Abstract
Purpose
Pulsatile gonadotropin-releasing hormone (GnRH) therapy and gonadotropin therapy (GT) were widely used for male patients with congenital hypogonadotropic hypogonadism (CHH), but their efficacy was not well compared before. We conducted this meta-analysis to compare the efficacy of restoring fertility using these two therapies.Materials and methods
PubMed, Web of Science, and Scopus were systematically searched for comparative studies evaluating the efficiency of GnRH therapy and GT for male patients with CHH. For continuous outcomes, the weighted mean difference (WMD) was used to measure the difference, whereas the risk ratio with 95% confidence interval was calculated for binary variables.Results
Overall, eight articles from seven studies with 420 patients enrolled were included in the analysis. Patients from the two different groups were determined to be comparable in age, proportion with Kallmann syndrome, percentage of cryptorchidism and pretreatment hormones (follicular-stimulating hormone, luteinizing hormone, and testosterone). GnRH therapy was related to a larger testicular volume (standardized mean difference=-1.43; p=0.01) and earlier spermatogenesis (WMD=-5.30 months; p=0.004) compared to GT. However, the difference in the rate of positive sperm detection (p=0.08), sperm concentration (p=0.37), and pregnancy rate (p=0.11) were not significant. Allergic reactions mostly occurred during GnRH therapy, while GT was related to a higher incidence of gynecomastia and acne.Conclusions
Compared to GT, GnRH was related to earlier spermatogenesis and less estradiol-related adverse reactions, although there were no significant differences in spermatogenesis rate, sperm concentration, and pregnancy rate. High-quality randomized controlled trials are needed for future research.Free full text

Spermatogenesis of Male Patients with Congenital Hypogonadotropic Hypogonadism Receiving Pulsatile Gonadotropin-Releasing Hormone Therapy Versus Gonadotropin Therapy: A Systematic Review and Meta-Analysis
Associated Data
Abstract
Purpose
Pulsatile gonadotropin-releasing hormone (GnRH) therapy and gonadotropin therapy (GT) were widely used for male patients with congenital hypogonadotropic hypogonadism (CHH), but their efficacy was not well compared before. We conducted this meta-analysis to compare the efficacy of restoring fertility using these two therapies.
Materials and Methods
PubMed, Web of Science, and Scopus were systematically searched for comparative studies evaluating the efficiency of GnRH therapy and GT for male patients with CHH. For continuous outcomes, the weighted mean difference (WMD) was used to measure the difference, whereas the risk ratio with 95% confidence interval was calculated for binary variables.
Results
Overall, eight articles from seven studies with 420 patients enrolled were included in the analysis. Patients from the two different groups were determined to be comparable in age, proportion with Kallmann syndrome, percentage of cryptorchidism and pretreatment hormones (follicular-stimulating hormone, luteinizing hormone, and testosterone). GnRH therapy was related to a larger testicular volume (standardized mean difference=−1.43; p=0.01) and earlier spermatogenesis (WMD=−5.30 months; p=0.004) compared to GT. However, the difference in the rate of positive sperm detection (p=0.08), sperm concentration (p=0.37), and pregnancy rate (p=0.11) were not significant. Allergic reactions mostly occurred during GnRH therapy, while GT was related to a higher incidence of gynecomastia and acne.
Conclusions
Compared to GT, GnRH was related to earlier spermatogenesis and less estradiol-related adverse reactions, although there were no significant differences in spermatogenesis rate, sperm concentration, and pregnancy rate. High-quality randomized controlled trials are needed for future research.
INTRODUCTION
Congenital hypogonadotropic hypogonadism (CHH) is a rare disorder caused by gonadotropin-releasing hormone (GnRH) deficiency or resistance [1]. Based on olfactory dysfunction, patients can be categorized into two subtypes, Kallmann syndrome (KalS), which is anosmic, and idiopathic hypogonadotropic hypogonadism (IHH), which maintains normal olfactory function [2]. Because of the GnRH secretion deficiency or resistance, children with CHH display absent or arrested puberty, and their development of sex characteristics and fertility are severely impeded.
Injections of testosterone can improve the development of sex organs and secondary sexual characteristics, but it can also inhibit spermatogenesis [3]. To restore fertility, gonadotropin therapy (GT) was developed, and the treatment of human chorionic gonadotropin (HCG) alone or in combination with human menopausal gonadotropin (HMG) was confirmed to be effective in the induction of spermatogenesis in CHH patients [4,5,6]. However, the HCG/HMG therapy was conducted by intramuscular or subcutaneous injection 2–3 times weekly and the long duration of therapy and inconvenience of frequent injections result in poor compliance.
In 1978, the mechanism of pulsatile secretion of GnRH was clarified by Belchetz et al [7]. In 1982, pulsatile GnRH was first applied to six IHH patients and successfully induced spermatogenesis in three patients [8]. Through a subcutaneously placed butterfly needle, GnRH was administrated in 90- or 120-minute intervals using a portable pump, and the pulsatile administration of medication more closely mimics the physiological conditions. Both pulsatile GnRH therapy and GT were recommended for the induction of male fertility [9]. However, although pulsatile GnRH therapy is closer to physiological conditions theoretically, whether it is superior to GT remains unclear. Several studies suggested that GnRH therapy enlarged testicular volume more efficiently [10,11,12], and the rate of spermatogenesis was significantly higher than GT [13]. Importantly, GnRH therapy was related to earlier spermatogenesis than GT [12,13,14]. However, several studies indicated that there was no difference between the two therapies [15,16]. In addition, several studies reported related side effects with highly inconsistent results [10,12,14,16].
Due to the controversial results regarding the efficacy of GnRH and GT, we performed this systematic review and meta-analysis to directly compare the efficacy and safety of these two therapy modalities.
MATERIALS AND METHODS
No previous protocol was published for this systematic review and meta-analysis.
1. Literature search
A comprehensive literature search was conducted for studies published from the inception of databases to February 10, 2020, in PubMed, Web of Science, and Scopus to identify studies comparing GnRH therapy to GT in CHH treatment.
Separate searches were carried out using diagnosis (hypothalamic hypogonadism, hypogonadotropic hypogonadism, CHH, IHH, Kallmann's syndrome) and intervention terms (GnRH, follicular-stimulating hormone [FSH], HCG, HMG). The detailed search string was listed in Supplement Table 1.
Titles and abstracts of articles identified by the keyword search were screened against the study selection criteria. Potentially relevant articles were evaluated of the full text. An additional manual search of references from identified studies was performed. Two independent reviewers screened all studies according to inclusion and exclusion criteria, and all disagreements were resolved by discussion with a third author.
2. Study selection criteria
Studies that met the following criteria were included in this meta-analysis:
1) The diagnosis of CHH was based on reliable evidence including history, hormone tests, and imaging tests.
2) The efficacy or safety of GnRH and GT in male patients was compared.
3) The therapy protocol was documented with detailed regimens.
4) The articles were written in English or Chinese with English abstracts.
5) The required data should be complete with confidence intervals (CIs), standard deviation (SD), or standard error.
Studies that met the following criteria were excluded:
1) The study was a review with no original data.
2) The study was a case report that reported the efficacy of GnRH therapy or GT with a limited sample size (less than 5).
3. Data extraction and quality assessment
Two reviewers independently extracted data from every study and evaluated methodological quality. The following information was extracted from each study: number of cases; proportion of KalS; proportion of cryptorchidism; age at initial treatment; duration of therapy and follow-up; pretreatment and posttreatment FSH, luteinizing hormone (LH), estradiol, testosterone, testicular volume; rate of spermatogenesis at the end of study; time to the first sperm detection; sperm concentration; rate of pregnancy; and events of side effects.
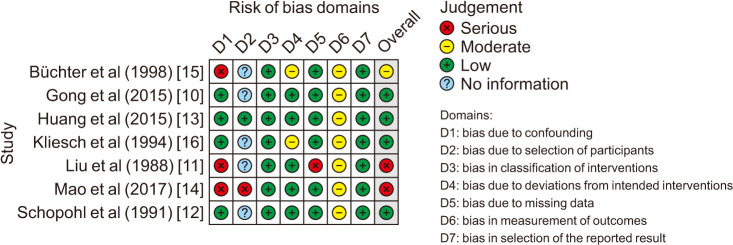
The quality of each study was determined using the ROBINS-1 tool, a tool recommended by Cochrane for assessing risk of bias in non-randomised studies of interventions [17]. The graph for risk of bias was generated with robvis tool [18].
4. Data analysis
A formal meta-analysis of studies comparing the efficacy (testosterone, testicular volume, rate of spermatogenesis, time to first sperm detection, sperm count, and rate of pregnancy) of GnRH therapy to GT for CHH was conducted (primary outcomes). Successful spermatogenesis was defined as the appearance of at least one sperm cell in the semen. Additionally, the side effects of the two treatments were compared (secondary outcomes). Besides, a subgroup analysis of the rate of spermatogenesis according to the presence of cryptorchidism was performed.
For outcomes of continuous variables, the weighted mean difference (WMD) was used to measure the difference, whereas the risk ratio (RR) with 95% CI was calculated for binary variables. Standardized mean difference (SMD) was used if the results were measured using different scales or the reported outcomes varied greatly. For studies reporting medians and ranges, a validated mathematical model was used to convert the median (range) to mean±SD [19].
When two publications reported the same study, relevant parameters were only counted once for the scope of the present analysis. For example, this approach was employed for the study published by Schopohl et al [12] and Schopohl [20]. When two studies reported by the same group contained overlapping patients, we only used the relevant data of the higher-quality study.
A fixed-effects model was used to calculate the pooled estimates if no significant heterogeneity was identified (I2<50%). Otherwise, a random-effects model was used. Additionally, a sensitivity analysis was also performed by changing effect model. Due to the limited number of included studies, the publication bias was evaluated by using Egger linear regression. All statistical analyses were performed by using Review Manager 5.3 (Cochrane Collaboration, Oxford, UK), except for the calculation of Egger's linear regression test, which was conducted in STATA ver. 12.0 (Stata Corp., College Station, TX, USA).
RESULTS
1. Characteristics of the included studies
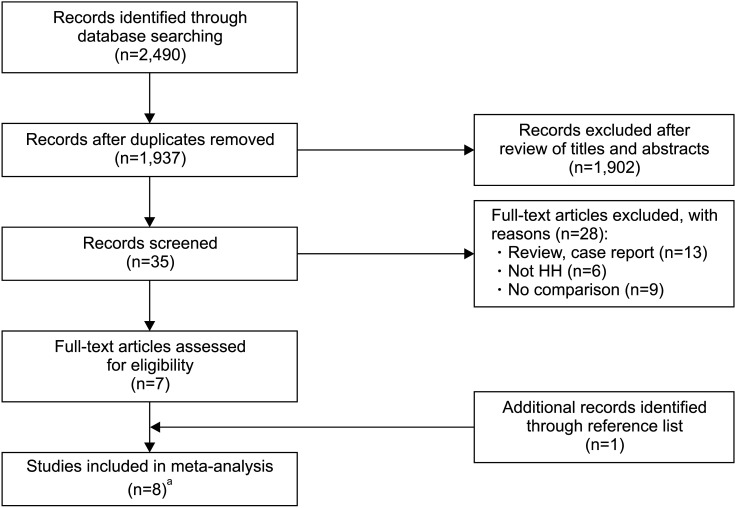
Overall, eight articles with 420 patients were included in the meta-analysis after screening (Fig. 1) [10,11,12,13,14,15,16,20]. Among these patients, 204 patients and 216 patients were diagnosed with KalS and IHH respectively. Of these studies, two articles reported the outcomes of the same research, and the relevant outcomes were used only once in our analysis [12,20]. Huang et al [13] and Mao et al [14] conducted two studies with overlapping data, as well as Büchter et al [15] and Kliesch et al [16]. When two studies were included in a single analysis, we used the data of Huang et al [13] and Kliesch et al [16] because of their higher quality.
The characteristics of the included studies are presented in Table 1. Most of these studies were conducted in Germany and China, and one was conducted in the USA in 1988. Four of the seven included studies were retrospective, and others were prospective. The combination of HCG and HMG for GT was used in most studies except for the study conducted by Gong et al [10], in which the patients were adolescent boys treated with HCG alone. Notably, most included studies were conducted based on limited sample size (the number of the GnRH cases was less than 10 in three studies).
Table 1
| Study | Study design | Study origin | GnRH regimens | GT regimen | GnRH/GT | Reported outcome | Testicular volume measurement | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Total cases (n) | Cases of KalS (n) | Mean age (y) | Median FU (mo) | |||||||
| Büchter et al (1998) [15] | RTP, SC | Germany | 5–20 μg every 120 minutes | HCG 1,000–2,500 IU twice a week and HMG 75–150 IU three times a week ( i.m. or s.c.) | 6/18 | 2/9 | 30.1/29.1 | NS | TV, RateS, TimeS, RateP | Palpation or US |
| Gong et al (2015) [10] | PRP, SC | China | 8–10 μg every 90 minutes | HCG 1,000–2,000 IU twice a week or every other day (i.m.) | 12/22 | 8/15 | 13.39/13.89 | NS | T, FSH, TV, AR | Orchidometer |
| Huang et al (2015) [13] | RTP, SC | China | 10 μg every 90 minutes | HCG 3,000 IU and HMG 75 IU twice a week (i.m.) | 40/52 | 20/29 | 22.4/20.5 | 8.2/9.2 | T, FSH, TV, RateS, TimeS, RateP | Orchidometer |
| HCG dose was adjusted according to T level. | ||||||||||
| Kliesch et al (1994) [16] | RTP, SC | Germany | 5–20 μg every 120 minutes | HCG 1,000–2,500 IU twice a week and HMG 75–150 IU three times a week (i.m. or s.c.) | 6/11 | 2/7 | 30.7/31.0 | 14.5/15.7 | FSH, TV, RateS, TimeS, SpC, RateP, AR | Palpation or US |
| Liu et al (1988) [11] | PRP, SC | USA | 10–20 μg every 120 minutes | HCG 2,000 IU and HMG (75 IU FSH and 75 IU LH) three times a week (i.m.) | 5/10 | 0/0 | 23.1/30.6 | 20.8/24.0 | T, FSH, TV, RateS | Orchidometer |
| Mao et al (2017) [14] | RTP, SC | China | 10 μg every 90 minutes | HCG 2,000 IU and HMG 75 IU twice a week (i.m.) | 20/182 | 11/84 | 27.1/21.5 | 15.6/28.7 | T, FSH, TV, RateS, TimeS, SpC, RateP, AR | Orchidometer |
| HCG dose was adjusted according to T level | ||||||||||
| Schopohl et al (1991) [12]a , Schopohl (1993) [20]a | PRP, SC | Germany | 4–16 μg every 90 or 120 minutes | HCG 2,500 IU three times a week initially and then reduced according to T and estradiol level | 18/18 | 8/9 | 21.1/23.6 | 9.3/14.4 | T, TV, RateS, TimeS, SpC, RateP, AR | Orchidometer |
| HMG 150 IU twice a week was added 2–3 months later and dosage increased according to testicular growth and sperm count (i.m.) | ||||||||||
GnRH: gonadotropin-releasing hormone, GT: gonadotropin therapy, CHH: congenital hypogonadotropic hypogonadism, KalS: Kallmann syndrome, FU: follow-up, RTP: retrospective, SC: single center, PRP: prospective, HCG: human chorionic gonadotropin, HMG: human menopausal gonadotropin, i.m.: intramuscular injection, s.c.: subcutaneous injection, NS: not specified, TV: testicular volume, RateS: rate of positive spermatogenesis, TimeS: time needed to induce spermatogenesis, RateP: rate of pregnancy, T: post-treatment testosterone, FSH: post-treatment follicular-stimulating hormone, AR: adverse reaction, SpC: sperm count, US: ultrasonography.
aThese two articles reported the same outcomes from the same study.
Most studies were of low and moderate bias (Fig. 2 and Supplement Table 2). The imbalanced sample sizes, follow-up durations, and baseline characteristics including initial testicular volume and age of the two arms might be the reasons for the relatively lower quality. In particularly, Büchter et al [15] and Gong et al [10] did not report their follow-up length, but the follow-up strategies were described, as every 3 to 6 months in Büchter et al's study [15] and every 3 months in Gong et al's study [10] until the treatment finished.
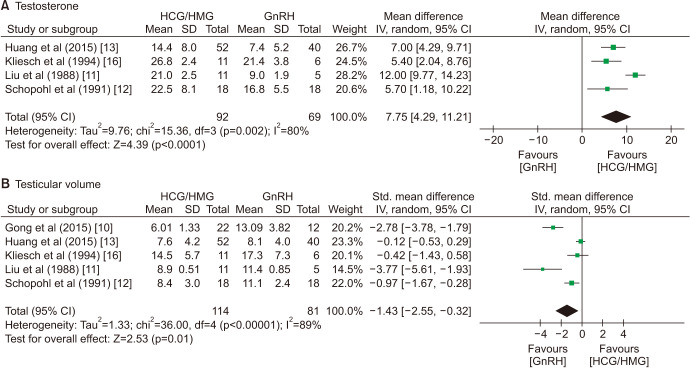
Two arms of the comparison were not significantly different in age (p=0.19), the proportion of KalS (p=0.53), and the percentage of cryptorchidism (p=0.78). Pretreatment hormones, including FSH (p=0.26), LH (p=0.11), and testosterone (p=0.65), were also similar in the GnRH group and GT group. Notably, the pretreatment testicular volume in the GnRH group was larger than that in the GT group (WMD=0.32 mL, 95% CI=0.08–0.57; p=0.01).
2. Efficacy of gonadotropin-releasing hormone and gonadotropin therapy
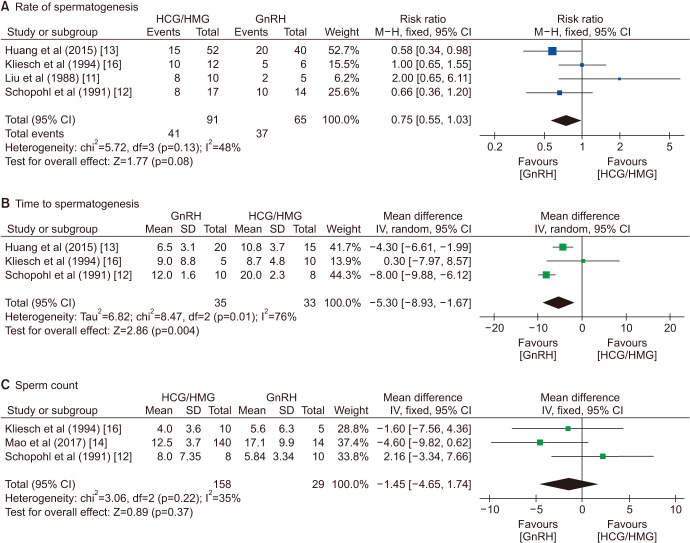
Although the plasma testosterone in the GT group was significantly higher than plasma testosterone in the GnRH group (WMD=7.75 nmol/L, 95% CI=4.29–11.21; p<0.0001), the testicular volumes increased considerably more in the GnRH group (SMD=−1.43, 95% CI=−2.55–-0.32; p=0.01; Fig. 3). There was no significant difference in the rate of positive spermatogenesis (RR=0.75, 95% CI=0.55–1.03; p=0.08), but GnRH treatment was related to earlier spermatogenesis (WMD=−5.30 months, 95% CI=−8.93–−1.67; p=0.004; Fig. 4). The sperm count (WMD=−1.45×106/mL, 95% CI=−4.65–1.74; p=0.37) and rate of pregnancy (RR=0.60, 95% CI=0.31–1.13; p=0.11) were not significantly different between the two groups (Fig. 4, ,55).
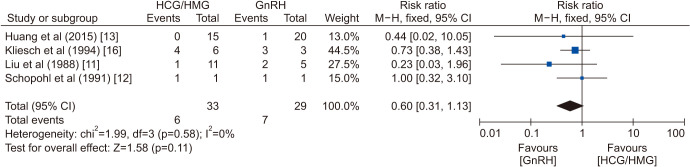
The subgroup analysis according to the presence of cryptorchidism showed that the rate of spermatogenesis remained similar for these two therapies in patients with or without cryptorchidism (Supplement Fig. 1, 2). Additionally, the presence of cryptorchidism was a predictor of the spermatogenesis rate of CHH patients (Supplement Fig. 3).
Considering that in Gong et al's study [10], only HCG was used for the GT group, a formal meta-analysis was also performed after excluding Gong at al's study [10]. The final outcome was consistent with the previous outcome and suggested that GnRH therapy was related to larger testicle volume.
3. Side effects of gonadotropin-releasing hormone and gonadotropin therapy
Adverse reactions reported during the therapy were allergy, breast development, and acne. The incidence of overall adverse reactions was comparable in the two groups (RR=1.55, 95% CI=0.56–4.35; p=0.40). However, the allergy occurred mostly during GnRH therapy (RR=37.93, 95% CI=7.03–204.74; p<0.0001), and the GT was related to gynecomastia and acne (RR=0.27, 95% CI=0.09–0.83; p=0.02; Fig. 6).
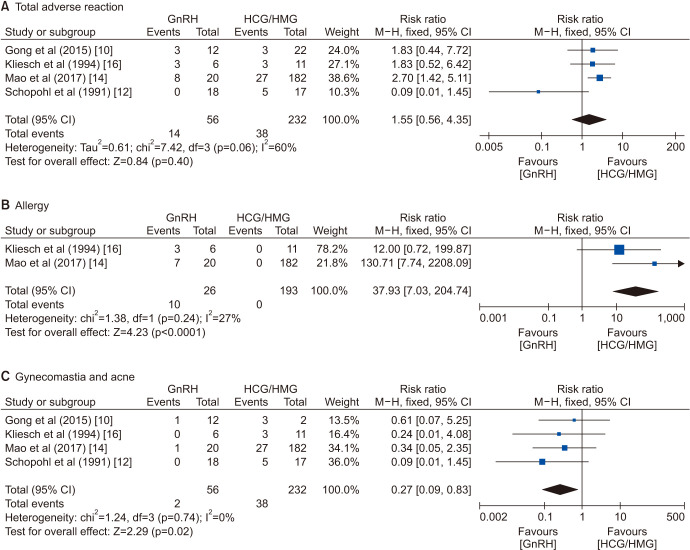
4. Publication bias and sensitivity analysis
All the egger's tests showed no significant publication bias.
Sensitivity analyses were conducted by changing effect model and the effect model changing did not alter any outcomes of this analysis.
DISCUSSION
To the best of our knowledge, this review is the first meta-analysis to compare GnRH therapy to GT in male CHH patients. In this analysis, we evaluated posttreatment physiological conditions, fertility, and adverse reactions. In conclusion, GnRH is related to larger testicular volume and earlier spermatogenesis, though the rates of spermatogenesis and pregnancy are similar in both groups.
1. Interpretation of outcomes
LH and FSH play an important role in the development of sperm and the levels of them could be normalized in the majority of CHH after pulsatile GnRH therapy [21]. Several hormone outcomes after pulsatile GnRH were reported in our included studies and all these results showed a greatly increased level of LH and FSH [10,13,14,16]. Compared to GT, GnRH therapy was more efficient in the normalization of LH and FSH levels [10,11]. However, due to the lack of relevant data, we were unable to conduct a quantitative analysis to compare the posttreatment level of LH and FSH after these two therapies.
Patients in the GnRH group had a lower testosterone concentration than the GT group. In the beginning, GnRH therapy aimed to maintain testosterone within the normal range [11,16]. However, later studies suggested that plasma testosterone levels of 8–10 nmol/L may be sufficient to induce spermatogenesis in CHH patients [13,14]. Pulsatile GnRH therapy can maintain the pulsatile secretion of gonadotropin by the pituitary gland and therefore stabilize the testosterone level. In the GT group, the fluctuation of testosterone was significant [22]. In addition, the GnRH receptors were found in spermatogonia, spermatocytes, and mature sperm [23]. Therefore, GnRH might be directly involved in spermatogenesis, sperm maturation, and fertilization [24]. These reasons might explain why GnRH therapy could efficiently induce spermatogenesis with a lower testosterone level. Notably, the normalization of testosterone could only be achieved by supraphysiological levels of LH and FSH in a subset of patients [25], and in specific populations, the level of testosterone is not correlated with the sperm concentration [26,27]. These studies suggested that the normalization of testosterone might be unbeneficial and unessential.
The ability to restore fertility in CHH patients is the critical efficacy of these two therapies, and whether GnRH therapy is related to a higher rate of spermatogenesis has not been determined. In our analysis, the testes were enlarged in both treatment groups, but GnRH therapy was related to a larger volume. Although the testicular volume may be related to fertility [5,28,29], the rate of spermatogenesis was not significantly different between the two groups. An analysis of 48 studies of HCG/HMG therapy and 16 studies of pulsatile GnRH therapy showed that the rate of spermatogenesis was 68% (95% CI=58%–77%) in HCG/HMG treatment, which is lower than that of 77% (95% CI=63%–87%) in GnRH therapy for patients with prepuberty-onset hypogonadotropic hypogonadism [30]. This analysis was mostly based on single-arm studies. Combined with our analysis, we are optimistic about pulsatile GnRH therapy. Moreover, it was suggested that GnRH therapy is related to earlier spermatogenesis, and GnRH therapy is associated with a shorter time to achieve sperm concentrations at various predetermined thresholds [14]. With the aid of assisted reproduction techniques such as intracytoplasmic sperm injection, shorter time to achieve pregnancy might also be practical for patients receiving GnRH therapy. Additionally, for these patients who failed to induce spermatogenesis after HCG/HMG therapy, GnRH still might be effective [31]. Therefore, patients who desire fertility may benefit more from GnRH therapy.
The sperm concentrations were compared in our analysis, and the sperm count in the GnRH group was slightly higher, though without statistical significance. In the included studies, the study conducted by Schopohl et al [12] was the only one that favored GT. In fact, in their research, one patient in the GT group had a sperm concentration of 26×106/mL, which was 2 to 12 fold higher than that of other patients. However, as the GnRH group was associated with a shorter time to achieve sperm concentrations at various predetermined thresholds [14], whether the final concentrations differ in the long-term treatment has not been determined. Several studies also compared sperm motility and morphology and obtained an insignificant difference [12,16].
There was no difference in the rate of pregnancy between the two groups (p=0.11). Büchter et al [15] and Kliesch et al [16] suggested that most pregnancies occurred with counts far below the normal range, and the sperm concentrations at pregnancy were similar. This might explain the similarity of pregnancy rate with different sperm concentrations because the low concentration of sperm is sufficient for pregnancy. Büchter et al [15] showed that GnRH therapy tended to be related to a shorter duration before pregnancy, but the difference was not significant because of the limited study size. A study with a larger size is still warranted to ascertain the effect of different therapies on the duration until pregnancy.
Although the overall incidence of adverse reactions was comparable in the two therapies, the specific adverse responses were different. GT was related to gynecomastia and acne, and the increased level of estradiol induced by HCG might be a reasonable explanation [32]. Allergy occurred mostly in GnRH therapy, and most of them were mild to moderate dermatological allergic reactions. Before the decision of treatment, these related adverse reactions should be considered, and the adverse responses during GnRH therapy seem to be more acceptable for patients.
The cost of treatment is an essential factor in clinical practice. The pulsatile GnRH therapy is much more expensive than the HCG/HMG therapy and the burden of drug and device cost could limit the use of GnRH therapy. Besides, the constant carrying of the pump, the refilling of medication, and frequent changing of injection sites also result in inconvenience for patients. However, with the decreasing of medication and device, more patients can afford the cost of treatment and the improved devices with smaller size and more volume could also reduce the inconvenience in the future. In addition, HCG/HMG therapy requires frequent hospital visits, twice to three times a week, which is also inconvenience for patients living in the place where the community doctors and family doctors are not available. Patients and doctors can choose the optimal treatment method by considering the efficacy and the patterns of these treatment.
2. Effect of cryptorchidism status
Cryptorchidism was reported to be a predictor for spermatogenesis in CHH patients [21,29]. Our analysis confirmed that cryptorchidism was related to a lower rate of spermatogenesis (Supplement Fig. 3). Mao et al [14] suggested that cryptorchidism was also related to a longer time to first sperm detection and a lower sperm concentration. Büchter et al [15] also reported a delayed duration until spermatogenesis, but the difference was not significant in their study. In particular, Büchter et al [15] noted that the two patients who failed spermatogenesis had bilateral cryptorchidism until 22 and 17 years of age, respectively. This finding suggests that early intervention is essential for patients with cryptorchidism.
According to the subgroup analysis (Supplement Fig. 1, 2), the rates of spermatogenesis did not differ between GnRH therapy and GT, regardless of the cryptorchidism status. The cryptorchidism status was unlikely to affect the clinical decision on therapy selection.
3. Strengths and limitations
Our study is the first meta-analysis that compared the effect and adverse reactions of GnRH therapy to GT based on comparative studies. We comprehensively evaluated the effect of these two therapies using various parameters. Subgroup analyses were also performed to assess the effect of cryptorchidism status.
There are several limitations to our study. First, some biases were inevitable because of their nonrandomized and retrospective nature. For example, in the retrospective study, testicular volumes were not assessed in a standardized manner or by the same clinician, leading to variation in determining testicular volumes. Also, the orchidometer was used in the majority of the included studies and the reliability could be compromised. Second, the follow-up time of some included studies was not long enough, and in some studies, the follow-up durations and sample sizes of two arms were not comparable. Third, the sample size of most included studies was small, and the two studies from the same group had the majority of included cases which could be a potential source of bias. Additionally, as an essential parameter, the time to pregnancy was not well-documented in most studies, and in future research, this parameter should be analyzed to compare the efficacy of different therapies. Fourth, the baseline features, such as age, of the patient population differed vastly among the included studies and the dosages of regimens were also different among the included researches. Finally, our conclusions were based on studies investigating HMG and should be cautiously applied to recombinant human FSH, which is widely used in CHH treatment.
CONCLUSIONS
Our study suggested that although the two therapies exhibited no significant difference in spermatogenesis rate, sperm count, and pregnancy rate, GnRH therapy was related to larger testicular size, shorter time to sperm detection, and less estradiol-related adverse reactions. More prospective, randomized studies with larger sample sizes are warranted to ascertain the advantages of pulsatile GnRH therapy.
ACKNOWLEDGEMENTS
This research was supported by National Natural Science Foundation of China (Grant Number: 81702518, 81500636) and Innovation Foundation of Huazhong University of Science and Technology (Grant Number 2019kfyXKJC06).
Footnotes
Conflict of Interest: The authors have nothing to disclose.
Author Contribution:
Conceptualization: XL, DM.
Data curation: CW, YZ, TW.
Formal analysis: GL, YZ, SW.
Funding acquisition: XL.
Project administration: XL.
Supervision: JL.
Writing — original draft: CW, GL.
Writing — review & editing: TW, SW.
Supplementary Materials
Supplementary materials can be found via https://doi.org/10.5534/wjmh.200043.
Forest plot of spermatogenesis rate of patients with cryptorchidism after gonadotropin-releasing hormone (GnRH) therapy compared to gonadotrophin therapy. HCG: human chorionic gonadotropin, HMG: human menopausal gonadotropin, M–H: Mantel–Haenszel, CI: confidence interval, df: degree of freedom.
Forest plot of spermatogenesis rate of patients without cryptorchidism after gonadotropin-releasing hormone (GnRH) therapy compared to gonadotrophin therapy. HCG: human chorionic gonadotropin, HMG: human menopausal gonadotropin, M–H: Mantel–Haenszel, CI: confidence interval, df: degree of freedom.
Forest plot of spermatogenesis rate after gonadotropin-releasing hormone (GnRH) and gonadotrophin therapy for patients with cryptorchidism compared to those without cryptorchidism. M–H: Mantel–Haenszel, CI: confidence interval, df: degree of freedom.
List of search strings in different databases
Risk of bias summary for included study according to ROBINS-1
References
Articles from The World Journal of Men's Health are provided here courtesy of Korean Society for Sexual Medicine and Andrology
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.5534/wjmh.200043
Article citations
Clinical manifestations and spermatogenesis outcomes in Chinese patients with congenital hypogonadotropic hypogonadism caused by inherited or de novo FGFR1 mutations.
Asian J Androl, 26(4):426-432, 09 Jan 2024
Cited by: 0 articles | PMID: 38227553 | PMCID: PMC11280213
Gonadotropins for pubertal induction in males with hypogonadotropic hypogonadism: systematic review and meta-analysis.
Eur J Endocrinol, 190(1):S1-S11, 01 Jan 2024
Cited by: 2 articles | PMID: 38128110 | PMCID: PMC10773669
Review Free full text in Europe PMC
Treatment progress of cryptozoospermia with Western Medicine and traditional Chinese medicine: A literature review.
Health Sci Rep, 6(1):e1019, 27 Dec 2022
Cited by: 2 articles | PMID: 36582629 | PMCID: PMC9793827
Pulsatile gonadotropin-releasing hormone: clinical applications of a physiologic paradigm.
F S Rep, 4(2 suppl):20-26, 02 Feb 2023
Cited by: 1 article | PMID: 37223766 | PMCID: PMC10201297
NGF and the Male Reproductive System: Potential Clinical Applications in Infertility.
Int J Mol Sci, 23(21):13127, 28 Oct 2022
Cited by: 7 articles | PMID: 36361912 | PMCID: PMC9655275
Review Free full text in Europe PMC
Go to all (6) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Pulsatile gonadotropin-releasing hormone therapy is associated with earlier spermatogenesis compared to combined gonadotropin therapy in patients with congenital hypogonadotropic hypogonadism.
Asian J Androl, 19(6):680-685, 01 Nov 2017
Cited by: 22 articles | PMID: 28051040 | PMCID: PMC5676428
Efficacy of Pulsatile Gonadotropin-Releasing Hormone Therapy in Male Patients: Comparison between Pituitary Stalk Interruption Syndrome and Congenital Hypogonadotropic Hypogonadism.
Endocr Pract, 28(5):521-527, 24 Feb 2022
Cited by: 1 article | PMID: 35218954
Pulsatile gonadotropin releasing hormone therapy for spermatogenesis in congenital hypogonadotropic hypogonadism patients who had poor response to combined gonadotropin therapy.
Arch Endocrinol Metab, 68:e230101, 01 May 2024
Cited by: 1 article | PMID: 38739523
Hormonal control of spermatogenesis in men: therapeutic aspects in hypogonadotropic hypogonadism.
Ann Endocrinol (Paris), 75(2):98-100, 29 Apr 2014
Cited by: 10 articles | PMID: 24793994
Review
Funding
Funders who supported this work.
Huazhong University of Science and Technology (1)
Grant ID: 2019kfyXKJC06
Innovation Foundation of Huazhong University of Science and Technology (1)
Grant ID: 2019kfyXKJC06
National Natural Science Foundation of China (2)
Grant ID: 81702518
Grant ID: 81500636

 1
1