Abstract
Importance
The goal of preclinical Alzheimer disease (AD) clinical trials is to move diagnosis and treatment to presymptomatic stages, which will require biomarker testing and disclosure.Objective
To assess the short-term psychological outcomes of disclosing amyloid positron emission tomography results to older adults who did not have cognitive impairment.Design, setting, and participants
This observational study included participants who were screening for a multisite randomized clinical trial that began on February 28, 2014, and is anticipated to be completed in 2022. Participants aged 65 to 85 years who had no known cognitive impairments underwent an amyloid positron emission tomography scan and learned their result from an investigator who used a protocol-specified process that included prescan education and psychological assessments. This report compares participants with elevated amyloid levels with at least 1 available outcome measure with participants who did not have elevated amyloid levels who enrolled in an observational cohort study and received further evaluations. Data were collected from April 2014 to December 2017 and analyzed from March 2019 to October 2019.Exposures
A personal biomarker result described as either an elevated or not elevated amyloid level.Main outcomes and measures
To assess the immediate and short-term psychological outcome of disclosure, the following validated measures were used: the Geriatric Depression Scale, the state items from the State-Trait Anxiety Inventory, and the Columbia Suicide Severity Rating Scale, as well as the Concerns About AD Scale and the Future Time Perspective Scale to assess changes in participants' perceived risk for AD and perceived remaining life span, respectively.Results
A total of 1167 participants with elevated amyloid levels and 538 participants with not elevated amyloid levels were included. Participants had a mean (SD) age of 71.5 (4.7) years, 1025 (60.1%) were women, and most were white (1611 [94.5%]) and non-Latino (1638 [96.1%]). Compared with participants who learned that they had a not elevated amyloid result, individuals who learned of an elevated amyloid result were no more likely to experience short-term increases in depression (mean [SD] change in the Geriatric Depression Scale score, 0.02 [1.3] vs 0.04 [1.3]; P = .90), anxiety (mean [SD] change in State-Trait Anxiety Inventory score, -0.02 [3.2] vs -0.15 [3.0]; P = .65), or suicidality (mean [SD] change in the Columbia Suicide Severity Rating Scale score, 0.0 [0.4] vs -0.01 [0.5]; P = .67). Participants with elevated amyloid levels had increased Concern About AD scores (raw change in scores: elevated amyloid group, 0.8 [3.9]; not elevated amyloid group, -0.4 [3.8]; P < .001). Participants with not elevated amyloid levels experienced a slight increase in Future Time Perspective score(mean [SD] score, 1.15 [7.4] points; P < .001); there was no change in time perspective among those receiving an elevated amyloid result (mean [SD] score, 0.33 [7.8] points).Conclusions and relevance
In this observational preclinical AD study, participants who learned they had elevated amyloid levels did not experience short-term negative psychological sequelae compared with persons who learned they did not have elevated amyloid levels.Free full text

Short-term Psychological Outcomes of Disclosing Amyloid Imaging Results to Research Participants Who Do Not Have Cognitive Impairment
Key Points
Question
Can Alzheimer disease biomarker results be safely shared with older research participants who do not have cognitive impairments?
Findings
In this observational study, disclosure of amyloid imaging results using a structured process in a clinical trial did not result in clinically meaningful short-term adverse psychological reactions but did change participants’ perceived risk of developing Alzheimer disease.
Meaning
In this study, in the short term, amyloid imaging results were safely disclosed to older adults who did not have cognitive impairment.
Abstract
Importance
The goal of preclinical Alzheimer disease (AD) clinical trials is to move diagnosis and treatment to presymptomatic stages, which will require biomarker testing and disclosure.
Objective
To assess the short-term psychological outcomes of disclosing amyloid positron emission tomography results to older adults who did not have cognitive impairment.
Design, Setting, and Participants
This observational study included participants who were screening for a multisite randomized clinical trial that began on February 28, 2014, and is anticipated to be completed in 2022. Participants aged 65 to 85 years who had no known cognitive impairments underwent an amyloid positron emission tomography scan and learned their result from an investigator who used a protocol-specified process that included prescan education and psychological assessments. This report compares participants with elevated amyloid levels with at least 1 available outcome measure with participants who did not have elevated amyloid levels who enrolled in an observational cohort study and received further evaluations. Data were collected from April 2014 to December 2017 and analyzed from March 2019 to October 2019.
Exposures
A personal biomarker result described as either an elevated or not elevated amyloid level.
Main Outcomes and Measures
To assess the immediate and short-term psychological outcome of disclosure, the following validated measures were used: the Geriatric Depression Scale, the state items from the State-Trait Anxiety Inventory, and the Columbia Suicide Severity Rating Scale, as well as the Concerns About AD Scale and the Future Time Perspective Scale to assess changes in participants’ perceived risk for AD and perceived remaining life span, respectively.
Results
A total of 1167 participants with elevated amyloid levels and 538 participants with not elevated amyloid levels were included. Participants had a mean (SD) age of 71.5 (4.7) years, 1025 (60.1%) were women, and most were white (1611 [94.5%]) and non-Latino (1638 [96.1%]). Compared with participants who learned that they had a not elevated amyloid result, individuals who learned of an elevated amyloid result were no more likely to experience short-term increases in depression (mean [SD] change in the Geriatric Depression Scale score, 0.02 [1.3] vs 0.04 [1.3]; P =
= .90), anxiety (mean [SD] change in State-Trait Anxiety Inventory score, –0.02 [3.2] vs –0.15 [3.0]; P
.90), anxiety (mean [SD] change in State-Trait Anxiety Inventory score, –0.02 [3.2] vs –0.15 [3.0]; P =
= .65), or suicidality (mean [SD] change in the Columbia Suicide Severity Rating Scale score, 0.0 [0.4] vs –0.01 [0.5]; P
.65), or suicidality (mean [SD] change in the Columbia Suicide Severity Rating Scale score, 0.0 [0.4] vs –0.01 [0.5]; P =
= .67). Participants with elevated amyloid levels had increased Concern About AD scores (raw change in scores: elevated amyloid group, 0.8 [3.9]; not elevated amyloid group, –0.4 [3.8]; P
.67). Participants with elevated amyloid levels had increased Concern About AD scores (raw change in scores: elevated amyloid group, 0.8 [3.9]; not elevated amyloid group, –0.4 [3.8]; P <
< .001). Participants with not elevated amyloid levels experienced a slight increase in Future Time Perspective score(mean [SD] score, 1.15 [7.4] points; P
.001). Participants with not elevated amyloid levels experienced a slight increase in Future Time Perspective score(mean [SD] score, 1.15 [7.4] points; P <
< .001); there was no change in time perspective among those receiving an elevated amyloid result (mean [SD] score, 0.33 [7.8] points).
.001); there was no change in time perspective among those receiving an elevated amyloid result (mean [SD] score, 0.33 [7.8] points).
Conclusions and Relevance
In this observational preclinical AD study, participants who learned they had elevated amyloid levels did not experience short-term negative psychological sequelae compared with persons who learned they did not have elevated amyloid levels.
Introduction
Alzheimer disease (AD) is a progressive neurodegenerative condition that results in mild cognitive impairment, dementia, and death.1,2,3 Alzheimer disease is diagnosed when patients demonstrate cognitive symptoms.4
Longitudinal studies using positron emission tomography (PET) and other biological markers (biomarkers) have revealed that AD pathology may be present in the brain for years before the onset of cognitive impairment.5,6,7 The stage of AD in which biomarkers are present but cognitive impairment is absent has been termed preclinical AD. Criteria for preclinical AD were proposed for use in research to advance development of AD therapies.8,9 Disclosure of biomarker results to older adults who do not have cognitive impairment is not currently recommended in clinical practice.10 Disclosure is justified in preclinical AD trials because of the need to ensure trial feasibility, because of the generalizability of these experiences to an eventual clinical practice, and to respect the self-determination of participants, who may wish to know their biomarker result before committing to take an investigational therapy.11,12
If successful, preclinical AD trials will transform the diagnosis and treatment of AD. Biomarker test results will indicate treatment strategies for individuals who do not have cognitive impairment. Preclinical AD trials are therefore testing 2 clinically important questions: the safety and efficacy of potential disease-delaying treatments and the safety and outcome of disclosing AD biomarker results to older adults who do not have cognitive impairment. Here, we examine the second question, the short-term psychological sequelae of disclosing amyloid biomarker results to older participants who do not have cognitive impairment and were screened for the Anti-Amyloid Treatment in Asymptomatic AD (A4) Study.13
Methods
The A4 Study Design and Visit Schedule
The A4 Study is a phase 3 registration trial (NCT02008357) of the monoclonal antibody against monomeric amyloid β, solanezumab, in participants meeting criteria for preclinical AD. Written informed consent was provided by participants prior to any research activities being performed in the A4 study. Institutional review board approval was secured at the participating sites.
The design and preliminary biomarker results from the A4 screening process have been reported elsewhere.14 Here, we focus specifically on the disclosure of amyloid PET imaging results to participants, which occurred across screening and baseline visits (Figure 1). At visit 1, participants provided self-reported demographic characteristics, such as age, sex, education, family history of dementia, and the Cognitive Function Instrument (CFI),15 a subjective cognitive function scale. Participants completed predisclosure outcome measures, including the 15-item Geriatric Depression Scale (GDS; range, 0-15 points),16 the 6 state items of the State-Trait Anxiety Scale (STAI; each item was scored on a 4-point Likert scale, from not at all [1] to very much [4]; range, 6-24 points),17,18 and the Columbia Suicide Severity Rating Scale (CSSRS).19 The CSSRS summary score was based on the highest level of suicidality reported (range, 0-9 points).20 Additional instruments collected at visit 1 included an adapted Concerns About AD Scale, in which participants indicate their level of agreement (on a Likert scale from strongly disagree [1] to strongly agree [5]; range, 6-30 points) with 6 statements about their perceived probability of developing AD (eg, “I believe I will someday develop Alzheimer’s disease dementia”) and their level of concern for that outcome (eg, “My concern about developing Alzheimer’s disease dementia is greater than my concern about other medical problems”).21 The 10-item Future Time Perspective Scale assesses agreement (from very untrue [1] to very true [7]; range, 10-70 points) with perceptions of one’s future (eg, “I have the sense that time is running out”).
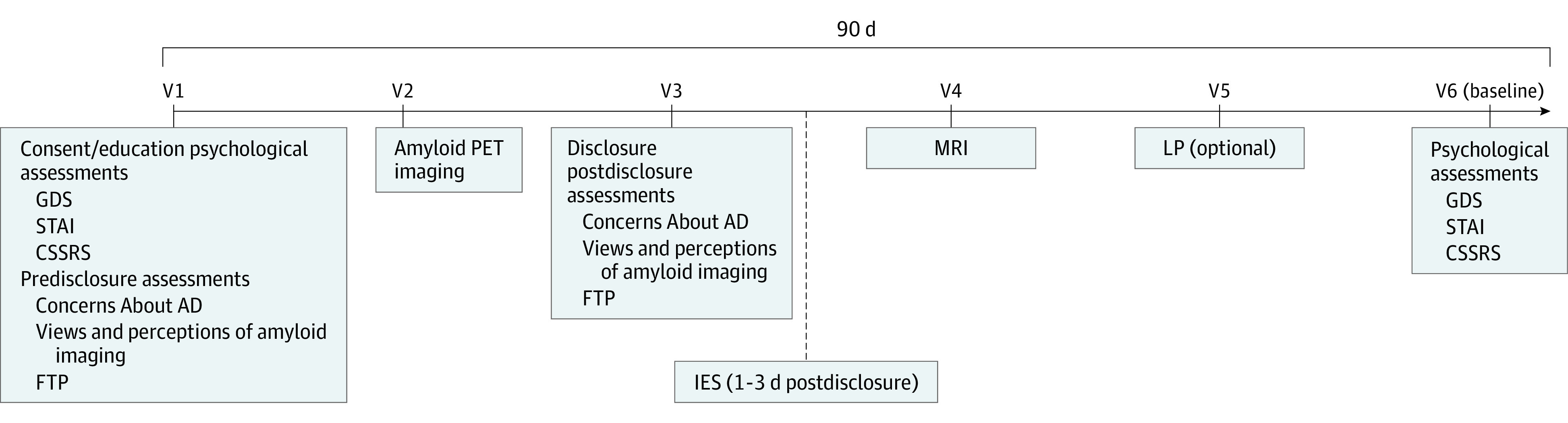
Screening required 4 to 5 visits, depending on whether participants completed the optional lumbar puncture. Education, informed consent, and psychological assessment were completed at visit 1. Amyloid results were disclosed at visit 3. Immediately after disclosure, associated assessments were performed (the Concerns About AD and the Future Time Perspective [FTP] scales); the Impact of Events Scale (IES) was collected by telephone within 72 hours of disclosure for all participants. Participants with elevated and not elevated amyloid levels selected for the Longitudinal Evaluation of Amyloid Risk and Neurodegeneration trial underwent a baseline assessment at visit 6, when postdisclosure psychological assessments were collected. CSSRS indicates Columbia Suicide Severity Rating Scale; GDS, Geriatric Depression Scale; LP, lumbar puncture; MRI, magnetic resonance imaging; PET, positron emission tomography; STAI, State-Trait Anxiety Scale; V, visit.
At visit 2, eligible participants underwent amyloid imaging. Individuals determined by visual reading or standardized uptake value ratios to have elevated brain amyloid levels were eligible for randomization in the A4 trial. Among individuals determined to have not elevated amyloid levels, a subset were enrolled at sites participating in the Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) cohort study (NCT02488720). Participants in LEARN otherwise met the same eligibility criteria for the A4 trial.
Participants received their amyloid imaging results at visit 3. They then completed postdisclosure Concerns About AD and Future Time Perspective scales. Sites performed telephone follow-up with participants 24 to 72 hours after disclosure and collected responses to the Impact of Events Scale (IES).22 The IES uses 15 items (range, 0-75 points) to assess intrusive thoughts and avoidance (eg, “I thought about it when I didn’t mean to”), specific to the person’s amyloid PET result.
Participants with elevated amyloid levels and participants in the LEARN trial with not elevated amyloid levels underwent visit 6, including the postdisclosure GDS, STAI, and CSSRS assessments. These analyses were limited to (1) participants who had elevated amyloid levels with at least 1 of CSSRS, STAI, or GDS at visit 6 and (2) participants who did not have elevated amyloid levels; enrolled in the observational LEARN cohort; and had at least 1 of the CSSRS, STAI, or GDS measures at visit 6.
Disclosure Process
The A4 study used a process to disclose amyloid PET results to participants23 that began with education about preclinical AD. Education was initiated with the informed consent process (visit 1) and was aided by the A4 Study Brochure, which outlined the goals and procedures of the trial. Site investigators were trained and certified centrally prior to performing disclosure and used the teach-back method to confirm participant comprehension.24 The disclosure process specified that imaging be performed on a separate day from consent and disclosure. Certified investigators performed in-person disclosures at visit 3 after reviewing psychological assessments from visit 1 and ensuring that the participant remained willing to learn their biomarker information.
The A4 disclosure process outlined additional monitoring for participants at increased risk (ie, GDS scores of 6 to 10, STAI scores of 17 to 19, or a history of suicidal ideations) and exclusion or consultation with study leadership for participants with higher scores (ie, GDS scores >10, STAI scores >19, or a history of suicidal behaviors). Exclusion was therefore ultimately based on site investigator discretion. The time allowed to complete the A4 screening process was 90 days.
Statistical Methods
The characteristics of the groups at screening visit 1 were compared using Fisher exact tests for categorical variables and 2-sample t tests for continuous variables. To assess whether amyloid status and other covariates were associated with psychological outcomes even before PET imaging and disclosure at visit 1, a rank regression analysis was conducted, controlling for age, sex, scores on the CFI, and a family history of dementia. To examine whether groups with vs without elevated amyloid levels differed in their psychological responses to disclosure, analysis of covariance (ANCOVA) models were used, with change in scores on the outcome measures (GDS and STAI) as the dependent variable and the amyloid group as the independent variable of interest, after adjusting for predetermined covariates, including age, sex, scores on the screening CFI, and family history of dementia. Secondary analyses compared the frequency with which participants experienced changes to predetermined scores on the GDS (>6), STAI (>17), or CSSRS (>0). These cutpoints are frequently used as exclusion criteria in clinical trials and to indicate clinically relevant severities. A small number of participants with elevated amyloid levels (n =
= 156) were not included in our analyses because they lacked all of the GDS, STAI, or CSSRS measurements at either the predisclosure or postdisclosure visit. The participants who were included and excluded from the elevated group were compared using Fisher exact tests and 2-sample t tests, as appropriate, to assess possible differences in these groups. For all models, we have reported the estimates, standard errors, and P values. For these analyses, a P
156) were not included in our analyses because they lacked all of the GDS, STAI, or CSSRS measurements at either the predisclosure or postdisclosure visit. The participants who were included and excluded from the elevated group were compared using Fisher exact tests and 2-sample t tests, as appropriate, to assess possible differences in these groups. For all models, we have reported the estimates, standard errors, and P values. For these analyses, a P <
< .05 was considered statistically significant. Because of the exploratory nature of these analyses, no adjustment for multiple comparisons were performed. All analyses were performed using the statistical software R version 3.6.1 (R Foundation for Statistical Computing). Data were collected from April 2014 to December 2017 and analyzed from March 2019 to October 2019.
.05 was considered statistically significant. Because of the exploratory nature of these analyses, no adjustment for multiple comparisons were performed. All analyses were performed using the statistical software R version 3.6.1 (R Foundation for Statistical Computing). Data were collected from April 2014 to December 2017 and analyzed from March 2019 to October 2019.
Results
Participants
A total of 1167 participants with elevated amyloid levels and 538 participants with not elevated amyloid levels were analyzed (mean [SD] age, 71.5 [4.7] years; 1025 women [60.1%]; and 1611 white individuals [94.5%] and 1638 individuals of non-Latino ethnicity [96.1%]). Table 1 describes demographic characteristics of individuals in the elevated amyloid vs not elevated amyloid groups. Expected differences between the groups were apparent. The participants with elevated amyloid levels, compared with those with not elevated levels, were slightly older (mean [SD] age, 71.9 [4.8] years vs 70.5 [4.3] years; P <
< .001), more often carried apolipoprotein E (APOE) ε4 (679 of 1167 [58.2%] vs 123 of 538 [22.0%]; P
.001), more often carried apolipoprotein E (APOE) ε4 (679 of 1167 [58.2%] vs 123 of 538 [22.0%]; P <
< .001), and more often reported a family history of dementia (878 of 1167 [75.2%] vs 355 of 538 [66.0%]; P
.001), and more often reported a family history of dementia (878 of 1167 [75.2%] vs 355 of 538 [66.0%]; P <
< .001). The participants with elevated amyloid who were included vs excluded were similar, except that excluded participants were older (mean [SD] ages, 71.9 [4.8] vs 73.4 [5.2] years) (data not shown).
.001). The participants with elevated amyloid who were included vs excluded were similar, except that excluded participants were older (mean [SD] ages, 71.9 [4.8] vs 73.4 [5.2] years) (data not shown).
Table 1.
| Characteristic | Participants with elevated amyloid levels (n = = 1167) 1167) | Participants with not elevated amyloid levels (n = = 538) 538) | P value |
|---|---|---|---|
| Age, mean (SD) [range] | 71.9 (4.8) [65.0-85.7] | 70.5 (4.3) [65.0-85.6] | <.001a |
| Male sex, No. (%) | 472 (40.4) | 208 (38.7) | .49b |
| White race, No. (%) | 1105 (94.7) | 506 (94.1) | >.99c |
| Latino ethnicity, No. (%) | 34 (2.9) | 18 (3.3) | .83b |
| Education, mean (SD) [range] | 16.6 (2.8) [7.0-30.0] | 16.8 (2.6) [8.0-30.0] | .12a |
| Carrying APOE ε4, No. (%) | 679 (58.2) | 123 (22.9) | <.001b |
| Family history of Alzheimer disease, No. (%) | 878 (75.2) | 355 (66.0) | <.001b |
| CFI of patient, mean (SD) [range] | 2.36 (2.2) [0.0-14.0] | 1.80 (1.9) [0.0-9.64] | <.001a |
| CFI of partner, mean (SD) [range] | 1.48 (2.0) [0.0-14.0] | 1.14 (1.7) [0.0-10.2] | <.001a |
| FTP, mean (SD) [range] | 44.3 (10.4) [15-70] | 45.6 (10.6) [18-70] | .02a |
| Concerns About AD scale score, mean (SD) [range] | 21.7 (4.5) [8.0-30.0] | 20.6 (4.5) [8.0-30.0] | <.001a |
| GDS, median (IQR) [range] | 1.0 (0.0-2.0) [0-10] | 0.0 (0.0-1.0) [0-12] | .49a |
| STAI, median (IQR) [range] | 10.0 (8.0-12.0) [6-22] | 10.0 (7.0-12.0) [6-22] | .57a |
| CSSRS, median (IQR) [range] | 0.04 (0.0-0.0) [0-9] | 0.02 (0.0-0.0) [0-5] | .58a |
Abbreviations: APOE, apolipoprotein E; CFI, Cognitive Function Inventory; CSSRS, Columbia Suicide Severity Rating Scale; FTP, Future Time Perspective Scale; GDS, Geriatric Depression Scale; STAI, State-Trait Anxiety Inventory.
Prior to imaging and disclosure, no differences between the groups were apparent on the CSSRS, GDS, or STAI. After adjusting for covariates, the amyloid group was not associated with higher GDS or STAI scores, but higher CFI scores were associated with higher scores on both scales (estimate [SE], GDS, 74.4 [5.0]; P <
< .001; STAI, 46.4 [5.6]; P
.001; STAI, 46.4 [5.6]; P <
< .001), and female sex was associated with higher STAI scores (estimate [SE], −102.9 [23.8]; P
.001), and female sex was associated with higher STAI scores (estimate [SE], −102.9 [23.8]; P <
< .001) (eTable 1 in the Supplement). Those who would later learn they had elevated amyloid levels showed higher predisclosure scores on the Concerns About AD Scale (mean [SD] scores, 21.7 [4.5] vs 20.6 [4.5]; P
.001) (eTable 1 in the Supplement). Those who would later learn they had elevated amyloid levels showed higher predisclosure scores on the Concerns About AD Scale (mean [SD] scores, 21.7 [4.5] vs 20.6 [4.5]; P <
< .001) and the CFI (mean [SD] scores, 2.36 [2.2] vs 1.80 [1.0]; P
.001) and the CFI (mean [SD] scores, 2.36 [2.2] vs 1.80 [1.0]; P <
< .001) and lower scores on the Future Time Perspective Scale (mean [SD] scores, 44.3 [10.4] vs 45.6 [10.6]; P
.001) and lower scores on the Future Time Perspective Scale (mean [SD] scores, 44.3 [10.4] vs 45.6 [10.6]; P =
= .02).
.02).
Disclosure Outcomes
The mean (SD) times between disclosure and psychological assessments were 41.6 (23.2; range, 2-189) days and 56.6 (53.5; range, 0-391) days for the elevated amyloid and not elevated amyloid groups, respectively. Disclosure of elevated amyloid status was not associated with short-term adverse psychological reactions (Figure 2). Controlling for covariates, amyloid group was not associated with change on the GDS or STAI (Table 2). Age was associated with greater change in GDS score (estimate [SE], 0.02 [0.01]; P =
= .01), and a family history of AD was associated with greater change in STAI (estimate [SE], −0.34 [0.17]; P
.01), and a family history of AD was associated with greater change in STAI (estimate [SE], −0.34 [0.17]; P =
= .04) after disclosure.
.04) after disclosure.
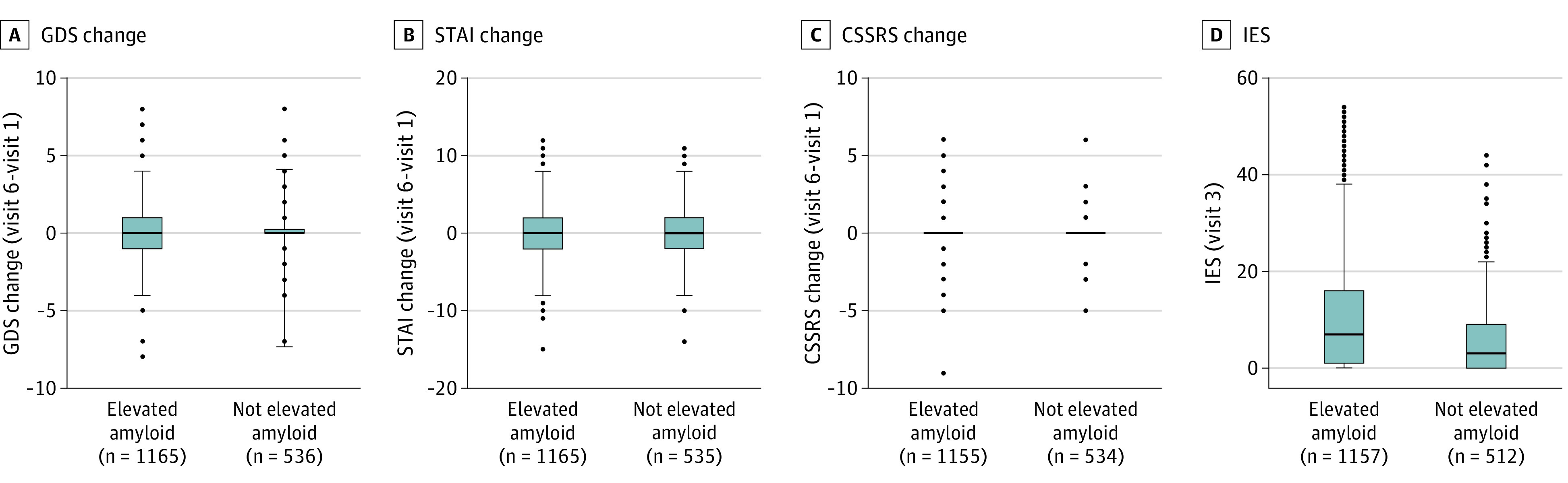
Box plots of the group (elevated amyloid levels vs not elevated amyloid levels) changes are plotted for the Geriatric Depression Scale (GDS) (A), State-Trait Anxiety Scale (STAI) (B), and Columbia Suicide Severity Rating Scale (CSSRS) (C). D, The group data from the Impact of Events Scale (IES), collected by telephone 24 to 72 hours after amyloid level disclosure.
Table 2.
| Characteristic | Δ in GDS | Δ in STAI | IES | |||
|---|---|---|---|---|---|---|
| Estimate (SE) | P value | Estimate (SE) | P value | Estimate (SE) | P value | |
| Intercept | –1.31 (0.51) | .01 | 0.38 (1.17) | .75 | 18.29 (3.73) | <.001 |
| Groupsb | –0.01 (0.07) | .90 | –0.07 (0.16) | .65 | 4.35 (0.53) | <.001 |
| Age | 0.02 (0.01) | .01 | –0.00 (0.02) | .95 | –0.17 (0.05) | <.001 |
| CFI | –0.02 (0.02) | .18 | –0.02 (0.04) | .63 | 0.33 (0.11) | .004 |
| Sexc | –0.00 (0.07) | .95 | –0.20 (0.15) | .19 | –3.32 (0.49) | <.001 |
| Family historyd | 0.05 (0.07) | .47 | –0.34 (0.17) | .04 | 0.84 (0.54) | .12 |
Abbreviations: CFI, Cognitive Function Instrument; GDS, Geriatric Depression Scale; IES, Impact of Events Scale; STAI, State-Trait Anxiety Inventory.
Overall, the proportions of scores in the range of clinical significance were extremely low (eTable 2 in the Supplement). There were no suicide attempts or suicide behaviors. New-onset suicidal ideations were infrequent and not statistically different between the groups. Ten individuals (0.9%) in the elevated amyloid group, compared with 5 (0.9%) in the not elevated amyloid group, experienced an increase to a GDS score of more than 6. Seventeen individuals (1.5%) in the elevated amyloid group, compared with 10 (1.9%) in the not elevated amyloid group, experienced an increase to STAI scores of more than 17. Six of 9 participants with predisclosure GDS scores of more than 6 who learned that they had an elevated amyloid result and 7 of 8 who learned they had a not elevated amyloid result had GDS scores that declined to less than 6 after disclosure. Nineteen of 24 participants with STAI scores of more than 17 who learned they had an elevated amyloid result and 11 of 13 who learned they had a not elevated amyloid result had STAI scores that declined to less than 17 after disclosure.
Those with elevated amyloid levels demonstrated greater intrusive thoughts on the IES, compared with participants with not elevated amyloid levels (mean [SD] scores, 10.2 [10.8] vs 5.9 [7.6]; P <
< .001; Figure 2). The difference remained after controlling for prespecified covariates (Table 2). Younger age (estimate [SE], –0.17 [0.05]; P
.001; Figure 2). The difference remained after controlling for prespecified covariates (Table 2). Younger age (estimate [SE], –0.17 [0.05]; P <
< .001), higher CFI score (estimate [SE], 0.33 [0.11]; P
.001), higher CFI score (estimate [SE], 0.33 [0.11]; P =
= .004), and female sex (estimate [SE], –3.32 [0.49]; P
.004), and female sex (estimate [SE], –3.32 [0.49]; P <
< .001) were also associated with greater IES scores after disclosure. The proportion of IES scores suggesting mild distress (score, 9-25: those with elevated amyloid levels, 407 of 1155 [35.3%]; not elevated amyloid levels, 119 of 534 [23.2%]) or moderate distress (score, >25: those with elevated amyloid levels, 114 [9.8%]; not elevated amyloid levels, 16 [3.1%]) were higher in the elevated amyloid group (eTable 2 in the Supplement).
.001) were also associated with greater IES scores after disclosure. The proportion of IES scores suggesting mild distress (score, 9-25: those with elevated amyloid levels, 407 of 1155 [35.3%]; not elevated amyloid levels, 119 of 534 [23.2%]) or moderate distress (score, >25: those with elevated amyloid levels, 114 [9.8%]; not elevated amyloid levels, 16 [3.1%]) were higher in the elevated amyloid group (eTable 2 in the Supplement).
Scores on the Concerns About AD Scale increased in participants with elevated amyloid and decreased in those with not elevated amyloid levels after disclosure (raw change in scores: elevated amyloid group, 0.8 [3.9]; not elevated amyloid group, –0.4 [3.8]; ANCOVA point estimate [SE], 1.28 [0.21]; P <
< .001). Figure 3 illustrates the group changes in the proportion of participants endorsing the highest levels of agreement on the individual scale items. Those with elevated amyloid levels did not demonstrate a change on the Future Time Perspective Scale after disclosure; those with not elevated amyloid levels increased in score (mean [SD] scores, elevated amyloid group, 0.33 [7.8] points; not elevated amyloid group, 1.15 [7.4] points; P
.001). Figure 3 illustrates the group changes in the proportion of participants endorsing the highest levels of agreement on the individual scale items. Those with elevated amyloid levels did not demonstrate a change on the Future Time Perspective Scale after disclosure; those with not elevated amyloid levels increased in score (mean [SD] scores, elevated amyloid group, 0.33 [7.8] points; not elevated amyloid group, 1.15 [7.4] points; P <
< .001).
.001).
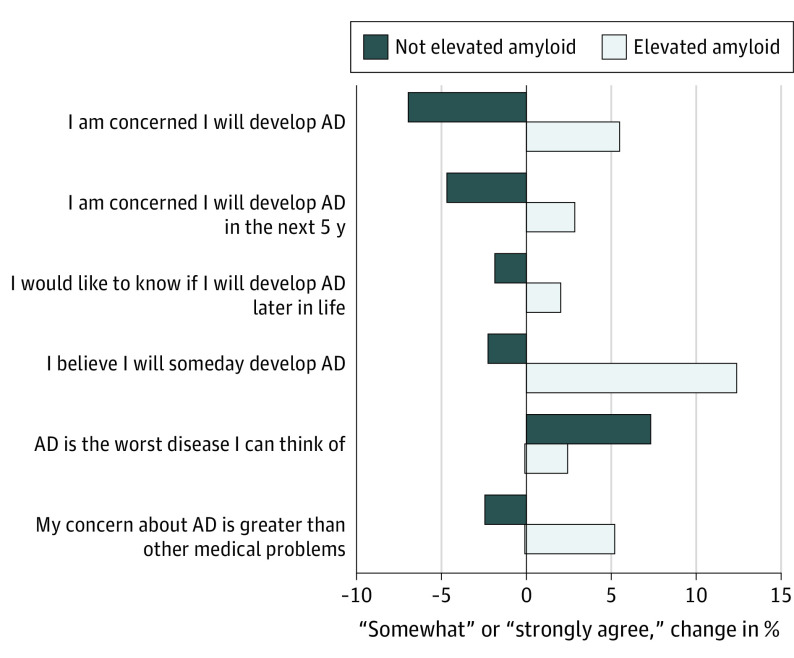
The changes in the proportion of participants endorsing the 2 most extreme levels of agreement (somewhat and strongly agree) on the Concerns About AD Scale are plotted for the elevated amyloid group (n =
= 1167) and the not elevated amyloid group (n
1167) and the not elevated amyloid group (n =
= 538). Bars above the x-axis represent an increase in the proportion of individuals acknowledging the highest level of concern about AD; bars below the x-axis represent a decrease in the proportion of individuals acknowledging the highest level of concern.
538). Bars above the x-axis represent an increase in the proportion of individuals acknowledging the highest level of concern about AD; bars below the x-axis represent a decrease in the proportion of individuals acknowledging the highest level of concern.
Discussion
In a preclinical AD trial that used a process of education and assessment of pretest psychological well-being, we found that trial participants who did not have cognitive impairment and received an elevated amyloid result were no more likely than those receiving a not elevated amyloid result to experience depression, anxiety, or catastrophic reactions in the short term. This study, the largest experience in AD biomarker disclosure to date,25 confirms and extends previous studies26,27,28,29 and supports a process for future similar clinical trials, specifically education and psychological assessment prior to AD biomarker testing and disclosure.
In contrast with previous studies assessing the safety of AD risk factor disclosure (eg, APOE genotypes),30 the A4 study protocol did not categorically exclude participants for specific scores on the CSSRS, GDS, or STAI. A small number of individuals with suicidal ideations, GDS scores of more than 6, or STAI scores of more than 17 prior to amyloid imaging were enrolled. Most of these individuals’ scores declined to scores less than these thresholds after disclosure.
These short-term results are encouraging, but studies with longer follow-up will be essential to confirm the safety of AD biomarker disclosure. Studies in smaller numbers of individuals with elevated amyloid levels support safety through 6 and 12 months.26,28 In a nested qualitative study of a subset of participants in the A4 trial (50 with elevated amyloid levels and 30 with not elevated amyloid levels), however, approximately 20% of participants with elevated amyloid levels acknowledged considering suicide or physician-assisted death if they experience cognitive decline or become a burden to others.31 These experiences emphasize the importance of the longitudinal evaluation of psychological outcomes being gathered in the A4 study.
Although amyloid disclosure did not result in depression, anxiety, or suicidality, it did affect participants in the A4 trial in the short term. Individuals receiving a not elevated amyloid result had reduced concerns about AD. Postdisclosure changes on the Future Time Perspective Scale indicated that the not elevated amyloid group also increased their expected future time. These data support results from 2 qualitative studies32,33 that a common emotional reaction to learning of a not elevated amyloid result was relief. Combined with the observation that those receiving an elevated amyloid result experienced no change in Future Time Perspective Scale scores, these results suggest that most participants in the A4 study were expecting to be eligible.
Individuals with elevated amyloid levels understood that their biomarker result conferred increased risk for AD dementia (Figure 3).33,34 This perception of increased risk, perhaps notably, did not appear to be associated with adverse psychological reactions, namely anxiety or depression. Whether such associations develop over time will be a key area of future research.
Participants receiving an elevated amyloid result, compared with participants receiving a not elevated amyloid result, reported more intrusive thoughts and avoidance on the IES. Scores among those with elevated amyloid levels were similar to scores observed after disclosure of APOE genotypes and even autosomal-dominant AD gene sequence variation status.35 The absolute difference in scores between the groups (approximately 4 points) was relatively small, given that the range of the IES is 0 to 75 points. Furthermore, IES scores were greater than 0 for most participants (eTable 2 in the Supplement), indicating that at least some aspect of the experience was derived from the process of learning any information about brain amyloid status, in addition to the specific result. In a qualitative study, participants in the A4 trial frequently acknowledged the unique sensitivity around amyloid imaging and disclosure, strongly distinguishing it from other medical tests. Specifically, participants explained that AD biomarker tests have a unique risk of stigma.33
The precision afforded by the large sample sizes in the A4 study led to statistical significance for numerically small differences between the groups (eg, a 1-point difference on the Future Time Perspective Scale). Longitudinal analyses of the A4 data will reveal whether these outcomes are fleeting reactions or separation between groups that increases over time. It will be essential to assess in future preclinical AD trials whether these and other reactions to amyloid information are unique to the population enrolled in the A4 study, which is to our knowledge the first trial of its kind.
In the future, biomarker testing may be used to identify individuals with preclinical AD for targeted therapies. In this setting, efficient and safe disclosure methods will be necessary and must also prepare patients and their families for a potential disease course that includes cognitive decline and dementia. The current results, although not generalizable to diverse clinical settings or the general population, suggest that trial participants internalize biomarker information without experiencing immediate mental health sequelae. Whether this information may also be motivating—leading to changes in diet, exercise, and other lifestyle modifications, as well as important long-term decisions and life planning33,36,37—will be another important area of future research.
As expected from previous population-based cohort studies, participants with elevated amyloid levels were older, more often carried APOE ε4, more frequently had a family history of dementia, and had greater subjective cognitive complaints at baseline.38 There were no differences in depression and anxiety between the groups prior to imaging and disclosure, despite previous demonstrations of longitudinal associations of amyloid and neuropsychiatric symptoms in preclinical AD.39,40 Notably, participants did differ in their Future Time Perspective Scale scores and level of concerns about AD prior to being told their amyloid status.
Limitations
The A4 study is not a randomized study to test the safety of amyloid disclosure. Differences between the elevated and not elevated amyloid groups present prior to disclosure cannot be entirely accounted for when interpreting the results. Such differences contributed to some of the observed results (eg, those with higher CFI scores were more likely to demonstrate higher IES scores). Participants in the A4 trial may be unique compared with eventual clinical practice or even future preclinical AD trials in their demographics, motivation, altruism, risk tolerance, and psychological status associated with AD. The A4 study sample was primarily composed of non-Hispanic white participants. Given especially that underrepresented groups, such as African American and Latino individuals, are at increased risk for AD,41 there is an urgent need to understand whether disclosure is associated with similar outcomes, including safety measures, in these groups. The not elevated amyloid group in this study represented only a subset of those disclosed a not elevated amyloid result and were largely self-selected based on their willingness to participate (although available results in the larger not elevated amyloid population were highly consistent [data not shown]). There was a differential time between disclosure and data collection in the not elevated amyloid group compared with the elevated amyloid group. Addressing these limitations to generalizability will be critical to future preclinical AD trials, especially if disclosure is not implemented by the same rigorous, protocol-defined process performed by experts,10 as was the case in the A4 study. Similarly, the A4 study included only individuals willing to learn their amyloid imaging results, further limiting generalizability to the general population. Data were missing for approximately 9% of participants and were not missing at random. Finally, analyses focused exclusively on participants, but biomarker information may also affect family members. Understanding the psychological effects of disclosure on partners at the time of the study, who may eventually become caregivers,42 will require further research.
Conclusions
These results support the short-term safety of research disclosure of amyloid imaging results to participants who do not have cognitive impairments in the setting of preclinical AD trials. Research into the longitudinal outcomes of biomarker disclosure will be essential to guide future clinical trials and clinical practice.
Notes
Supplement.
eTable 1. Multivariable assessments of visit 1 scores on psychological scales.
eTable 2. Raw psychological scale data.
References
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamaneurol.2020.2734
Read article for free, from open access legal sources, via Unpaywall:
https://jamanetwork.com/journals/jamaneurology/articlepdf/2769022/jamaneurology_grill_2020_oi_200058_1607448141.57528.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1001/jamaneurol.2020.2734
Article citations
Building expert consensus regarding sharing of individual research results in Alzheimer's disease research: a Delphi study protocol.
BMJ Open, 14(8):e089242, 24 Aug 2024
Cited by: 0 articles | PMID: 39181557 | PMCID: PMC11344503
Psychosocial implications of learning amyloid PET results in an observational cohort.
Alzheimers Dement, 20(9):6579-6589, 11 Aug 2024
Cited by: 1 article | PMID: 39129396 | PMCID: PMC11497643
Amyloid PET disclosure in subjective cognitive decline: Patient experiences over time.
Alzheimers Dement, 20(9):6556-6565, 01 Aug 2024
Cited by: 1 article | PMID: 39087383 | PMCID: PMC11497681
Reasons for undergoing amyloid imaging among diverse enrollees in the A4 study.
Alzheimers Dement, 20(9):6060-6069, 23 Jul 2024
Cited by: 1 article | PMID: 39041310 | PMCID: PMC11497770
Protocol for a double-blind placebo-controlled randomised controlled trial assessing the impact of oral semaglutide in amyloid positivity (ISAP) in community dwelling UK adults.
BMJ Open, 14(6):e081401, 21 Jun 2024
Cited by: 1 article | PMID: 38908839 | PMCID: PMC11328662
Go to all (53) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (2)
- (1 citation) ClinicalTrials.gov - NCT02488720
- (1 citation) ClinicalTrials.gov - NCT02008357
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Analysis of Psychological Symptoms Following Disclosure of Amyloid-Positron Emission Tomography Imaging Results to Adults With Subjective Cognitive Decline.
JAMA Netw Open, 6(1):e2250921, 03 Jan 2023
Cited by: 12 articles | PMID: 36637820 | PMCID: PMC9857261
Immediate Reactions to Alzheimer Biomarker Disclosure in Cognitively Unimpaired Individuals in a Global Truncated Randomized Trial.
Neurol Clin Pract, 14(2):e200265, 12 Jan 2024
Cited by: 1 article | PMID: 38585443
Cognitively unimpaired adults' reactions to disclosure of amyloid PET scan results.
PLoS One, 15(2):e0229137, 13 Feb 2020
Cited by: 58 articles | PMID: 32053667 | PMCID: PMC7018056
Association of Factors With Elevated Amyloid Burden in Clinically Normal Older Individuals.
JAMA Neurol, 77(6):735-745, 01 Jun 2020
Cited by: 151 articles | PMID: 32250387 | PMCID: PMC7136861
Funding
Funders who supported this work.
NCATS NIH HHS (1)
Grant ID: UL1 TR001414
NIA NIH HHS (6)
Grant ID: P50 AG016573
Grant ID: R01 AG063689
Grant ID: P30 AG010124
Grant ID: P30 AG066519
Grant ID: K24 AG035007
Grant ID: U19 AG010483
 1,2,3,4
1,2,3,4 




