Abstract
Background
Although antivirals remain important for the treatment COVID-19, methods to assess treatment efficacy are lacking. Here, we investigated the impact of remdesivir on viral dynamics and their contribution to understanding antiviral efficacy in the multicenter Adaptive COVID-19 Treatment Trial 1, which randomized patients to remdesivir or placebo.Methods
Longitudinal specimens collected during hospitalization from a substudy of 642 patients with COVID-19 were measured for viral RNA (upper respiratory tract and plasma), viral nucleocapsid antigen (serum), and host immunologic markers. Associations with clinical outcomes and response to therapy were assessed.Results
Higher baseline plasma viral loads were associated with poorer clinical outcomes, and decreases in viral RNA and antigen in blood but not the upper respiratory tract correlated with enhanced benefit from remdesivir. The treatment effect of remdesivir was most pronounced in patients with elevated baseline nucleocapsid antigen levels: the recovery rate ratio was 1.95 (95% CI, 1.40-2.71) for levels >245 pg/mL vs 1.04 (95% CI, .76-1.42) for levels <245 pg/mL. Remdesivir also accelerated the rate of viral RNA and antigen clearance in blood, and patients whose blood levels decreased were more likely to recover and survive.Conclusions
Reductions in SARS-CoV-2 RNA and antigen levels in blood correlated with clinical benefit from antiviral therapy.Clinical trial registration
NCT04280705 (ClinicalTrials.gov).Free full text

SARS-CoV-2 RNA and Nucleocapsid Antigen Are Blood Biomarkers Associated With Severe Disease Outcomes That Improve in Response to Remdesivir
Abstract
Background
Although antivirals remain important for the treatment COVID-19, methods to assess treatment efficacy are lacking. Here, we investigated the impact of remdesivir on viral dynamics and their contribution to understanding antiviral efficacy in the multicenter Adaptive COVID-19 Treatment Trial 1, which randomized patients to remdesivir or placebo.
Methods
Longitudinal specimens collected during hospitalization from a substudy of 642 patients with COVID-19 were measured for viral RNA (upper respiratory tract and plasma), viral nucleocapsid antigen (serum), and host immunologic markers. Associations with clinical outcomes and response to therapy were assessed.
Results
Higher baseline plasma viral loads were associated with poorer clinical outcomes, and decreases in viral RNA and antigen in blood but not the upper respiratory tract correlated with enhanced benefit from remdesivir. The treatment effect of remdesivir was most pronounced in patients with elevated baseline nucleocapsid antigen levels: the recovery rate ratio was 1.95 (95% CI, 1.40–2.71) for levels >245 pg/mL vs 1.04 (95% CI, .76–1.42) for levels <245 pg/mL. Remdesivir also accelerated the rate of viral RNA and antigen clearance in blood, and patients whose blood levels decreased were more likely to recover and survive.
Conclusions
Reductions in SARS-CoV-2 RNA and antigen levels in blood correlated with clinical benefit from antiviral therapy.
Clinical Trial Registration
NCT04280705 (ClinicalTrials.gov).
The Adaptive COVID-19 Treatment Trial 1 (ACTT-1) was one of the earliest randomized placebo-controlled trials conducted during the COVID-19 pandemic and the first to demonstrate the clinical efficacy of the antiviral remdesivir [1]. In that study, remdesivir shortened the time to recovery in patients hospitalized with COVID-19 and lowered risk of disease progression and death among those requiring oxygen supplementation. Today, remdesivir remains one of few safe and effective antiviral treatment options for COVID-19 that retains activity against all known variants of concern [2]. However, fundamental questions that could better inform its clinical use remain unanswered. In particular, our understanding of remdesivir's impact on viral dynamics and our strategies to identify patients who would benefit from treatment are unclear. One approach to improve these gaps in knowledge is through the development of prognostic laboratory markers to guide therapy. Yet, the specific biomarkers that may aid in these goals remain unknown.
We analyzed viral and immunologic biomarkers from ACTT-1 to identify subsets of patients who derive greater benefit from remdesivir. In doing so, we compared 3 viral assays (1) to determine if any could measure the antiviral effects of remdesivir and (2) to assess if reductions in viral load early in the course of treatment affected severe disease outcomes. To optimize the precision and accuracy of these measurements, we utilized sensitive assays that quantitated viral RNA and antigen in blood (plasma RNA and serum nucleocapsid antigen) [3–5] and the upper respiratory tract (RNA), as well as SARS-CoV-2–specific antibody responses. We also evaluated interleukin 6 (IL-6) and C-reactive protein (CRP): 2 inflammatory biomarkers associated with worse disease [6–8].
METHODS
Study Design and Participants
The original ACTT-1 protocol and study design have been described [1]. This analysis was conducted with data and biospecimens from ACTT-1 participants who received at least 1 day of treatment with remdesivir or placebo and from whom biospecimens were available for measurement of plasma viral RNA, upper respiratory tract viral RNA, serum viral nucleocapsid, IL-6, and CRP—all collected at baseline and at least 1 other time point within the 6 days following randomization (Supplementary Figure 1). Written informed consent was obtained from each patient or legally authorized representative. Patients were grouped into 2 categories based on disease severity and ordinal scale group per the National Institute of Allergy and Infectious Diseases:
Moderate/severe disease: patients were hospitalized but not critically ill and consisted of those in group 4 (not requiring supplemental oxygen) or group 5 (requiring low-flow supplemental oxygen)
Critical disease: patients required care in the intensive care unit and consisted of those in group 6 (requiring high-flow oxygen or noninvasive ventilation) or group 7 (requiring invasive mechanical ventilation) [1]
These groupings were based on an a priori hypothesis that antiviral treatment would demonstrate better efficacy earlier in the course of illness, when viral replication is suspected to be higher. Symptom duration was dichotomized at 5 days based on our hypothesis that this time point could differentiate changes in viral load given prior reports of improved efficacy with early remdesivir use [1].
Details for our a priori hypotheses regarding viral and inflammatory biomarkers were defined in a statistical analysis plan (supplementary material). Serum, plasma, and upper respiratory tract specimens were collected at designated visits during hospitalization, including study days 1 (baseline), 3, and 5 (ie, prior to and at 2 and 4 days after treatment initiation, respectively). All laboratory assays were performed centrally.
Measurement of SARS-CoV-2 RNA and Nucleocapsid
Oropharyngeal swabs were preferentially collected over nasopharyngeal swabs when available; both were collectively categorized as upper respiratory specimens. For the primary analysis, samples collected from baseline through study day 5 were included. Upper respiratory and plasma viral RNA was measured with the Roche cobas SARS-CoV-2 RT-PCR assay on a Roche cobas 6800 instrument via the SeraCare AccuPlex SARS-CoV-2 Verification Panel as the exogenous standards and calibrators. Validation of the quantitative viral load assay in plasma was performed with spike-in of clinical materials, standards, and calibrators into reconstituted commercial plasma (Lyphochek 2211-08; Bio-Rad). For quantitative calculations, similar to prior studies [9], values below the assay limit of quantification (LOQ) of 62 copies/mL (1.79 log10 copies/mL) were imputed as half the LOQ (31 copies/mL or 1.49 log10 copies/mL) when RNA was detected but not quantifiable and one-quarter the LOQ (15.5 copies/mL or 1.19 log10 copies/mL) when RNA was not detected.
SARS-CoV-2 nucleocapsid levels were determined in 90 µL of serum in duplicate with a quantitative Simoa assay (Quanterix) at baseline through study day 5. Simoa (single-molecule array) is a novel and ultrasensitive microparticle-based enzyme-linked immunosorbent assay platform capable of precise quantification of specific protein and/or antibody targets within the femtomolar range of concentrations [10]. The lower level of quantification for the SARS-CoV-2 nucleocapsid assay is 3 pg/mL. For quantitative calculations, values below that level were imputed as half this value (1.5 pg/mL).
Measurement of Immunologic Biomarkers
Baseline anti–SARS-CoV-2 spike IgG antibody levels were measured with 10 µL of serum in duplicate via a semiquantitative Simoa assay (Quanterix); seropositivity was defined as patients having measurements above the assay cutoff of 770 ng/mL. IL-6 and CRP were measured from serum in duplicate via electrochemiluminescence (Meso Scale Discovery) per the manufacturer's instructions.
Clinical and Virologic End Points
The primary clinical outcome of ACTT-1 was time to recovery, defined as the point at which a patient met criteria for improvement to ordinal scale 1 (not hospitalized, without limitation of activities), 2 (not hospitalized, with limitation of activities but not requiring new supplemental oxygen), or 3 (hospitalized but no longer requiring ongoing medical care; ie, still hospitalized for infection control or placement reasons). Patients who did not recover failed to progress to ordinal score 3, 2, or 1 over the course of the study. Mortality through day 28 was a secondary clinical end point. Virologic end points were evaluated as the daily rate of change in viral load and as changes in viral load trajectories.
Statistical Analyses
A statistical analysis plan (supplementary material) was written prior to analysis of the unblinded data, and it was amended once to add prespecified analyses for plasma viral RNA and anti–SARS-CoV-2 antibody measurements. Clinical and demographic characteristics of study participants were summarized with descriptive statistics. Differences in distributions of viral assays were compared with the Mann-Whitney U test. Correlations were estimated by Pearson correlation coefficient. Log-rank tests assessed differences in survival and recovery times through methods applied in the original study [1]. Kaplan-Meier curves are shown. Time-to-event analyses were supplemented by proportional hazards models and the Fine-Gray method for competing risk for recovery, with death as a competing risk [11]. To evaluate longitudinal changes in biomarkers, linear regression models were fit to all available values for each patient to estimate the patient-specific daily rate of decline as a derived variable; sensitivity analyses per linear mixed effects models were estimated when appropriate. Additionally, slopes were dichotomized to determine viral load trajectories, defined as increasing (persistently positive) for positive slopes (or zero slopes when a baseline value was above the LOQ) or decreasing (persistently negative) for negative slopes (or zero slopes when a baseline value was below the LOQ). Primary analyses adjusted for potential baseline imbalances in measurements of viral load (ie, prior to receipt of remdesivir or placebo) by inclusion of baseline viral load as a covariate in a linear regression model. Tests of proportions were conducted with the chi-square test. Wald confidence intervals were used for all primary analyses. All P values were 2-sided. All analyses were conducted in R statistical software and confirmed independently by 2 biostatisticians. Figures were produced with R software (ggplot2 and forester packages [12]) and Prism (GraphPad).
RESULTS
Study Design and Participants
We tested a substudy cohort of 642 ACTT-1 participants who provided specimens at baseline and at least 1 subsequent time point within the first 6 days following randomization (Supplementary Figure 1). Demographic and clinical characteristics were generally well balanced between the participants in the placebo arm (n = 319) and remdesivir arm (n = 323), as well as between the overall cohort and original ACTT-1 study population (Table 1, Supplementary Table 1). The median age was 59 years; the median time from symptom onset was 9 days; and mortality was 9% vs 11% in the remdesivir and placebo arms, respectively. Biomarker analyses were stratified by disease severity, comparing patients with moderate/severe disease (ie, patients considered noncritically ill who required no or minimal oxygen supplementation) with those who presented with critical disease (ie, patients considered critically ill who required high-flow oxygen, mechanical ventilation, or extracorporeal membranous oxygenation).
Table 1.
Demographic and Clinical Characteristics of the Study Population at Baseline
| Placebo | Remdesivir | |||
|---|---|---|---|---|
| Included (n = 319) | Total (n = 516) | Included (n = 323) | Total (n = 532) | |
| Age, y, median (IQR) | 59 (47-68) | 60 (49-70) | 59 (48-69) | 59 (49-69) |
| Male sex | 205 (64.26) | 328 (63.57) | 210 (65.02) | 347 (65.23) |
| Race and ethnicity | ||||
 Asian Asian | 30 (9.40) | 56 (10.85) | 42 (13.00) | 79 (14.85) |
 Black or African American Black or African American | 84 (26.33) | 114 (22.09) | 73 (22.60) | 105 (19.74) |
 White White | 167 (52.35) | 286 (55.43) | 168 (52.01) | 273 (51.32) |
 Ethnicity: Hispanic or Latino Ethnicity: Hispanic or Latino | 69 (21.63) | 114 (22.09) | 89 (27.55) | 132 (24.81) |
| Clinical characteristics | ||||
 Any comorbidities Any comorbidities | 255 (79.94) | 417 (80.81) | 271 (83.90) | 435 (81.77) |
 Days of symptoms, median (IQR) Days of symptoms, median (IQR) | 9 (7-13) | 9 (7-13) | 9 (6-12) | 9 (6-12) |
| Baseline ordinal scale a | ||||
 4 4 | 38 (11.91) | 63 (12.21) | 39 (12.07) | 75 (14.10) |
 5 5 | 114 (35.74) | 202 (39.15) | 148 (45.82) | 231 (43.42) |
 6 6 | 56 (17.55) | 98 (18.99) | 42 (13.00) | 94 (17.67) |
 7 7 | 111 (34.80) | 153 (29.65) | 94 (29.10) | 132 (24.81) |
| Disease severity subgroups b | ||||
 Moderate/severe Moderate/severe | 152 (47.65) | 265 (51.36) | 187 (57.89) | 306 (57.52) |
 Critical Critical | 167 (52.35) | 251 (48.64) | 136 (42.11) | 226 (42.48) |
Patients in our primary biomarker analysis cohort (Included) vs all patients from the original Adaptive COVID-19 Treatment Trial 1 (Total). Data are presented as No. (%) unless noted otherwise.
aNational Institute of Allergy and Infectious Diseases categorization of COVID-19 disease severity.
bModerate/severe disease is a combined subgroup of ordinal scales 4 and 5. Critical disease is a combined subgroup of ordinal scales 6 and 7.
Comparison of Baseline Measures of Viral Load and Inflammatory Biomarkers
The majority of participants had quantifiable viral loads at study entry irrespective of assay used: 87.9% (564/642) for serum viral nucleocapsid, 85.2% (547/642) for upper respiratory tract viral RNA, and 56.9% (365/642) for plasma viral RNA. Patients with critical disease had higher baseline plasma and upper respiratory tract viral RNA levels as compared with patients with moderate/severe disease: plasma viral RNA median, 2.40 vs 1.49 log10 copies/mL, P < .001; upper respiratory tract viral RNA median, 3.64 vs 3.26 log10 copies/mL, P = .002 (Figure 1AA, Supplementary Tables 2 and 3). Patients with critical disease also presented later in illness with modestly protracted symptoms (mean days, 10.5 vs 9.3; P = .002). Viral loads were lower among patients reporting longer durations of symptoms prior to enrollment and, on average, decreased over the early period of observation among placebo recipients (Supplementary Figure 2).
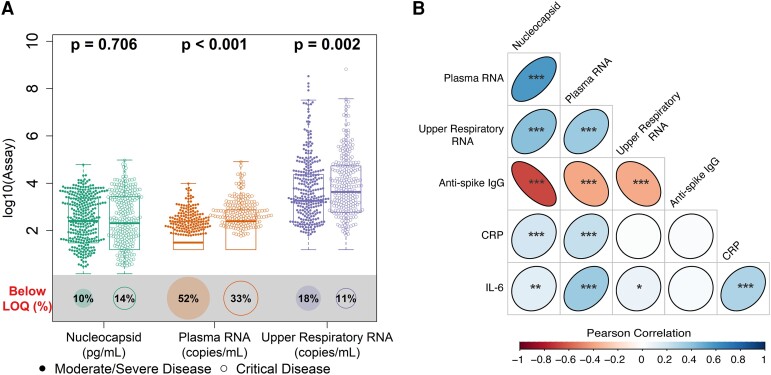
Distribution of baseline viral loads by disease severity and correlation with other biomarkers. A, Distribution of baseline viral loads by assay categorized by disease severity. Box plots indicate the median (line) and IQR (box). Wilcoxon P values assess differences in medians between disease severity groups, and proportions of samples are shown below each assay's limit of quantification (LOQ; illustrated by size of bottom circle). RNA values below the assay LOQ of 1.79 log10 copies/mL were set as half the LOQ (1.49 log10 copies/mL) when measurements were above the lower limit of detection and one-quarter the LOQ (1.19 log10 copies/mL) when RNA was not detected; nucleocapsid antigen values below the assay LOQ of 3 pg/mL were set as half the LOQ (1.5 pg/mL). B, Matrix heat map of Pearson correlations between baseline biomarker levels among all patients in the primary cohort. *P < .05. **P < .01. ***P < .001. Coefficient estimates are provided in Supplementary Table 4. CRP, C-reactive protein; IL-6, interleukin 6.
Plasma viral RNA and serum nucleocapsid levels correlated with inflammatory biomarkers (Figure 1BB, Supplementary Table 4); modest but stronger correlations were seen between these blood viral biomarkers and CRP (Pearson correlation, r = 0.237 for plasma RNA and r = 0.189 for nucleocapsid; both P < .001) and IL-6 (r = 0.361 for plasma RNA and r = 0.122 for nucleocapsid; P < .001 and P = .002, respectively), in contrast to upper respiratory tract viral RNA. Anti–SARS-CoV-2 spike antibodies were detected in 57.3% patients, and antibody levels negatively correlated with all 3 measures of viral load.
Higher Baseline Viral Loads Were Associated With Worse Clinical Outcomes
Patients with higher baseline viral loads were less likely to recover or survive (Figure 2, Supplementary Table 5). Cox proportional hazard models showed that higher baseline viral loads were associated with an increased risk of mortality (plasma viral RNA: hazard ratio (HR), 4.44 [95% CI, 1.41–13.96]; upper respiratory tract viral RNA: HR, 7.80 [95% CI, 1.70–35.82]; serum viral nucleocapsid: HR, 3.92 [95% CI, 1.11–13.86]) and lower likelihood of recovery (plasma viral RNA: recovery rate ratio [RRR], 0.42 [95% CI, .33–.53]; upper respiratory tract viral RNA: RRR, 0.51 [95% CI, .40–.64]; serum viral nucleocapsid: RRR, 0.53 [95% CI, .42–.66]) among patients with moderate/severe disease (Table 2). Higher baseline plasma viral RNA levels also correlated with a lower likelihood of recovery among patients who were critically ill (RRR, 0.582 [95% CI, .44–.78]), a finding not observed with other measures of viral load. Elevated IL-6 levels were similarly associated with a lower likelihood of survival and recovery across all categories of disease severity.
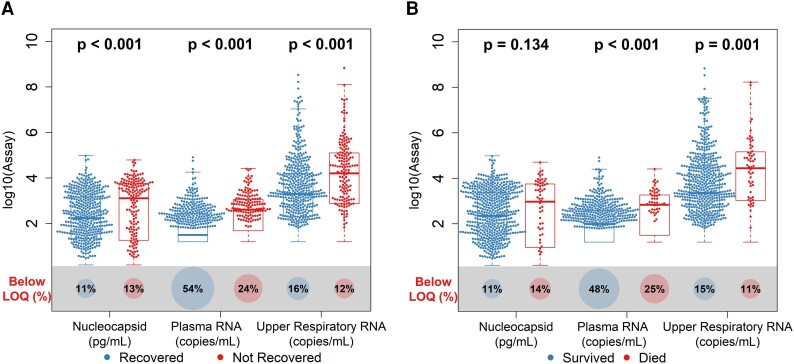
Distribution of baseline viral loads by assay according to the clinical outcome: A, recovery; B, mortality. Box plots indicate the median (line) and IQR (box). Wilcoxon P values assess differences in medians between disease severity groups, and proportions of samples are shown below each assay's limit of quantification (LOQ; illustrated by size of bottom circle). RNA values below the assay LOQ of 1.79 log10 copies/mL were set as half the LOQ (1.49 log10 copies/mL) when measurements were above the lower limit of detection and one-quarter the LOQ (1.19 log10 copies/mL) when RNA was not detected; nucleocapsid antigen values below the assay LOQ of 3 pg/mL were set as half the LOQ (1.5 pg/mL).
Table 2.
Likelihood of Recovery or Death Based on Baseline Viral Load or Inflammatory Biomarker
| Recovery | Mortality | |||||
|---|---|---|---|---|---|---|
| Disease Severity: Biomarker | RRR | 95% CI | P Value | HR | 95% CI | P Value |
| Moderate/severe | ||||||
| Nucleocapsid, log10 pg/mL | 0.53 | .42–.66 | <.001 | 3.92 | 1.11–13.86 | .034 |
| RNA, log10 copies/mL | ||||||
 Plasma Plasma | 0.41 | .33–.53 | <.001 | 4.44 | 1.41–13.96 | .011 |
 Upper respiratory Upper respiratory | 0.50 | .40–.64 | <.001 | 7.80 | 1.70–35.82 | .008 |
| Inflammatory Biomarkers, log10 pg/mL | ||||||
 CRP CRP | 0.83 | .67–1.04 | .106 | 1.79 | .65–4.95 | .259 |
 IL-6 IL-6 | 0.53 | .42–.67 | <.001 | 3.68 | 1.32–10.29 | .013 |
| Critical | ||||||
| Nucleocapsid, log10 pg/mL | 0.77 | .58–1.03 | .078 | 1.07 | .61–1.87 | .805 |
| RNA, log10 copies/mL | ||||||
 Plasma Plasma | 0.58 | .44–.78 | <.001 | 1.53 | .83–2.85 | .175 |
 Upper respiratory Upper respiratory | 0.84 | .63–1.12 | .239 | 1.12 | .64–1.98 | .687 |
| Inflammatory Biomarkers, log10 pg/mL | ||||||
 CRP CRP | 0.74 | .55–1.00 | .050 | 1.75 | .93–3.29 | .085 |
 IL-6 IL-6 | 0.39 | .28–.53 | <.001 | 3.55 | 1.41–8.96 | .007 |
Cox model estimates of HR for mortality and RRR based on baseline elevation of viral load or inflammatory biomarker (dichotomized at baseline median) when adjusted for treatment arm.
Abbreviations: CRP, C-reactive protein; HR, hazard ratio; IL-6, interleukin 6; RRR, recovery rate ratio
Patients With Elevated Blood Viral Loads at Baseline Recovered Faster With Remdesivir
Among participants with moderate/severe disease, those with higher baseline serum viral nucleocapsid levels recovered faster when treated with remdesivir vs those who received placebo (RRR, 1.95 vs 1.04 for levels above and below the cohort median of 2.39 log10 pg/mL; for interaction, P = .01; Figure 3AA). A similar trend was seen for patients with higher baseline plasma viral RNA levels (RRR, 1.95 vs 1.24 for levels above and below the cohort median of 2.09 log10 copies/mL; for interaction, P = .06). Remdesivir also shortened the time to recovery when given earlier in illness. Among patients with moderate/severe disease who were treated within 5 days of initial symptoms, 93% vs 54% recovered by day 28 in the remdesivir and placebo arms, respectively (RRR, 3.0; 95% CI 1.70–5.28), as opposed to 94% vs 88% among those treated 5 days after symptom onset (RRR, 1.27; 95% CI, .99–1.63; for interaction, P = .006; Supplementary Table 6). Similar findings were observed if symptom duration was analyzed as a continuous variable (Supplementary Table 7). There was no differential treatment effect for remdesivir on mortality across subgroups of viral load (Figure 3BB) or symptom duration. Additionally, although patients with antispike antibodies had improved clinical outcomes when compared with individuals who were seronegative, clinical benefit from remdesivir did not vary by baseline antibody status.
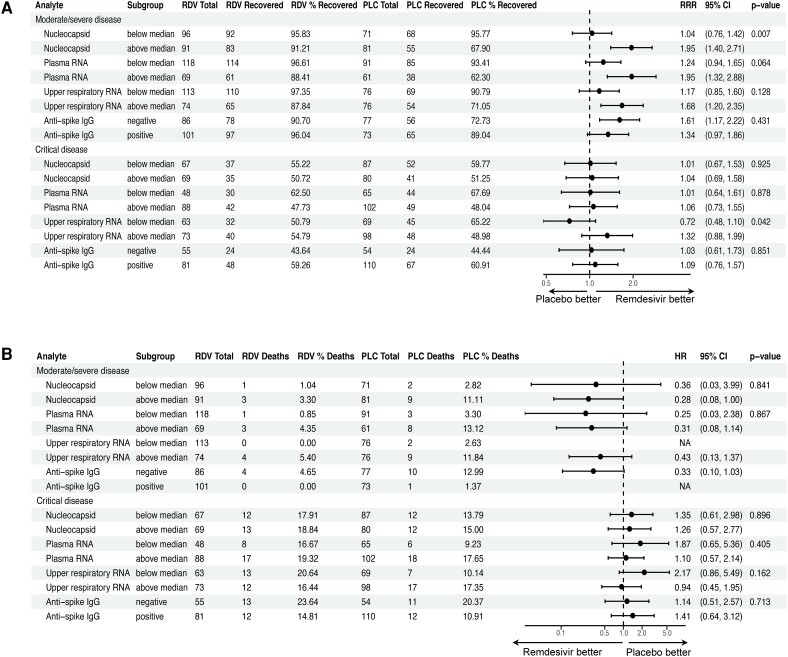
Impact of remdesivir on clinical outcomes according to viral load subgroups. Forest plots illustrate subgroup analyses of Cox models to evaluate for a differential effect of remdesivir on (A) recovery and (B) mortality based on baseline viral load or presence of antispike antibodies. Viral load measurements were dichotomized at median values per assay: 2.39 log10 pg/mL (245 pg/mL) for serum nucleocapsid, 2.09 log10 copies/mL (123 copies/mL) for plasma RNA, and 3.41 log10 copies/mL (2570 copies/mL) for upper respiratory RNA. P values for treatment interaction are shown. HR, hazard ratio; PLC, placebo; RDV, remdesivir; RRR, recovery rate ratio.
Remdesivir Accelerated the Reduction of Viral Load in Blood
Relative to placebo, participants with moderate/severe disease who received remdesivir had larger averaged daily declines in levels of serum viral nucleocapsid (difference, −0.062 log10 pg/mL/d; P = .003) and plasma viral RNA (difference, −0.040 log10 copies/mL/d; P = .004; Figure 4AA, Supplementary Table 8); sensitivity analyses based on linear mixed effects models demonstrated comparable results (Supplementary Table 9). Reductions in blood viral RNA and antigen were greater among patients treated within 5 days of initial symptoms (Supplementary Table 10). Remdesivir similarly reduced the likelihood of an increasing viral load trajectory in blood during the first 5 days of treatment among patients with moderate/severe disease: the relative risk of an increasing viral load comparing remdesivir against placebo was 0.068 for serum viral nucleocapsid (P < .001) and 0.366 for plasma viral RNA (P = .006; Figure 4BB). In contrast, no effect of remdesivir was observed on the upper respiratory tract viral RNA levels among patients with moderate/severe disease. No significant changes in viral loads were observed in patients who were critically ill.
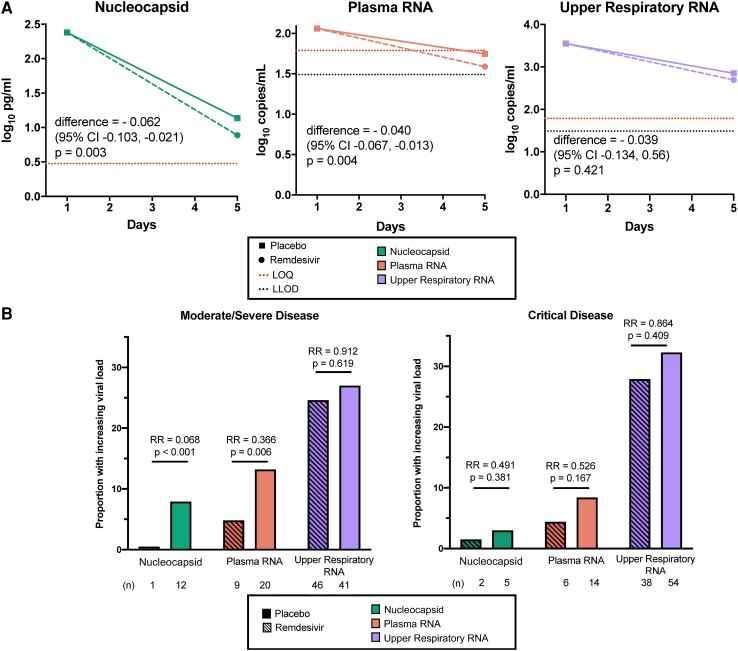
Virologic efficacy of remdesivir vs placebo. A, Differences in mean daily rate of change of viral load among patients with moderate/severe disease by treatment arm. P values are shown for differences in adjusted daily rate of change when normalized to baseline level. For calculations, RNA values below the assay limit of quantification (LOQ) of 1.79 log10 copies/mL were set as half the LOQ (1.49 log10 copies/mL) when measurements were above the lower limit of detection (LLOD) and one-quarter the LOQ (1.19 log10 copies/mL) when RNA was not detected. B, Bar plot of patients with increasing or persistently elevated viral loads measured through 5 days of treatment. Relative risk (RR) of an increasing viral load comparing remdesivir and placebo recipients are shown with corresponding P values.
Reductions in Viral Load Were Associated With Improved Clinical Outcomes
Patients with declines in plasma viral RNA or serum viral nucleocapsid over the first 5 days had improved times to clinical recovery as compared with individuals without declines (Figure 5, Supplementary Table 11). This association was seen in patients with moderate/severe disease (P = .001 for plasma viral RNA, P = .018 for serum viral nucleocapsid) and those with critical disease (P = .004 for plasma viral RNA, P = .013 for serum viral nucleocapsid). Notably, no patient who was critically ill and exhibited increasing serum levels of viral nucleocapsid recovered. Decreasing trajectories of plasma viral RNA and serum viral nucleocapsid were similarly associated with reduced mortality across both disease severity subgroups (Supplementary Figure 3, Supplementary Table 12). In contrast, the trajectories of upper respiratory viral RNA had weaker associations with clinical outcomes in patients with moderate/severe disease and failed to show any association with outcomes among patients with critical illness.
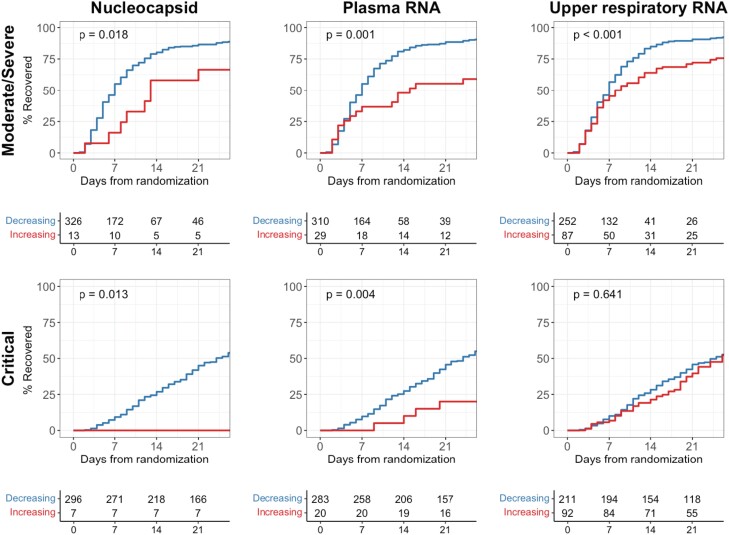
Time to recovery comparing patients with increasing vs decreasing viral loads. Kaplan-Meier curve analyses compare patients with decreasing viral loads (blue) and those with increasing or persistently elevated viral loads (red) through the first 5 days of treatment. P values represent test of the log-rank statistic.
DISCUSSION
In this analysis of a large-scale, phase 3, randomized clinical trial, we hypothesized that accelerated reductions in peripheral SARS-CoV-2 viral load would correlate with improved patient outcomes. By assessing 3 methods to quantify viral load, we demonstrated that the 2 blood measurements (viral RNA and nucleocapsid antigen) more consistently associated with the clinical risk of severe disease outcomes and the benefits of antiviral therapy when compared with upper respiratory RNA.
ACTT-1 was the first randomized placebo-controlled trial to demonstrate the efficacy of remdesivir in improving recovery in patients hospitalized with COVID-19. Since its completion, several other trials have corroborated the clinical efficacy of remdesivir in similar and disparate populations [13, 14]. However, because identifying the specific patients within this broader population who are likely to benefit from treatment has continued to prove challenging, we hypothesized that augmenting commonly used categorizations of disease severity with laboratory assessments might offer new insights. We previously showed that simple hematologic parameters in combination with baseline disease severity better predicted risk of disease progression [15]. Here, we report that SARS-CoV-2 biomarkers in the peripheral blood may also offer new insights. In particular, the associated benefits of remdesivir were most pronounced among patients who were not critically ill with elevated serum nucleocapsid antigen levels (>2.39 log10 pg/mL) or shorter durations of symptoms, with a similar favorable trend seen in patients with higher levels of plasma viral RNA (>2.09 log10 copies/mL). Moreover, early trajectories of both these viral analytes correlated with clinical outcomes. These findings support the hypothesis that antivirals are more effective when used earlier in illness and that unmitigated viral replication (or an inability to decrease peripheral viral loads) is associated with worse prognoses.
Although upper respiratory specimens are commonly used to measure viral loads in clinical and research settings, measurements in peripheral blood may offer several advantages. In our study, plasma viral RNA and serum nucleocapsid assays more consistently associated with mortality and recovery across a wider range of disease severity when compared with upper respiratory RNA measurements. Plasma RNAemia as a correlate of worse disease has been reported with other respiratory viral infections [16, 17], and several studies have similarly found it to be predictive of critical illness and poorer outcomes in COVID-19 [18–22]. Here, we demonstrate that SARS-CoV-2 RNAemia may serve as a useful prognostic biomarker among patients without critical illness, although sensitivity may decline in mild or early illness where plasma RNA levels may be low or absent [23]. In contrast, nucleocapsid antigen may be a more sensitive marker earlier in infection [3, 24–29], and in our study it was present in a considerably higher proportion of patients when compared with plasma viral RNA (87.9% vs 56.9%), potentially pointing to the added value of measuring circulating antigen levels concurrently with plasma viral RNA. In a similar cohort of patients, plasma antigenemia in ranges comparable to those that we observed correlated with poorer outcomes, including worse pulmonary disease and prolonged hospitalizations [5]. Peripheral blood biomarkers may provide new insights into COVID-19 pathogenesis: IL-6 and CRP correlated better with viral RNA and antigen measurements in blood than with viral RNA levels in the upper respiratory tract, suggesting that the presence and magnitude of viremia better reflect the immune dysregulation associated with worse patient outcomes [30–32].
To the best of our knowledge, ours is one of the first studies to demonstrate the systemic antiviral efficacy of remdesivir as compared with placebo in the setting of a randomized controlled trial. We observed that 5 days of remdesivir accelerated declines and improved trajectories of viral biomarkers in blood but not in the upper respiratory tract. With rare exception [33], multiple prospective clinical trials of remdesivir similarly did not find any antiviral impact of remdesivir on upper respiratory tract viral RNA levels [34–37], supporting the assertion that this specimen type may be less sensitive for in vivo monitoring of antiviral therapies and/or that the antiviral effect of remdesivir may be relatively modest in hospitalized patients, particularly those treated later in illness. Nonetheless, the concordant reductions in peripheral viral RNA and antigen levels that we observed suggest possible detection of an in vivo inhibition of viral replication [27, 38, 39]. These findings may hint at an underlying rationale, such as reduced concentrations of remdesivir in lung tissue [40] and/or spillover from primary sites of replication, wherein the peripheral blood offers a better compartment in which to measure treatment responses in real-world settings. Note that ACTT-1 was conducted prior to recommendations for the use of immunomodulating therapies to temper hyperinflammatory responses in severe COVID-19 disease. As such, it remains to be seen if the antiviral effects of remdesivir may even be greater among patients receiving immunosuppressive therapies (eg, corticosteroids), which may prolong the duration of viral replication and shedding [41].
Our study has some limitations. First, our analyses were predefined but exploratory and not adjusted for multiple comparisons. Second, some patients did not contribute samples, which may have been missing at random due in part to the overwhelming burden placed on the medical system at the time when ACTT-1 was conducted. However, the proportion of recoveries and ventilator-free survivals captured in our study were comparable to those observed in the original cohort (Supplementary Table 1), suggesting that any missingness was not largely driven by outcomes. Additionally, sensitivity analyses that included all patients with a baseline viral load (Supplementary Tables 3 and 5) or assessed for cohort selection bias (Supplementary Tables 13 and 14) supported our primary findings. Third, restricting enrollment to hospitalized patients may have selected for overall lower baseline viral loads, potentially underestimating the strength of associations with clinical outcomes, particularly among patients who presented later in illness (Supplementary Figure 2A). Fourth, quantitative polymerase chain reaction and Simoa assays may not be available in all settings and would benefit from further study to validate diagnostic cutoffs and better determine their routine clinical applicability. Last, as ACTT-1 enrolled unvaccinated patients during the first wave of the pandemic, our findings may vary by factors such as immune status and SARS-CoV-2 variant. Yet, growing evidence suggests that plasma viral RNA and nucleocapsid antigen correlate with clinical parameters across more recent viral variants [5, 42].
In summary, in this analysis of a randomized clinical trial, we report that SARS-CoV-2 plasma RNA and nucleocapsid antigen have potential as surrogate biomarkers for evaluating the benefit of antiviral therapy and warrant further study. As remdesivir is one of the few available treatments that retains activity against circulating variants of concern [43–45], an understanding of its real-world antiviral effects and their ability to inform clinical outcomes remains important. Our study may also offer insights into strategies that could be applied to the evaluation of other antiviral drug candidates.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Contributor Information
Kanal Singh, National Institute of Allergy and Infectious Diseases, Bethesda.
Kevin Rubenstein, Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research,
Viviane Callier, Clinical Monitoring Research Program Directorate, Frederick National Laboratory for Cancer Research,
Katy Shaw-Saliba, National Institute of Allergy and Infectious Diseases, Bethesda.
Adam Rupert, National Laboratory for Cancer Research, Frederick, Maryland.
Robin Dewar, National Laboratory for Cancer Research, Frederick, Maryland.
Sylvain Laverdure, National Laboratory for Cancer Research, Frederick, Maryland.
Helene Highbarger, National Laboratory for Cancer Research, Frederick, Maryland.
Perrine Lallemand, National Laboratory for Cancer Research, Frederick, Maryland.
Meei-Li Huang, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington,
Keith R Jerome, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington, Fred Hutchinson Cancer Center, Seattle, Washington.
Reigran Sampoleo, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington,
Margaret G Mills, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington,
Alexander L Greninger, Department of Laboratory Medicine and Pathology, School of Medicine, University of Washington,
Kavita Juneja, Gilead Sciences, Inc, Foster City, California.
Danielle Porter, Gilead Sciences, Inc, Foster City, California.
Constance A Benson, University of California San Diego,
Walla Dempsey, National Institute of Allergy and Infectious Diseases, Bethesda.
Hana M El Sahly, Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, Texas.
Chris Focht, The Emmes Company, Rockville, Maryland.
Nikolaus Jilg, Massachusetts General Hospital and Brigham and Women's Hospital, Harvard Medical School, Boston.
Catharine I Paules, Division of Infectious Diseases, Milton S. Hershey Medical Center, Penn State Health, Hershey, Pennsylvania.
Rekha R Rapaka, Center for Vaccine Development and Global Health, School of Medicine, University of Maryland, Baltimore.
Timothy M Uyeki, Influenza Division, Centers for Disease Control and Prevention, Atlanta, Georgia.
H Clifford Lane, National Institute of Allergy and Infectious Diseases, Bethesda.
John Beigel, National Institute of Allergy and Infectious Diseases, Bethesda.
Lori E Dodd, National Institute of Allergy and Infectious Diseases, Bethesda.
the Adaptive COVID-19 Treatment Trial (ACTT-1) Study Group Members
:
Notes
Author contributions. K. S., K. R., V. C., and L. E. D. designed the study and analyzed the data. C. A. B., W. D., H. M. E. S., C. F., N. J., C. I. P., R. R. R., T. M. U., H. C. L., J. B., and L. E. D. conducted the clinical trial. K. S.-S., A. R., R. D., S. L., H. H., P. L., M.-L. H., K. R. J., R. S., M. G. M., and A. L. G. conducted the biomarker experiments. K. S., K. R., V. C., A. L. G., K. J., D. P., C. A. B., W. D., H. M. E. S., C. F., N. J., C. I. P., R. R. R., T. M. U., H. C. L., J. B., and L. E. D. wrote the manuscript.
Data and materials availability. All data associated with this study are present in the article or supplementary materials.
Disclaimer. The views expressed are those of the authors and do not necessarily reflect the official position of the National Institutes of Health or the Centers for Disease Control and Prevention.
Financial support . This analysis used data from ACTT-1 (10.1056/nejmoa2007764). ACTT-1 was sponsored and primarily funded by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH). This trial has been funded in part with federal funds from the NIAID and the National Cancer Institute of the NIH (contract HHSN261200800001E 75N910D00024, task order 75N91019F00130/75N91020F00010) and by the Defense Health Program, Department of Defense. This trial has been supported in part by the NIAID of the NIH under awards UM1AI148684, UM1AI148576, UM1AI148573, UM1AI148575, UM1AI148452, UM1AI148685, UM1AI148450, and UM1AI148689. The trial has also been funded in part by the governments of Denmark, Japan, Mexico, and Singapore. The trial site in South Korea received funding from the Seoul National University Hospital. Support for the London International Coordinating Centre was also provided by the United Kingdom Medical Research Council (MRC_UU_12023/23). Collaborating investigators involved with collection of data during the ACTT-1 trial are noted in the supplementary materials.
References
Articles from The Journal of Infectious Diseases are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/infdis/jiae198
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/jid/advance-article-pdf/doi/10.1093/infdis/jiae198/57321931/jiae198.pdf
Citations & impact
Impact metrics
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/162848645
Article citations
SARS-CoV-2 Plasma Antibody and Nucleocapsid Antigen Status Predict Outcomes in Outpatients With COVID-19.
Clin Infect Dis, 79(4):920-927, 01 Oct 2024
Cited by: 0 articles | PMID: 39018444
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Responses to a Neutralizing Monoclonal Antibody for Hospitalized Patients With COVID-19 According to Baseline Antibody and Antigen Levels : A Randomized Controlled Trial.
Ann Intern Med, 175(2):234-243, 21 Dec 2021
Cited by: 42 articles | PMID: 34928698 | PMCID: PMC9334931
Evaluation of the Effects of Remdesivir and Hydroxychloroquine on Viral Clearance in COVID-19 : A Randomized Trial.
Ann Intern Med, 174(9):1261-1269, 13 Jul 2021
Cited by: 66 articles | PMID: 34251903 | PMCID: PMC8279143
Beneficial effect of combinational methylprednisolone and remdesivir in hamster model of SARS-CoV-2 infection.
Emerg Microbes Infect, 10(1):291-304, 01 Dec 2021
Cited by: 34 articles | PMID: 33538646 | PMCID: PMC7919885
Remdesivir in COVID-19: A critical review of pharmacology, pre-clinical and clinical studies.
Diabetes Metab Syndr, 14(4):641-648, 12 May 2020
Cited by: 86 articles | PMID: 32428865 | PMCID: PMC7214279
Review Free full text in Europe PMC
Funding
Funders who supported this work.
CSRD VA (1)
Grant ID: I01 CX002688
London International Coordinating Centre
Medical Research Council (1)
Grant ID: MRC_UU_12023/23
NCI NIH HHS (1)
Grant ID: HHSN261200800001E 75N910D00024
NIAID NIH HHS (5)
Grant ID: UM1 AI148689
Grant ID: UM1 AI148576
Grant ID: K08 AI143923
Grant ID: UM1 AI148450
Grant ID: UM1 AI148685
NIH HHS
National Cancer Institute (10)
Grant ID: UM1AI148685
Grant ID: UM1AI148452
Grant ID: UM1AI148573
Grant ID: 75N91019F00130/75N91020F00010
Grant ID: UM1AI148450
Grant ID: UM1AI148689
Grant ID: HHSN261200800001E 75N910D00024
Grant ID: UM1AI148575
Grant ID: UM1AI148576
Grant ID: UM1AI148684





