. L.; Butler T.; Zhang L.; Xu J.; Vela J. E.; Feng J. Y.; Ray A. S.; Kim C. U. Synthesis and Antiviral Activity of a Series of 1′-Substituted 4-Aza-7,9-Dideazaadenosine C-Nucleosides. Bioorg. Med. Chem. Lett. 2012, 22 (8), 2705–2707. 10.1016/j.bmcl.2012.02.105. [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Articles from ACS Medicinal Chemistry Letters are provided here courtesy of American Chemical Society
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Article citations
A robust mouse model of HPIV-3 infection and efficacy of GS-441524 against virus-induced lung pathology.
Nat Commun, 15(1):7765, 05 Sep 2024
Cited by: 0 articles | PMID: 39237507 | PMCID: PMC11377736
SARS-CoV-2 RNA and Nucleocapsid Antigen Are Blood Biomarkers Associated With Severe Disease Outcomes That Improve in Response to Remdesivir.
J Infect Dis, 230(3):624-634, 01 Sep 2024
Cited by: 2 articles | PMID: 38657001 | PMCID: PMC11420797
Owner experience and veterinary involvement with unlicensed GS-441524 treatment of feline infectious peritonitis: a prospective cohort study.
Front Vet Sci, 11:1377207, 26 Jun 2024
Cited by: 1 article | PMID: 38988986 | PMCID: PMC11233523
Clinical pharmacodynamics of obeldesivir versus remdesivir.
Antimicrob Agents Chemother, 68(9):e0096924, 12 Aug 2024
Cited by: 0 articles | PMID: 39133123
Remdesivir treatment does not reduce viral titers in patients with COVID-19.
Antimicrob Agents Chemother, 68(8):e0085624, 18 Jul 2024
Cited by: 0 articles | PMID: 39023261
Go to all (105) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
HPA - The Human Protein Atlas (3)
- (1 citation) HPA - HPA046717
- (1 citation) HPA - CAB024930
- (1 citation) HPA - HPA044577
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Biotransformation and transplacental transfer of the anti-viral remdesivir and predominant metabolite, GS-441524 in pregnant rats.
EBioMedicine, 81:104095, 04 Jun 2022
Cited by: 7 articles | PMID: 35671622 | PMCID: PMC9166662
Oral prodrug of remdesivir parent GS-441524 is efficacious against SARS-CoV-2 in ferrets.
Nat Commun, 12(1):6415, 05 Nov 2021
Cited by: 62 articles | PMID: 34741049 | PMCID: PMC8571282
Population pharmacokinetics and exposure-clinical outcome relationship of remdesivir major metabolite GS-441524 in patients with moderate and severe COVID-19.
CPT Pharmacometrics Syst Pharmacol, 12(4):513-521, 22 Feb 2023
Cited by: 4 articles | PMID: 36798006 | PMCID: PMC10088080
Pharmacokinetic, Pharmacodynamic, and Drug-Interaction Profile of Remdesivir, a SARS-CoV-2 Replication Inhibitor.
Clin Pharmacokinet, 60(5):569-583, 30 Mar 2021
Cited by: 61 articles | PMID: 33782830 | PMCID: PMC8007387
Review Free full text in Europe PMC
Funding
Funders who supported this work.
American Cancer Society (1)
Grant ID: RSG-15-145-01-CDD





