Abstract
Importance
For more than a decade, sorafenib has been the only systemic treatment option for patients with advanced hepatocellular carcinoma (HCC). However, rapid progress over the past few years led to approval of other angiogenesis inhibitors and several immune checkpoint blockers (ICBs) that have been added to the treatment armamentarium for advanced HCC. Moreover, the recent success of a combination of bevacizumab with atezolizumab signals an important change in the front-line treatment of HCC.Observations
This review summarizes rapidly emerging clinical data on the promise and challenges of implementing ICBs in HCC and discusses the unmet need of biomarkers to predict response or resistance to therapy. Two strategies to target immunosuppression in tumors are also discussed: one proven (vascular endothelial growth factor pathway inhibition) and one currently under investigation (transforming growth factor-β pathway inhibition). The rationale and preliminary evidence on how their inhibition may reprogram the immunosuppressive milieu and enhance the efficacy of ICBs in HCC are reviewed.Conclusion and relevance
The recent successes and failures of angiogenesis inhibitors and ICBs, alone and in combination, have provided important insights into how to implement this novel systemic therapy in HCC and led to new avenues to enhance immunotherapy efficacy in this disease.Free full text

The Current Landscape of Immune Checkpoint Blockade in Hepatocellular Carcinoma A Review
Abstract
IMPORTANCE
For more than a decade, sorafenib has been the only systemic treatment option for patients with advanced hepatocellular carcinoma (HCC). However, rapid progress over the past few years led to approval of other angiogenesis inhibitors and several immune checkpoint blockers (ICBs) that have been added to the treatment armamentarium for advanced HCC. Moreover, the recent success of a combination of bevacizumab with atezolizumab signals an important change in the front-line treatment of HCC.
OBSERVATIONS
This review summarizes rapidly emerging clinical data on the promise and challenges of implementing ICBs in HCC and discusses the unmet need of biomarkers to predict response or resistance to therapy. Two strategies to target immunosuppression in tumors are also discussed: one proven (vascular endothelial growth factor pathway inhibition) and one currently under investigation (transforming growth factor-β pathway inhibition). The rationale and preliminary evidence on how their inhibition may reprogram the immunosuppressive milieu and enhance the efficacy of ICBs in HCC are reviewed.
CONCLUSION AND RELEVANCE
The recent successes and failures of angiogenesis inhibitors and ICBs, alone and in combination, have provided important insights into how to implement this novel systemic therapy in HCC and led to new avenues to enhance immunotherapy efficacy in this disease.
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a leading cause of cancer-related mortality. Early HCC can be treated curatively with surgery or ablation, but at advanced stages, available HCC treatments (eg, transarterial chemoembolization, systemic therapies) are merely palliative.1 The development of the multityrosine kinase inhibitor (mTKI) sorafenib represented the first systemic therapy for advanced HCC.2 While sorafenib was the only systemic therapy option for more than a decade, the field has evolved rapidly over the past 4 years.1 Four more agents succeeded in phase 3 trials and were eventually approved: lenvatinib mesylate (mTKI) in front-line treatment and regorafenib, cabozantinib S-malate (both mTKIs), and ramucirumab (anti-vascular endothelial growth factor [anti-VEGF] receptor(R)2) in second-line treatment.1 In addition, immune checkpoint blockers (ICBs) against the programmed cell death protein (PD)-1 and cytotoxic T lymphocyte antigen 4 have been approved for HCC in second-line treatment.3–5 Fueled by this progress, a large number of studies are currently testing ICBs worldwide, alone or in combination with other systemic or locoregional therapies.
There is a rationale supporting the use of immunotherapy in liver cancer.6 While HCC could be immunogenic, the tumor cells and the infiltrating stromal and immune cells promote an immunosuppressive tumor microenvironment (TME), including by upregulation of immune checkpoint molecules on their surface. Moreover, the tolerogenic liver environment, as well as chronic inflammation caused by the underlying liver disease present in most patients with HCC, further enhance immunosuppression, which enables the cancer cells to evade immune surveillance and potentially resist ICB treatment.6
In this review, we summarize recent clinical data on the use of ICBs in HCC and discuss the need for biomarkers to estimate the probable response or resistance to immunotherapy. We also elaborate on the roles of 2 of the pathways known to contribute to tumor immunosuppression: the VEGF and transforming growth factor (TGF)-β pathways. We summarize the rationale and preliminary evidence on how inhibition of these pathways may reprogram the immunosuppressive TME and enhance the efficacy of ICBs in HCC.
ICBs in Advanced HCC
Several ICBs have been tested in clinical phase 1, 2, and 3 trials in advanced HCC, either alone or in combination with targeted therapies or other ICBs. Response rates to ICB monotherapy ranged from 15% to 23% and increased to approximately 30% after combination treatment (Table 13–5,7–16 and Table 2).17–19 Based on durable antitumor responses from phase 2 trials of nivolumab and pembrolizumab (both anti–programmed cell death protein 1 [PD-1] antibodies) and nivolumab with ipilimumab (anti–cytotoxic T lymphocyte antigen-4 antibody) combination in HCC, the US Food and Drug Administration granted conditional approval for these ICBs.3–5,7,8 The CheckMate 040 study tested nivolumab alone or with ipilimumab and reported an overall response rate (ORR) of 22.5% for sorafenib-naive and 18.7% for sorafenib-experienced patients for nivolumab and 33% for the nivolumab-ipilimumab combination; median overall survival (OS) rates were 29 months (sorafenib naive), 15 months (sorafenib experienced), and 23 months (nivolumab-ipilimumab combination).3,5,7,8 The KEYNOTE-224 study investigated pembrolizumab in sorafenib-experienced patients and demonstrated an ORR of 17% and a median OS of 13 months.4
Table 1.
Selected Phase 1/2 Trials of Immune Checkpoint Blockers in Advanced Hepatocellular Carcinomaa
| Source | Treatment (No. of patients) | Prior sorafenib (%) | ORR (%) | TTP/PFS, mo | OS, mo |
|---|---|---|---|---|---|
| Monotherapy trials | |||||
| El-Khoueiry et al,3 Crocenzi et al,7 Sangro et al,8 2017 | Nivolumab (80) | 0 | 22.5b | NR/NR | 28.6 |
| El-Khoueiry et al,3 Crocenzi et al,7 Sangro et al,8 2017 | Nivolumab (182) | 100 | 18.7b | NR/NR | Approximately 15 |
| Wainberg et al,9 2017 | Durvalumab (40) | 92.5 | 10b | NR/2.7 | 13.2 |
| Zhu et al,4 2018 | Pembrolizumab (104) | 100 | 17b | 4.9/4.9 | 12.9 |
| Qin et al,102020 | Camrelizumab (217) | 72.8 | 14.7b | NR/2.1 | 13.8 |
| Combination therapy trials | |||||
| Kelley et al,11 2017 | Durvalumab + tremelimumab (40) | 75.0 | 25b | NR/NR | NR |
| Yau et al,5 2019 | Nivolumab + ipilimumab (148)c | 99 | 31–32b | NR/NR | 12.5–22.8 |
| Lee et al,12 2019 | Atezolizumab + bevacizumab (104) | 0 | 36b | NR/7.3 | 17.1 |
| Lee et al,13 2019 | Atezolizumab + bevacizumab (60) vs atezolizumab (59) | 0 | 20 vs 17b | NR/5.6 vs 3.4; HR 0.55 (80% CI, 0.40–0.74); P = .01 | NR |
| Llovet et al,14 2019 | Pembrolizumab + lenvatinib (67) | 6 | 52.2d | 11.8/9.7 | 20.4 |
| Yau et al,15 2020 | Nivolumab + cabozantinib S-malate (36) vs nivolumab + ipilimumab + cabozantinib S-malate (35) | 53 vs 66 | 14 vs 31b | NR/5.4 vs NR/6.8 | 21.5 vs NR |
Abbreviations: NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
Table 2.
Randomized Phase 3 Trials of Immune Checkpoint Blockers in Advanced Hepatocellular Carcinoma
| Source | Treatment (No. of patients) | Main efficacy and safety results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| OS | PFS | ORR | Grade 3–4 TRAEs | ||||||
| Median | HR (95% CI) | P value | Median | HR (95% CI) | P value | ||||
| First-line | |||||||||
| Yau et al,17 2019 (CheckMate 459) | Nivolumab (371) vs sorafenib (372) | 16.4 vs 14.7 mo | 0.85 (0.72–1.02) | .08a | 3.7 vs 3.8 mo | 0.93 (0.79–1.10) | 15% vs 7% (RECIST v1.1) | 22% vs 49% | |
| Finn etal,18 2020 (IMbravel50) | Atezolizumab + bevacizumab (336) vs sorafenib (165) | NE vs 13.2 mo | 0.58 (0.42–0.79) | <.001b | 6.8 vs 4.3 mo | 0.59 (0.47–0.76) | <.001b | 27% vs 12% (RECIST v1.1); 33% vs 13% (mRECIST) | 36% vs 46% |
| Second-line | |||||||||
| Finn et al,19 2020 (KEYNOTE-240) | Pembrolizumab (278) vs placebo (135) | 13.9 vs 10.6 mo | 0.781 (0.611–0.998) | .02a | 3.0 vs 2.8 mo | 0.775 (0.609–0.987) | .02a | 18.3% vs 4.4% (RECIST v1.1) | 18.6% vs 7.5% |
Abbreviations: HR, hazard ratio; mRECIST, modified Response Evaluation Criteria in Solid Tumors; NE, not estimable; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1; TRAEs, treatment-related adverse events; TTP, time to progression.
Despite the positive signals from phase 1/2 studies, 2 subsequent randomized phase 3 trials testing nivolumab and pembrolizumab in advanced HCC failed to meet their primary end points.17,19 The CheckMate 459 study compared nivolumab vs sorafenib in the first-line setting in advanced HCC (Table 2).17 The predefined threshold of significance for the primary end point OS was not reached (hazard ratio [HR], 0.85; P = .09). However, the median OS was substantially longer with nivolumab (16.4 vs 14.7 months) and was the longest ever seen for any drug monotherapy in advanced HCC. While relatively low, the ORR was double with nivolumab vs sorafenib (15% vs 7%), and 4% of the patients achieved a complete response. Nivolumab also showed an improved safety profile and quality of life compared with sorafenib.17 Another randomized phase 3 trial (KEYNOTE-240) tested pembrolizumab vs placebo in sorafenib-experienced patients with HCC and also missed the predefined significance levels for its coprimary end points of OS and progression-free survival (PFS) (Table 2).19 Median OS for pembrolizumab vs placebo was 13.9 vs 10.6 months (HR, 0.78; P = .02), and median PFS was 3.0 vs 2.8 months (HR, 0.78; P = .02). Pembrolizumab was well tolerated and improved ORR vs placebo (18.3 vs 4.4%); median duration of response was 13.8 months.19 The failure of both phase 3 trials despite clear activity of the ICBs could be explained by the unexpectedly long median OS in the control arms, which was likely impacted by poststudy treatment (including ICBs).19 Another phase 3 trial testing pembrolizumab vs placebo after previous sorafenib therapy in Asian patients is ongoing (KEYNOTE-394).20
The setbacks for ICB monotherapy indicated that combinations with additional agents might be necessary to enhance ICBs’ efficacy. In a randomized phase 3 trial,18 first-line atezolizumab (anti-PD-ligand [L]1 antibody) plus bevacizumab (anti-VEGF antibody) significantly improved the coprimary end points median OS vs sorafenib (not estimable vs 13.2 months; HR, 0.58; P < .001) and PFS (6.8 vs 4.3 months; HR, 0.59; P < .001) (Table 2). The safety profile was also more favorable, with fewer treatment-related grade 3 to 4 adverse events in the combination arm. These data are especially important given the limited impact of bevacizumab in HCC reported previously.21
Thus, despite recent successes, much remains to be understood for optimal integration of ICBs in HCC. Safety and efficacy of ICBs have been researched in combination ICB studies. Large studies have confirmed the durability of responses seen in smaller studies, but both phase 3 trials testing anti-PD-1 monotherapy failed. Clearly, patient selection using predictive biomarkers would be highly desirable. Moreover, the impressive outcome data seen with the combination of nivolumab with ipilimumab and atezolizumab with bevacizumab demonstrate that combinatorial approaches represent valid strategies to increase the efficacy of immunotherapy in HCC.
Potential Predictors of Response
Even though combination with targeted therapy nearly doubled the ORR of ICB, still more than half of the patients did not respond.18 Moreover, ICBs can cause severe immune-related adverse events as well as hyper progression (accelerated tumor growth) as a new pattern of progression during PD-1/PD-L1-targeted therapy (8% in HCC).22,23 Patient selection based on biomarkers could help to maximize the efficacy and reduce the number of patients who may not benefit or even be harmed from ICBs.
To our knowledge, no predictive biomarkers for ICB response currently exist. Several potential biomarkers have been proposed based on exploratory end points in HCC trials. These biomarkers mainly include PD-L1 expression, tumor mutational burden, and specific genomic alterations.
Expression of PD-L1 on immunohistochemistry is routinely being used for patient stratification in non–small cell lung cancer or gastric cancer; however, some patients with PD-L1–negative tumors respond to ICBs, and PD-L1 expression did not correlate with response in other tumor types.24 In HCC, tumoral PD-L1 expression (cutoff ≥1%) was not predictive for response to nivolumab or pembrolizumab.3,4,17 The combined positive score (PD-L1 on tumor and immune cells), assessed in only a subset of patients (n = 52), was associated with response to pembrolizumab and PFS.4
High tumor mutational burden (ie, number of nonsynonymous single nucleotide variants) may increase the likelihood of ICB response, as seen in some patients with ICB-treated cancer.24 Moreover, tumors with high microsatellite instability have a high tumor mutational burden, making them more likely to be sensitive to ICBs. Pembrolizumab was granted approval for any microsatellite instability–high or mismatch repair deficient tumors by the US Food and Drug Administration in 2017 and became the first drug to be approved with a tumor-agnostic indication.24,25 In HCC, tumor mutational burden is generally low, and its utility as a biomarker to predict response to ICB is not supported by available data.26 Similarly, the prevalence of microsatellite instability–high status is rare in HCC.27
Activated Wnt/β-catenin signaling has been associated with immune exclusion (cold, non–T-cell inflamed tumors) in HCC and proposed as potential biomarker of resistance to immunotherapy.28,29 However, this observation needs prospective confirmation.
On the basis of these findings, biomarker-associated patient selection for ICB may increase the likelihood of durable responses, but, to our knowledge, no such biomarkers have been confirmed for HCC. Based on the current clinical evidence, a predictive model that incorporates several factors (genetic and microenvironmental) may be more likely to estimate the probability of response to immunotherapy than a single biomarker.24
Targeting the Immune TME
Several immune and stromal cells of the TME directly or indirectly orchestrate antitumor immunity. Tumor-infiltrating CD4+ and CD8+ effector T lymphocytes are thought to mediate responses to ICBs. These cells are primed in the draining lymph nodes through tumor antigen presentation by dendritic cells. In addition, natural killer cells have the ability to directly recognize tumor cells and also contribute to antitumor immunity. Tumor-associated endothelial cells and the aberrant tumor vasculature hinder trafficking of immune effector cells while promoting the recruitment of immunosuppressive cell types. These cell types include regulatory T cells and myeloid-derived suppressor cells, which suppress effector T-cell proliferation, function, and cytotoxicity. Tumor-associated macrophages display different phenotypes; M1-like TAMs are considered to have antitumor activity, while M2-like tumor-associated macrophages exert immunosuppressive and tumor-promoting effects. Cancer-associated fibroblasts contribute to immunosuppression by inhibiting T-cell function and secretion of extracellular matrix, which represents a physical barrier to T-cell infiltration.30–32
Each of these components represents a potential target to reprogram the immunosuppressive TME. Herein, we focus on VEGF and TGF-β signaling—2 immunosuppressive pathways that are characteristic of HCC and modulate several cell types of the TME—as targets for reprogramming the HCC TME and enhance ICB efficacy.
Targeting VEGF Signaling
Hepatocellular carcinoma is a highly vascularized tumor that exploits the active formation of new blood vessels (angiogenesis) to grow and disseminate.33 The VEGF pathway is a key regulator of tumor angiogenesis and upregulated in most cancer types.34 All 5 targeted therapies with proven efficacy against HCC inhibit VEGF signaling,1 which supports the notion that this pathway mediates HCC progression.
In addition to its widely studied role in promoting angiogenesis, VEGF can directly affect immune cells of both myeloid and lymphoid lineage and promote immune evasion in different tumor types (Figure 1).30 For example, VEGF can impair maturation and function of dendritic cells, which are key antigen-presenting cells,35,36 and promote accumulation of regulatory T cells and myeloid-derived suppressor cells.37,38 In addition, VEGF can directly and indirectly inhibit infiltration and function of cytotoxic T lymphocytes,39,40 and increase PD-1 expression on intratumoral CD8+ T cells.41 Vascular endothelial growth factor indirectly affects immunity by increasing vessel permeability (leakiness), a main feature of the aberrant tumor vasculature.42,43 Leakiness impairs tumor blood flow and increases interstitial fluid pressure, consequently leading to hypoxia and acidosis,43 which promote immunosuppression by impairing the function of antigen-presenting cells and cytotoxic T lymphocytes, and by increasing accumulation of immunosuppressive cells and immune checkpoint expression.31,43,44
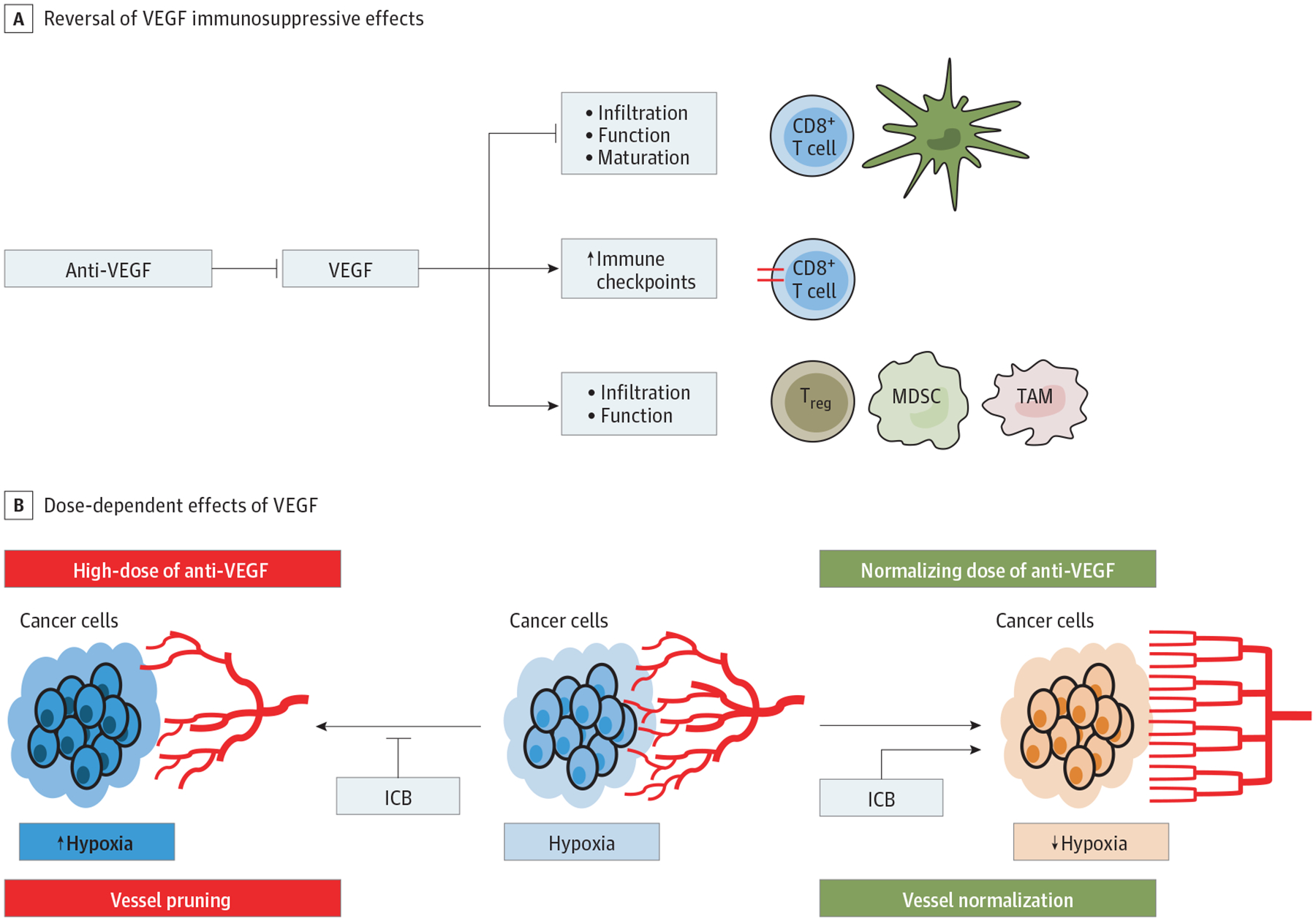
Effects of Anti-Vascular Endothelial Growth Factor (Anti-VEGF) Treatment on the Tumor Immune Microenvironment A, VEGF-targeted therapy can revert the immunosuppressive effects of VEGF. These effects include the inhibition of dendritic cell (DC) function and maturation, impairment of CD8+ T-cell function and infiltration, upregulation of immune checkpoint molecules, as well as the accumulation of immunosuppressive cell types, including tumor-associated macrophages (TAM), myeloid-derived suppressor cells (MDSC), and regulatory T cells (Treg). B, The effects of anti-VEGF treatment are dose-dependent. Higher doses lead to blood vessel pruning and thereby aggravate tumor hypoxia and acidosis, which supports tumor immune evasion. In contrast, low-dose anti-VEGF treatment may normalize the aberrant and dysfunctional tumor vasculature and thereby improve tumor perfusion, alleviate tumor hypoxia, reprogram the immunosuppressive milieu, and increase drug delivery of concomitant therapies, including immune checkpoint blockers (ICBs). Since anti-PD(L)-1 and anti–cytotoxic T lymphocyte antigen-4 antibodies may also normalize blood vessels and make them refractory to pruning by anti-VEGF(R) antibodies, even higher doses of anti-VEGF(R) may normalize tumor vessels when co-administered with immune checkpoint blockers (ICBs).
Inhibition of VEGF has also been shown to impact not just the vasculature, but also the immune TME of HCC, but the results have been inconsistent. Some preclinical studies showed that treatment with sorafenib, 30 mg/kg/d, decreased the intratumoral accumulation of regulatory T cells and myeloid-derived suppressor cells in subcutaneous and orthotopic murine HCC models.45,46 Another study showed that sorafenib, 10–90 mg/kg/d, triggered a natural killer cell response against HCC by inducing proinflammatory activity mediated in part by treatment-induced pyroptosis—a potentially immunogenic type of cell death—in tumor-associated macrophages.47 On the other hand, excessive vessel pruning caused by anti-VEGF therapy can increase tumor hypoxia and counteract these effects by promoting immunosuppression.43 In addition, antivascular effects can impair drug delivery to tumors and may thus reduce the efficacy of the anti-VEGF agent itself or concomitant ICBs.42 In experimental models of HCC, sorafenib, 40–50 mg/kg, treatment intensified tumor hypoxia and increased the accumulation of immunosuppressive cell types (eg, myeloid-derived suppressor cells, regulatory T cells, and M2-like macrophages) as well as PD-L1 expression in tumors.48,49 While anti-PD-1 therapy had no additional efficacy when combined with sorafenib, a triple combination with a CXCR4 inhibitor, that prevented polarization toward an immunosuppressive milieu, delayed tumor growth and reduced dissemination.49
Judicious dosing of anti-VEGF drugs to normalize the dysfunctional tumor vasculature instead of causing excessive vessel pruning may improve tumor perfusion, alleviate tumor hypoxia, and increase delivery of concomitant systemic therapy (eg, immunotherapy) (Figure 1).42 This hypothesis was supported by emerging clinical data: bevacizumab (anti-VEGF antibody) improved tumor vessel function in patients with rectal cancer,50 and increased tumor blood perfusion upon treatment with a VEGF receptor (R)-targeted TKI was associated with prolonged survival in patients with glioblastoma.51
Immune checkpoint blocker itself may normalize vascular structure and function when eliciting immune responses via CD4+ and CD8+ lymphocytes (Figure 1).52,53 Moreover, combination of lower, vascular-normalizing doses of anti-VEGF treatment with active immunotherapy showed efficacy in preclinical54 as well as clinical studies.55 In murine breast cancer models, lower doses of anti-VEGFR2 therapy induced vascular normalization and thereby alleviated tumor hypoxia, reprogrammed the immunosuppressive TME, and enhanced the efficacy of immunotherapy.54 A recent phase 1b trial reported encouraging efficacy data for the combination of regorafenib, 80 mg/d (half the standard dose), plus nivolumab (>35% ORR) in advanced gastric or colorectal cancer.55 A similar trial, using regorafenib, 80 mg/kg/d, and anti-PD1 antibodies is ongoing in HCC.56
Preclinical studies provided several insights into the effect of this treatment interaction in murine HCC models. One study showed that dual inhibition of VEGFR2 and PD-1 resulted in normalized vessel formation mediated by CD4+ T cells and was accompanied by augmented antitumor immune response and improved efficacy, including in ICB-resistant HCC models.57 Another study showed that lenvatinib—but not sorafenib—decreased the tumor infiltration by monocytes and macrophages, increased the numbers of CD8+ T cells, and augmented the antitumor effect of anti-PD-1 treatment in a subcutaneous murine model of HCC.58
In HCC, several trials are currently investigating anti-VEGF–based therapies in combination with ICBs (Table 3).59–86 Based on preliminary data, the combinations of lenvatinib plus pembrolizumab and bevacizumab plus atezolizumab have both been granted breakthrough therapy designation for advanced HCC by the US Food and Drug Administration (Table 1).12,14 The latter combination improved both coprimary end points (OS and PFS) over sorafenib alone in the first-line setting of advanced HCC in the recently reported IMbrave150 phase 3 study18 and is currently being investigated in another phase 3 study as an adjuvant therapy after resection or local ablation (IMbrave050).64 Lenvatinib plus pembrolizumab is currently being tested in a phase 3 front-line trial, and data are forthcoming.63
Table 3.
Selected Ongoing Studies Testing Immune Checkpoint Blockers in Combination With Anti-VEGF(R)-Targeted Therapies in Hepatocellular Carcinoma
| Drug (mechanism) | Phase, setting | Primary end point | Primary completion | ClinicalTrials.gov identifier |
|---|---|---|---|---|
| Atezolizumab (PD-L1 mAb) + cabozantinib S-malate (TKI) vs sorafenib (TKI) | Phase 3, advanced | PFS/OS | Q3 2020 | NCT0375579159 |
| TACE + durvalumab (PD-L1 mAb) +/− bevacizumab (VEGF mAb) vs TACE + placebos | Phase 3, intermediate | PFS | Q1 2021 | NCT0377895760 |
| Camrelizumab (PD-1 mAb) + apatinib (TKI) vs sorafenib (TKI) | Phase 3, advanced | OS, PFS | Q4 2021 | NCT0376429361 |
| Durvalumab (PD-L1 mAb) +/− bevacizumab (VEGF mAb) vs placebo | Phase 3, early adjuvant | RFS | Q2 2022 | NCT0384742862 |
| Prembrolizumab (PD-1 mAb) + lenvatinib (TKI) vs lenvatinib (TKI) | Phase 3, advanced | PFS/OS | Q3 2022 | NCT0371359363 |
| Atezolizumab (PD-L1 mAb) + bevacizumab (VEGF mAb) vs active surveillance | Phase 3, early adjuvant | RFS | Q1 2023 | NCT0410209864 |
| Nivolumab + ipilimumab vs sorafenib/lenvatinib | Phase 3, advanced | OS | Q3 2023 | NCT0403960765 |
| Nivolumab (PD-1 mAb) + lenvatinib (TKI) vs lenvatinib (TKI) | Phase 2/3, advanced | OS | Q1 2021 | NCT0404465166 |
| Sintilimab (PD-1 mAb) + IBI305 (VEGF mAb) vs sorafenib (TKI) | Phase 2/3, advanced | OS, ORR | Q4 2022 | NCT0379444067 |
| Camrelizumab + apatinib or FOLFOX4 or GEMOX | Phase 2 | Safety | Q4 2018 | NCT0309289568 |
| Sintilimab (PD-1 mAb) + anlotinib hydrochloride (TKI) | Phase 2, advanced | ORR, safety | Q4 2019 | NCT0405215269 |
| Nivolumab (PD-1 mAb) + sorafenib (TKI) | Phase 2, advanced | MTD, ORR | Q2 2020 | NCT0343989170 |
| Toripalimab (PD-1 mAb) + lenvatinib (TKI) + systemic chemotherapy | Phase 2, advanced | 6-mos PFS | Q4 2020 | NCT0417017971 |
| Nivolumab (PD-1 mAb) + lenvatinib (TKI) | Phase 2, advanced | ORR, safety | Q3 2021 | NCT0384120172 |
| Atezolizumab (PD-L1 mAb) + bevacizumab (VEGF mAb) | Phase 2, advanced | ORR | Q4 2021 | NCT0418007273 |
| HAIC + camrelizumab (PD-1 mAb) + apatinib (TKI) | Phase 2, advanced | ORR | Q4 2021 | NCT0419188974 |
| Sintilimab (PD-1 mAb) + lenvatinib (TKI) | Phase 2, advanced | ORR | Q3 2022 | NCT0404280575 |
| TACE + durvalumab (PD-L1 mAb) + bevacizumab (VEGF mAb) | Phase 2, intermediate and advanced | 6-mo PFS | Q4 2022 | NCT0393783076 |
| Camrelizumab (PD-1 mAb) + apatinib (TKI) | Phase 1/2, advanced | OS | Q4 2018 | NCT0294232977 |
| Pembrolizumab (PD-1 mAb) + sorafenib (TKI) | Phase 1/2, advanced | ORR | Q3 2020 | NCT0321141678 |
| Toripalimab (PD-1 mAb) + sorafenib (TKI) | Phase 1/2, advanced | 6-mos PFS, safety | Q4 2020 | NCT0406994979 |
| Durvalumab (PD-L1 mAb) + tivozanib (TKI) | Phase 1/2, advanced | Safety | Q3 2021 | NCT0397061680 |
| Nivolumab (PD-1 mAb) + regorafenib (TKI) | Phase 1/2, advanced | Safety | Q4 2022 | NCT0417055681 |
| Pembrolizumab (PD-1 mAb) + lenvatinib (TKI) | Phase 1, advanced | Safety, ORR, DOR | Q3 2019 | NCT0300692682 |
| Spartalizumab (PD-1 mAb) + sorafenib (TKI) | Phase 1, advanced | Safety | Q1 2020 | NCT0298844083 |
| Nivolumab (PD-1 mAb) + lenvatinib (TKI) | Phase 1, advanced | Safety | Q2 2020 | NCT0341892284 |
| Pembrolizumab (PD-1 mAb) + regorafenib (TKI) | Phase 1, advanced | Safety | Q3 2020 | NCT0334729285 |
| Nivolumab (PD-1 mAb) + cabozantinib (TKI) | Phase 1, early neoadjuvant | Safety, completion | Q1 2022 | NCT0329994686 |
Abbreviations: DOR, duration of response; FOLFOX4, fluorouracil, leucovorin, and oxaliplatin; GEMOX, gemcitabine and oxaliplatin; HAIC, hepatic arterial infusion chemotherapy; mAb, monoclonal antibody; MTD, maximum tolerated dose; ORR, overall response rate; OS, overall survival; PD-1, programmed cell death protein 1; PD-L1, programmed death 1 ligand 1; PFS, progression-free survival; Q, quarter; RFS, recurrence-free survival; TACE, transarterial chemoembolization; TKI, tyrosine kinase inhibitor; VEGF(R), vascular endothelial growth factor (receptor).
Taken together, the role of targeting the immune TME of HCC has been confirmed clinically using anti-VEGF/VEGFR therapy. The effects may be dose and agent dependent when using mTKIs. The use of lower, vascular-normalizing doses of anti-VEGF therapies is supported by emerging clinical data,55 but needs further confirmation in larger studies in HCC. This mechanistic complexity notwithstanding, given that combining anti-VEGF and ICB treatment is the new standard of care in HCC, future therapeutic approaches will have to improve on this new backbone treatment.
Targeting TGF-β Signaling
The TGF-β pathway has complex functions in the liver tissue, where it regulates homeostasis. Dysregulated TGF-β signaling is involved in the pathogenesis of several liver diseases, including HCC development. Moreover, TGF-β has a dual role in carcinogenesis as it can act as a tumor suppressor, particularly in early tumor stages, but can also promote tumor progression and dissemination in more advanced cancers. Several components of the TME, including fibroblasts, immune cells, and extracellular matrix components, mediate these context-dependent functions.87,88
Regulation of tumor immune evasion is also seen with TGF-β, especially in advanced tumor stages.88 The immunosuppressive effects are mediated by dampening T-cell responses, but TGF-β may also affect other immune cells.88 For example, TGF-β inhibits natural killer cell function and increases the number of regulatory T cells.89,90 Another action of TGF-β is promotion of antigen-specific T-cell exhaustion by upregulating PD-1.91 In addition, this cytokine affects tumor-associated macrophages by inducing an M2-like (tumor-promoting) phenotype.92 The expression of VEGF in different cell types of the TME is induced by TGF-β, including immune, tumor, and stromal cells.93,94 This crosstalk between TGF-β and VEGF signaling may further promote TGF-β–mediated immunosuppression.
Moreover, the profibrotic effects of TGF-β can indirectly contribute to immunosuppression. TGF-β activates myofibroblasts and upregulates the deposition of extracellular matrix proteins in the tumor,95 which may act as a physical barrier and lead to exclusion of effector T cells (Figure 2).96
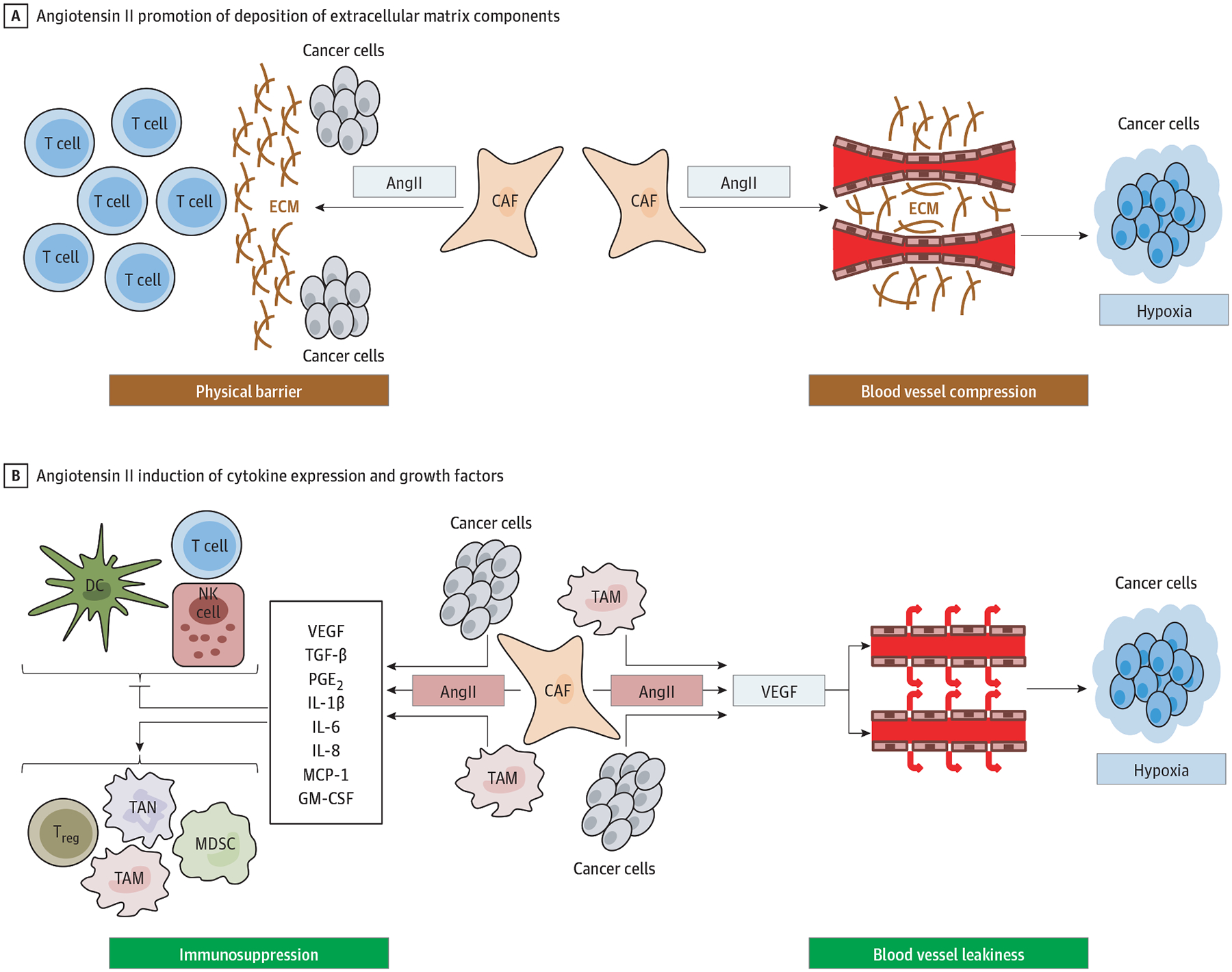
The Angiotensin II (AngII)/AngII Type I Receptor Axis Promotes Tumor Immune Evasion by Affecting Cancer Cells as Well as Various Stromal Cells A, AngII activates profibrotic pathways and promotes the deposition of extracellular matrix (ECM) components from fibroblasts. ECM acts as a physical barrier to T-cell infiltration, which hampers an antitumor immune response. ECM also leads to blood vessel compression, which impairs tumor perfusion and aggravates tumor hypoxia and acidosis. The hypoxic and acidic milieu further promotes immunosuppressive mechanisms. B, AngII also induced the secretion of different cytokines and growth factors from cancer and stromal cells. These cytokines inhibit function and accumulation of dendritic cells (DC), natural killer (NK) cells, and T-cells, and promote the accumulation of immunosuppressive cell types, including regulatory T cells (Treg), tumor-associated macrophages (TAM), and neutrophils (TAN), and myeloid derived suppressor cells (MDSC). Finally, tumor hypoxia is further aggravated by AngII-mediated upregulation of vascular endothelial growth factor (VEGF), which increases vascular leakiness and impairs tumor blood perfusion. CAF indicates cancer-associated fibroblast; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; MCP, monocyte chemoattractant protein-1; PGE2, prostaglandin E2; and TGF-β, transforming growth factor-β.
Activated TGF-β signaling was linked to an exhausted immune subclass in HCC (approximately 10% of cases) characterized by exhausted T cells, impaired cytotoxicity, M2-like tumor-associated macrophages, and an upregulation of immunosuppressive cytokines.28 In addition, 4 distinct clusters with different levels of TGF-β disruption were described recently with use of The Cancer Genome Atlas transcriptome sequencing database,97 and the highly activated TGF-β cluster overlapped with the exhausted immune subclass.28,97 Patients with the least disrupted TGF-β signaling had a better outcome than those with activated or inactivated TGF-β signaling. These data implicate TGF-β in immunotherapy resistance. A phase 2 study reported that high pretreatment plasma TGF-β levels correlated with HCC resistance to pembrolizumab.98
These data also suggest that TGF-β inhibition may reprogram the TME to enhance ICB efficacy, especially in HCCs with activated TGF-β signaling. In preclinical (non-HCC) tumor models, blockade of TGF-β reduced infiltration of regulatory effector T cells and myeloid-derived suppressor cells, facilitated T-cell infiltration, and enhanced the efficacy of anti-PD-L1 and anti–cytotoxic T lymphocyte antigen-4 treatment.96,99 Phase 2 studies testing the TGF-β receptor 1 kinase inhibitor galunisertib alone100 or combined with sorafenib101 showed acceptable safety and prolonged survival in advanced HCC, especially in patients who experienced a decrease in circulating TGF-β. The combination of galunisertib plus nivolumab is currently being investigated as second-line treatment in a phase 2 study.102 In addition, a phase 1 study is testing the combination of NIS793 (anti-TGF-β antibody) and spartalizumab (anti-PD-1 antibody) in patients with advanced cancers, including HCC.103
In addition to directly targeting TGF-β, TGF-β activity can be downregulated using inhibitors of the angiotensin II (AngII)/AngII type I receptor (AT1R) axis of the renin-angiotensin system.104,105 Apart from reducing TGF-β expression, blocking AngII/AT1R signaling can also prevent other immunosuppressive effects of the renin-angiotensin system (Figure 2).31 Inhibition of AT1R normalized the desmoplastic stroma in several preclinical tumor models and consequently decreased solid stress and thereby improved vascular perfusion and reduced hypoxia.104–106 Inhibition of AT1R also reduced infiltration of tumor-associated neutrophils and regulatory T cells and increased CD8+ T-cell infiltration in pancreatic tumors of obese mice.107 In patients with pancreatic cancer, gene expression profiles indicated reduced activation of Wnt signaling in lisinopril users.108 These data suggest that inhibition of the renin-angiotensin system could have the potential to enhance ICB efficacy and may help to overcome primary resistance to immunotherapy, which may be associated with activated Wnt/β-catenin signaling in HCC.28,29 In preclinical breast and pancreatic cancer models, AngII/AT1R blockade reprogrammed the immunosuppressive TME and improved the efficacy of ICBs directed against PD-1 and cytotoxic T lymphocyte antigen-4.106 A phase 2 study is currently investigating the AT1R blocker losartan plus nivolumab in combination with chemoradiotherapy in patients with pancreatic cancer.109 In HCC, the use of renin-angiotensin system inhibitors decreased tumor growth preclinically110 and was associated with improved outcome in patients with advanced HCC treated with sorafenib.111
In summary, TGF-β signaling is often upregulated in HCC and contributes to an immunosuppressive TME, mainly by inhibiting effector T-cell function. The profibrotic effects of TGF-β also contribute to immunosuppression by inhibiting immune cell infiltration. The combination of TGF-β (or renin-angiotensin system) inhibition and ICBs is currently being tested in early phase clinical trials. Whether these approaches will be effective in reprogramming the immunosuppressive TME of HCC will need prospective confirmation.
Conclusions and Future Perspectives
Immune checkpoint blocker monotherapy with nivolumab and pembrolizumab did not show significant benefit in randomized phase 3 trials in HCC.17,19 The hypothesis that efficacy of immunotherapy in HCC can be safely achieved by using combination therapies was confirmed by the recent success of dual VEGF/PD-L1 blockade in front-line (IMbrave150 study) treatment.18 This breakthrough signals an important change in the standard treatment for advanced HCC. Moreover, the success of this therapy will directly affect the future use of currently approved targeted therapies in first- and second-line treatment. These drugs have been tested against sorafenib or in sorafenib-experienced patients, respectively, but not in patients who have previously received anti-VEGF/PD-L1 treatment. The patterns of tumor recurrence are expected to differ. Thus, establishing the optimal treatment sequence of anti-VEGF(R) antibodies, mTKIs, and ICBs will become an important challenge in the management of HCC. This new first-line treatment will also impact ongoing clinical studies as well as the design of future trials in advanced HCC, and perhaps the implementation of immunotherapy with other treatment modalities (eg, surgery, radiotherapy, and transarterial chemoembolization) at earlier stages of the disease.
Another challenge will be addressing treatment resistance. Even though dual VEGF/PD-L1 blockade doubled the response rates, more than two-thirds of the patients still do not respond. Whether targeting other pathways, such as TGF-β or AT1R, will be effective with ICBs in these patients needs to be demonstrated.112
Estimating the probability of response or resistance to immunotherapy remains a challenge. Identification of biomarkers will help to improve patient outcomes and reduce adverse effects and economic burden of these treatments. Currently, there is no clinically available biomarker to estimate response to ICBs. Tumor tissue should routinely be gathered within clinical HCC trials to better characterize the TME and identify potential biomarkers.
The underlying cause of HCC may also have implications for ICB response. Chronic inflammation, as seen in patients with viral hepatitis, induces the expression of immune checkpoint molecules and promoteseffectorT-cellexhaustion.113 Results from phase 3 trials demonstrated higher efficacy of ICBs in patients with underlying viral disease vs other etiologic factors, including nonalcoholic fatty liver disease.17,18 Nonalcoholic fatty liver disease can cause CD4+ T-cell loss and induce protumor effects in natural killer T cells, CD8+ T-cells, and helper T17 cells.114 In preclinical models, nonalcoholic fatty liver disease impaired the efficacy of immunotherapy.115 Given the expected surge in nonalcoholic fatty liver disease–associated HCC and reduction in viral-related disease, these observations warrant further investigation of the immune cell landscape of HCC with respect to the cause of the disease.
These challenges not with standing, the systemic therapy of HCC has rapidly changed with the development of multiple antiangiogenic agents and immunotherapies over the past 3 to 4 years, raising expectations for unprecedented durable responses and increased survival in this aggressive cancer.
Conflict of Interest Disclosures:
Dr Pinter is an investigator for Bayer, Bristol Myers Squibb, Lilly, and Roche; he received speaker honoraria from Bayer, Bristol Myers Squibb, Eisai, Lilly, and MSD; he is a paid consultant for Bayer, Bristol Myers Squibb, Eisai, Ipsen, Lilly, MSD, and Roche; he received travel support from Bayer and Bristol Myers Squibb. Dr Jain received honorarium from Amgen and consultant fees from Chugai, Ophthotech, Merck, SPARC, SynDevRx, and XTuit. Dr Jain owns equity in XTuit, Enlight, SPARC, SynDevRx, and Accurius Therapeutics and serves as a paid member of the boards of trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. He is a member of the scientific advisory board of Accurius Therapeutics. He is listed as an inventor on US Patents: 2011329638 (issued April 13, 2017) and application 16/063,353 (pending). The research of Dr Jain is supported by National Institutes of Health grants P01-CA080124, R35-CA197743, R01-CA208205, and U01-CA224173, and by the National Foundation for Cancer Research, Harvard Ludwig Cancer Center, Advanced Medical Research Foundation, Jane’s Trust Foundation, and Koch Institute/DF/HCC Bridge Project. Dr Duda received consultant fees from Bayer, Simcere, and Bristol Myers Squibb, and research grants from Bayer, Exelixis, and Bristol Myers Squibb. Dr Duda’s research is supported by Department of Defense awards W81XWH-19-1-0284 and W81XWH-19-1-0482.
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1001/jamaoncol.2020.3381
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8265820
Citations & impact
Impact metrics
Article citations
Exosomal PDL1 Suppresses the Anticancer Activity of CD8<sup>+</sup> T Cells in Hepatocellular Carcinoma.
Anal Cell Pathol (Amst), 2024:1608582, 09 Oct 2024
Cited by: 0 articles | PMID: 39421264 | PMCID: PMC11483647
Immunotherapy in liver cancer: overcoming the tolerogenic liver microenvironment.
Front Immunol, 15:1460282, 03 Sep 2024
Cited by: 0 articles | PMID: 39295859 | PMCID: PMC11409253
Review Free full text in Europe PMC
Gastrointestinal Cancer Patient Derived Organoids at the Frontier of Personalized Medicine and Drug Screening.
Cells, 13(16):1312, 06 Aug 2024
Cited by: 0 articles | PMID: 39195202 | PMCID: PMC11352269
Review Free full text in Europe PMC
Transarterial Embolization Enhances Programmed Cell Death Ligand 1 Expression and Influences CD8<sup>+</sup>T Lymphocytes Cytotoxicity in an Orthotopic Hepatocellular Carcinoma Rat Model.
Cardiovasc Intervent Radiol, 47(10):1372-1381, 05 Aug 2024
Cited by: 1 article | PMID: 39103638
A necroptosis-regulated model from single-cell analysis that predicts survival and identifies the Pivotal role of MAGEA6 in hepatocellular carcinoma.
Heliyon, 10(18):e37711, 13 Sep 2024
Cited by: 0 articles | PMID: 39315163 | PMCID: PMC11417173
Go to all (165) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials (Showing 28 of 28)
- (1 citation) ClinicalTrials.gov - NCT04039607
- (1 citation) ClinicalTrials.gov - NCT03937830
- (1 citation) ClinicalTrials.gov - NCT03211416
- (1 citation) ClinicalTrials.gov - NCT03755791
- (1 citation) ClinicalTrials.gov - NCT03713593
- (1 citation) ClinicalTrials.gov - NCT04102098
- (1 citation) ClinicalTrials.gov - NCT04044651
- (1 citation) ClinicalTrials.gov - NCT03006926
- (1 citation) ClinicalTrials.gov - NCT03841201
- (1 citation) ClinicalTrials.gov - NCT03847428
- (1 citation) ClinicalTrials.gov - NCT03970616
- (1 citation) ClinicalTrials.gov - NCT04170556
- (1 citation) ClinicalTrials.gov - NCT04170179
- (1 citation) ClinicalTrials.gov - NCT03764293
- (1 citation) ClinicalTrials.gov - NCT04180072
- (1 citation) ClinicalTrials.gov - NCT04052152
- (1 citation) ClinicalTrials.gov - NCT04042805
- (1 citation) ClinicalTrials.gov - NCT03794440
- (1 citation) ClinicalTrials.gov - NCT02942329
- (1 citation) ClinicalTrials.gov - NCT02988440
- (1 citation) ClinicalTrials.gov - NCT03778957
- (1 citation) ClinicalTrials.gov - NCT03299946
- (1 citation) ClinicalTrials.gov - NCT03439891
- (1 citation) ClinicalTrials.gov - NCT03092895
- (1 citation) ClinicalTrials.gov - NCT03347292
- (1 citation) ClinicalTrials.gov - NCT04069949
- (1 citation) ClinicalTrials.gov - NCT03418922
- (1 citation) ClinicalTrials.gov - NCT04191889
Show less
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Angiogenesis and immune checkpoint dual blockade: Opportunities and challenges for hepatocellular carcinoma therapy.
World J Gastroenterol, 28(42):6034-6044, 01 Nov 2022
Cited by: 6 articles | PMID: 36405383 | PMCID: PMC9669824
Review Free full text in Europe PMC
The Treatment Landscape of Advanced Hepatocellular Carcinoma.
Curr Oncol Rep, 24(7):917-927, 26 Mar 2022
Cited by: 18 articles | PMID: 35347594
Review
Immune checkpoint inhibitors for hepatocellular carcinoma - A game changer in treatment landscape.
J Formos Med Assoc, 121(8):1371-1383, 07 Apr 2022
Cited by: 3 articles | PMID: 35400583
Review
Immunotherapy for advanced hepatocellular carcinoma: a focus on special subgroups.
Gut, 70(1):204-214, 03 Aug 2020
Cited by: 126 articles | PMID: 32747413 | PMCID: PMC7788203
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NCI NIH HHS (3)
Grant ID: R35 CA197743
Grant ID: R01 CA208205
Grant ID: P01 CA080124





