Abstract
Background
Venous sinus stenting (VSS) is an accepted and minimally invasive treatment for adult idiopathic intracranial hypertension (IIH) associated with lateral sinus stenosis (LSS). The efficacy and safety of venous sinus stenting (VSS) in children with IIH has not been established.Methods
This is a retrospective analysis of IIH patients 18 years of age or younger with LSS treated with VSS at our institution. Included patients have fulminant disease course or are refractory or intolerant to medical management.Results
Eight patients were identified; 4 males and 4 females. Mean age is 13.4 years (range 4-18). All patients had severe headaches, 5 had blurred vision, 3 had diplopia and 3 had pulsatile tinnitus. Papilledema was present in 4 patients. Three patients had prior surgical procedures. Four patients were intolerant to medical management, 3 were refractory and 1 had fulminant course. Cerebral venography demonstrated severe stenosis of the dominant sinus in 6 patients and of bilateral co-dominant sinuses in 2 patients. Six patients had intrinsic stenosis and 2 had extrinsic stenosis. Venous sinus stenting (VSS) resulted in improvement of symptoms, papilledema and normalization of CSF opening pressure in 7 patients. No immediate complications were observed. Mean follow-up period is 21 months (range 6-42). Two patients required re-stenting; one responded well and the other had persistent symptoms and underwent subsequent surgical procedures of CSF diversion, suboccipital decompression and duraplasty which were also ineffective.Conclusion
VSS may provide a viable option for pediatric IIH patients who are intolerant to medication, have failed conservative management or prior surgical interventions, or present with fulminant disease.Free full text

Management of idiopathic intracranial hypertension in children utilizing venous sinus stenting
Abstract
Background
Venous sinus stenting (VSS) is an accepted and minimally invasive treatment for adult idiopathic intracranial hypertension (IIH) associated with lateral sinus stenosis (LSS). The efficacy and safety of venous sinus stenting (VSS) in children with IIH has not been established.
Methods
This is a retrospective analysis of IIH patients 18 years of age or younger with LSS treated with VSS at our institution. Included patients have fulminant disease course or are refractory or intolerant to medical management.
years of age or younger with LSS treated with VSS at our institution. Included patients have fulminant disease course or are refractory or intolerant to medical management.
Results
Eight patients were identified; 4 males and 4 females. Mean age is 13.4 years (range 4–18). All patients had severe headaches, 5 had blurred vision, 3 had diplopia and 3 had pulsatile tinnitus. Papilledema was present in 4 patients. Three patients had prior surgical procedures. Four patients were intolerant to medical management, 3 were refractory and 1 had fulminant course. Cerebral venography demonstrated severe stenosis of the dominant sinus in 6 patients and of bilateral co-dominant sinuses in 2 patients. Six patients had intrinsic stenosis and 2 had extrinsic stenosis. Venous sinus stenting (VSS) resulted in improvement of symptoms, papilledema and normalization of CSF opening pressure in 7 patients. No immediate complications were observed. Mean follow-up period is 21
years (range 4–18). All patients had severe headaches, 5 had blurred vision, 3 had diplopia and 3 had pulsatile tinnitus. Papilledema was present in 4 patients. Three patients had prior surgical procedures. Four patients were intolerant to medical management, 3 were refractory and 1 had fulminant course. Cerebral venography demonstrated severe stenosis of the dominant sinus in 6 patients and of bilateral co-dominant sinuses in 2 patients. Six patients had intrinsic stenosis and 2 had extrinsic stenosis. Venous sinus stenting (VSS) resulted in improvement of symptoms, papilledema and normalization of CSF opening pressure in 7 patients. No immediate complications were observed. Mean follow-up period is 21 months (range 6–42). Two patients required re-stenting; one responded well and the other had persistent symptoms and underwent subsequent surgical procedures of CSF diversion, suboccipital decompression and duraplasty which were also ineffective.
months (range 6–42). Two patients required re-stenting; one responded well and the other had persistent symptoms and underwent subsequent surgical procedures of CSF diversion, suboccipital decompression and duraplasty which were also ineffective.
Conclusion
VSS may provide a viable option for pediatric IIH patients who are intolerant to medication, have failed conservative management or prior surgical interventions, or present with fulminant disease.
Introduction
Venous sinus stenting (VSS) for patients with idiopathic intracranial hypertension (IIH) and significant cerebral lateral sinus stenosis (LSS) has become increasingly accepted as a minimally invasive treatment option in patients requiring treatment escalation beyond medical therapy.1 VSS has been shown to reduce intracranial pressure (ICP) by alleviating venous outflow obstruction. This reduction in ICP directly ameliorates associated signs and symptoms of intracranial hypertension including headaches, diplopia, tinnitus, and papilledema.2–4
While treatment options for adult IIH include medical management with acetazolamide (ACTZ), serial lumbar punctures, ventriculoperitoneal and lumboperitoneal shunting, optic sheath fenestration, subtemporal decompression, and, more recently, venous sinus stenting, the role of these treatments in the pediatric population is not well established. Clinical trial data to support any of these interventions for the pediatric population is lacking, and indications for when to use each treatment therapy is not clear. Weight loss and acetazolamide are the most common approaches initially.5,6 Failure of conservative medical management necessitates more aggressive therapies, especially in the context of severe papilledema and visual deterioration despite appropriate medical therapy. Symptoms of IIH in the pediatric population are primarily headache and blurred vision, reported in approximately 96 percent and 71 percent of patients respectively, with significant papilledema present in 87 percent.7
There has been limited data to support any specific surgical interventions due to the paucity of pediatric patients with IIH or due to the potential under-diagnosis in the pediatric population. In patients refractory to medical management, multiple surgical techniques have been employed to help prevent vision loss.8 Cerebrospinal fluid diversion techniques, which have been the standard of care as they rapidly normalize ICP, have significant revision rates and associated complications.9,10 The role of optic nerve sheath fenestration for pediatric IIH consists mostly of small case series, with one study showing continued edema or vision loss in 5/12 patients requiring fenestration of the fellow eye.11,12
There is a growing body of literature validating venous sinus stenting as a durable and successful treatment for adult patients with IIH. Stent related reductions in ICP can lead to resolution or improvement of papilledema and visual field loss, and symptomatic improvement of headaches and pulsatile tinnitus.4 To assess whether this adult data might be extended to other patient populations in whom IIH is diagnosed, we evaluated our own institutional experience placing venous sinus stents in pediatric patients with medically refractory IIH.
Methods
Institutional Review Board approval was obtained. This is a retrospective analysis of IIH patients 18 years of age or younger treated with VSS at our institution. Verbal consent was obtained for inclusion in this study by the patient’s parents. Patients were diagnosed with IIH using Friedman’s 2013 update to the modified Dandy criteria.13 Lumbar puncture (LP) was performed under fluoroscopy in the lateral decubitus position, preferentially using local anesthesia, but general anesthesia was required for patients younger than 15
years of age or younger treated with VSS at our institution. Verbal consent was obtained for inclusion in this study by the patient’s parents. Patients were diagnosed with IIH using Friedman’s 2013 update to the modified Dandy criteria.13 Lumbar puncture (LP) was performed under fluoroscopy in the lateral decubitus position, preferentially using local anesthesia, but general anesthesia was required for patients younger than 15 years old.
years old.
All patients were considered for surgical treatment of IIH on the basis of one or more of the following conditions: the presence of papilledema and vision loss despite maximally tolerated medical management for 3 months or prior surgical treatment for IIH, debilitating headaches despite maximally tolerated medical management for 3 months or prior surgical treatment, and fulminant IIH. Maximal medical management of IIH was defined as the maximum tolerated dose of acetazolamide or topiramate, managed by the treating pediatric neurologists and or neuro-ophthalmologists. Fulminant IIH was defined as acute visual field loss to within the central 5° and/or a decrement in visual acuity to less than or equal to 20/50 in either eye, in the presence of grade 4 or 5 papilledema. The patients with MRV findings of greater than 50 percent bilateral lateral sinus stenosis or unilateral stenosis of a dominant lateral sinus were considered for VSS in lieu of other surgical options such as CSF diversion surgery.
months or prior surgical treatment for IIH, debilitating headaches despite maximally tolerated medical management for 3 months or prior surgical treatment, and fulminant IIH. Maximal medical management of IIH was defined as the maximum tolerated dose of acetazolamide or topiramate, managed by the treating pediatric neurologists and or neuro-ophthalmologists. Fulminant IIH was defined as acute visual field loss to within the central 5° and/or a decrement in visual acuity to less than or equal to 20/50 in either eye, in the presence of grade 4 or 5 papilledema. The patients with MRV findings of greater than 50 percent bilateral lateral sinus stenosis or unilateral stenosis of a dominant lateral sinus were considered for VSS in lieu of other surgical options such as CSF diversion surgery.
Patient demographics, body-mass index (BMI), symptoms, CSF opening pressure, papilledema (Frisén scale), medication type and dosage were obtained from our database. Data on LSS localization and severity were collected from contrast-enhanced MR venography (MRV) and catheter venography before and after stenting. The procedural records were reviewed to document trans-stenotic pressure gradient, stent sizes, procedural complications, and post-stenting venous outflow.
Catheter venography and manometry
Venography and manometry were performed as previously described in our adult patients.4 Briefly, using a trans-femoral venous approach, a 5 French guide catheter was advanced over a 0.035″ guide-wire to the internal jugular vein close to the jugular bulb. Next, a 0.027″ microcatheter was navigated through the guide catheter over a 0.014″ microwire into the superior sagittal sinus (SSS). Venography was performed to validate LSS. Then intra-sinus pressures of the SSS, transverse and sigmoid sinuses and the jugular bulb were measured by connecting the microcatheter to a pressure transducer. Trans-stenotic gradient (TSG) measurements were preferentially performed using local anesthesia, but general anesthesia was necessary for patients younger than 15 years old because they could not tolerate the procedure awake. Severe stenosis was defined as a luminal stenosis of more than 70%. To proceed with stent placement, patient must have both severe stenosis and TSG threshold of 8
years old because they could not tolerate the procedure awake. Severe stenosis was defined as a luminal stenosis of more than 70%. To proceed with stent placement, patient must have both severe stenosis and TSG threshold of 8 mm
mm Hg when measuring under local anesthesia and 6
Hg when measuring under local anesthesia and 6 mm
mm Hg when measuring under general anesthesia, since general anesthesia has been shown to lower intracranial venous pressures.14,15
Hg when measuring under general anesthesia, since general anesthesia has been shown to lower intracranial venous pressures.14,15
Venous sinus stenting
Venous sinus stenting was performed under general anesthesia. The femoral venous sheath was exchanged for a 0.088″ guide catheter which was advanced over a 5 French diagnostic catheter and a 0.035″ guide-wire to the internal jugular vein on the side of intended stenting. The diagnostic catheter and guide-wire were then removed and a 0.072″ distal access catheter was advanced over a combination of a 0.027″ microcatheter and a 0.014″ microwire through the guide catheter into the proximal transverse sinus. The microcatheter and microwire were further advanced into the SSS and the microwire was then removed. Using an exchange-length 0.014″ microwire positioned in the SSS, the microcatheter was removed and appropriately sized (6–9 mm × 40
mm × 40 mm) Precise Pro Rx Nitinol stents (Cordis, Miami Lakes, FL) were advanced and deployed in the lateral sinus across the stenosis, as previously described for our adult patients.4 A single stent was used for short stenosis and a longer stent construct with two overlapping stents was used for longer stenosis. If the lateral sinuses were codominant with bilateral stenosis, the stent was placed in the nondominant cerebral hemisphere. Post-stent venous sinus pressures were measured, and ipsilateral common carotid artery angiography was performed through contralateral femoral arterial access to ensure that there was no delay in cortical venous drainage in the stented lateral sinus. After stenting, if applicable, the patient’s acetazolamide and topiramate were tapered as tolerated.
mm) Precise Pro Rx Nitinol stents (Cordis, Miami Lakes, FL) were advanced and deployed in the lateral sinus across the stenosis, as previously described for our adult patients.4 A single stent was used for short stenosis and a longer stent construct with two overlapping stents was used for longer stenosis. If the lateral sinuses were codominant with bilateral stenosis, the stent was placed in the nondominant cerebral hemisphere. Post-stent venous sinus pressures were measured, and ipsilateral common carotid artery angiography was performed through contralateral femoral arterial access to ensure that there was no delay in cortical venous drainage in the stented lateral sinus. After stenting, if applicable, the patient’s acetazolamide and topiramate were tapered as tolerated.
Antiplatelet protocol
Patients younger than 15 years of age were treated with aspirin 81 mg daily and clopidogrel 1
mg daily and clopidogrel 1 mg/kg daily starting five days prior to the procedure and were continued for 1 month. Subsequently, the patients continued aspirin 81
mg/kg daily starting five days prior to the procedure and were continued for 1 month. Subsequently, the patients continued aspirin 81 mg for 1-year post-stenting.
mg for 1-year post-stenting.
Patients older than 15 years of age were treated with aspirin 325 mg daily and clopidogrel 75
mg daily and clopidogrel 75 mg daily starting five days prior to the procedure and were continued for 1 month. Subsequently, the patients continued aspirin 325
mg daily starting five days prior to the procedure and were continued for 1 month. Subsequently, the patients continued aspirin 325 mg for 1-year post-stenting.
mg for 1-year post-stenting.
Follow-up
Patients were followed at regular intervals with neuro-ophthalmological assessments. MRV with contrast was obtained 3 months after stenting and then annually. Catheter venogram or angiogram was considered only if there was clinical and imaging concern for recurrent venous sinus stenosis.
Results
Eight patients fulfilled criteria and opted to proceed with venous sinus stenting. Patients’ demographic data and previous procedures are summarized in Table 1. The mean age was 13.4 years (range: 4–18 years). 4 patients were female and 4 were male. Average BMI was 24.1 kg/m2 (range: 15.3–34.2). Four patients (50.0%) were intolerant to medications, 1 patient (12.5%) had fulminant vision loss, and 3 patients (37.5%) were medically-refractory with persistent severe headaches. Three patients had undergone prior surgical procedures. Patient 1 had a prior suboccipital craniectomy, C1 laminectomy and duraplasty. Patient 4 had a prior cranial vault expansion surgery and Patient 7 had previously undergone bilateral optic nerve sheath fenestrations.
kg/m2 (range: 15.3–34.2). Four patients (50.0%) were intolerant to medications, 1 patient (12.5%) had fulminant vision loss, and 3 patients (37.5%) were medically-refractory with persistent severe headaches. Three patients had undergone prior surgical procedures. Patient 1 had a prior suboccipital craniectomy, C1 laminectomy and duraplasty. Patient 4 had a prior cranial vault expansion surgery and Patient 7 had previously undergone bilateral optic nerve sheath fenestrations.
Table 1.
Demographics, symptoms, previous treatments in pediatric IIH patients.
| Pt | Age (Y) | Gender | Treatment failure | Prior surgery | Longest F/U (mo.) | BMI | Medications | ||
|---|---|---|---|---|---|---|---|---|---|
| Pre-stent | Post-stent | Pre-stent | Post-stent | ||||||
| 1 | 4 | M | Intolerance | SOC/C1lam/D | 18 | 15.3 | 15.4 | None | None |
| 2 | 18 | F | Refractory | none | 42 | 30.9 | 30.7 | ACTZ 1.5 g/d g/d | ACTZ 1.0 g/d g/d |
| 3 | 15 | F | Fulminant | none | 8 | 26.8 | 25.1 | ACTZ 1.0 g/d g/d | None |
| 4 | 7 | M | Intolerance | CVE | 38 | 16.1 | 17.1 | None | None |
| 5 | 16 | F | Refractory | none | 17 | 19.4 | 17.8 | ACTZ 2.0 g/d g/d | None |
| 6 | 17 | F | Intolerance | none | 30 | 24.6 | 23.7 | ACTZ 1.0 g/d g/d | None |
| 7 | 16 | M | Refractory | R/L ONSF | 14 | 25.7 | 31.5 | ACTZ 2.0 g/d g/d | None |
| 8 | 14 | M | Intolerance | none | 6 | 34.2 | 33.5 | ACTZ 4.0 g/d g/d | TPM 100 mg/d mg/d |
CVE: cranial vault expansion; SOC/C1lam/D: suboccipital craniectomy, C1 laminectomy, duraplasty; ONSF: optic nerve sheath fenestration.
Pre-stent symptoms
Eight patients (100%) complained of severe headaches, 3 (37.5%) of severe pulsatile tinnitus (PST), 3 (37.5%) of diplopia from CN VI palsy, and 5 (62.5%) had subjective visual complaints (Table 2).
Table 2.
Response to stenting in pediatric IIH patients.
| Pt | Headache | Tinnitus | Diplopia | Papilledema (Grade) | ||||
|---|---|---|---|---|---|---|---|---|
| Pre-stent | Post-stent | Pre-stent | Post-stent | Pre-stent | Post-stent | Pre-stent | Post-stent | |
| 1 | Severe | Severe | – | – | – | – | 0 | 0 |
| 2 | Severe | Resolved | + | Resolved | + | Resolved | 4 | 0 |
| 3 | Severe | Resolved | + | Resolved | + | Resolved | 4 | 0 |
| 4 | Severe | Resolved | – | – | – | – | 1 | 0 |
| 5 | Severe | Mild | – | – | – | – | 0 | 0 |
| 6 | Severe | Resolved | – | – | – | – | 0 | 0 |
| 7 | Severe | Resolved | + | Resolved | + | Resolved | 1 | 0 |
| 8 | Severe | Resolved | – | – | – | – | 0 | 0 |
Pre-stent neuro-ophthalmologic data
Four patients had papilledema (50.0%), with 2 patients having Grade I and 2 patients having Grade IV Frisen papilledema (Table 2). Six out of 8 patients were taking ACTZ on presentation, with an average dose of 1.75 g per day among the patients taking ACTZ (range: 1.0–4.0
g per day among the patients taking ACTZ (range: 1.0–4.0 g/day), as summarized in Table 1.
g/day), as summarized in Table 1.
Pre-stent opening pressure (OP)
Seven out of 8 patients had lumbar punctures performed prior to stenting with a mean OP of 44.6 cm of water (range: 26–78) with a normal cerebrospinal fluid composition in all patients (Table 3). Patient 1’s family refused a pre-stent LP due to their concern for precipitating herniation as he had significant descent of his cerebellar tonsils on a previous brain MRI.
cm of water (range: 26–78) with a normal cerebrospinal fluid composition in all patients (Table 3). Patient 1’s family refused a pre-stent LP due to their concern for precipitating herniation as he had significant descent of his cerebellar tonsils on a previous brain MRI.
Table 3.
Data regarding cerebral venous sinus stenting in pediatric IIH patients.
| Pt | Stenosis details | Trans-stenotic gradient (mm Hg) | Opening pressure (cm H2O) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stented side | Dominant sinus | Type | Stent (mm) | Pre | Post | Decrease | Pre | Post | Decrease | % Decrease | |
| 1a | Right | Right | Intrinsic | 6 x 40 > > 6 x 40 6 x 40 | 7, 7 | 1, 2 | 6, 5 | Refused | 27b | – | – |
| 2c | Right | Co-dominant | Intrinsic | 8 x 40 > > 8 x 40 8 x 40 | 28, 17 | 6, 1 | 22, 16 | 78 | 24 | 54 | 69 |
| 3 | Right | Right | Extrinsic | 7 x 40, 8 x 40 | 34 | 1 | 33 | 65 | 22 | 43 | 66 |
| 4 | Left | Left | Intrinsic | 8 x 40 | 10 | 2 | 8 | 32 | 18 | 14 | 44 |
| 5 | Right | Right | Intrinsic | 8 x 40 | 14 | 0 | 14 | 39 | 14 | 25 | 64 |
| 6 | Right | Co-dominant | Extrinsic | 7 x 40, 8 x 40 | 10 | 1 | 9 | 26 | Refusedd | – | – |
| 7 | Right | Right | Intrinsic | 9 x 40 | 19 | 1 | 18 | 39 | 33.5e | 5.5 | 14 |
| 8 | Right | Right | Intrinsic | 8 x 40 | 9 | 0 | 9 | 33 | Refusedd | – | – |
| Avg.f | 16.1 | 1.5 | 14.6 | 44.6 | 22.7 | 28.3 | 51.4 | ||||
aPersistence of symptoms despite stenting, underwent VPS after stenting that also did not affect symptoms
bLP prior to second stenting, pt family refused all other LP procedures.
cRestenosis proximal to stent 2 yr after initial treatment, second stent placed, LP reflects pre-stenting LP to LP after 2nd stent placed.
yr after initial treatment, second stent placed, LP reflects pre-stenting LP to LP after 2nd stent placed.
dRefused post-stent LP because patients had resolution of symptoms post-stenting.
eVenogram after post-stent LP showed a non-significant trans-stenotic gradient of 5 mm Hg.
mm Hg.
fAverage values reflect only the first stenting procedure and only OP for pts that had LP before and after stenting.
Venography
Catheter venography demonstrated bilateral severe (>70%) stenosis of the lateral sinus in 2 patients with codominant venous sinus anatomy, and unilateral severe stenosis of dominant lateral sinus in the remaining 6 patients; 5 on the right and 1 on the left. The stenosis involved the distal transverse sinus and the transverse-sigmoid junction in all patients and was intrinsic in six patients. Pre- and post- venous sinus stenting results are summarized in Table 3. Mean pre-stent TSG was 16.1 mm Hg (range: 7–34
mm Hg (range: 7–34 mm Hg) decreasing to a mean of 1.5
mm Hg) decreasing to a mean of 1.5 mm Hg (range: 0–6
mm Hg (range: 0–6 mm Hg) post-stenting. Three patients (under 15 years of age) underwent manometry under general anesthesia with mean TSG of 8.7
mm Hg) post-stenting. Three patients (under 15 years of age) underwent manometry under general anesthesia with mean TSG of 8.7 mm Hg (range: 7–10), whereas 5 patients (15 years and older) had manometry under local anesthesia with mean TSG of 21
mm Hg (range: 7–10), whereas 5 patients (15 years and older) had manometry under local anesthesia with mean TSG of 21 mmHg (range: 10–34). Figures 1 and and22 illustrate pre-stenting MRV and venography images, and post-stenting venography images of patient 8 (intrinsic stenosis) and patient 6 (extrinsic stenosis), respectively.
mmHg (range: 10–34). Figures 1 and and22 illustrate pre-stenting MRV and venography images, and post-stenting venography images of patient 8 (intrinsic stenosis) and patient 6 (extrinsic stenosis), respectively.
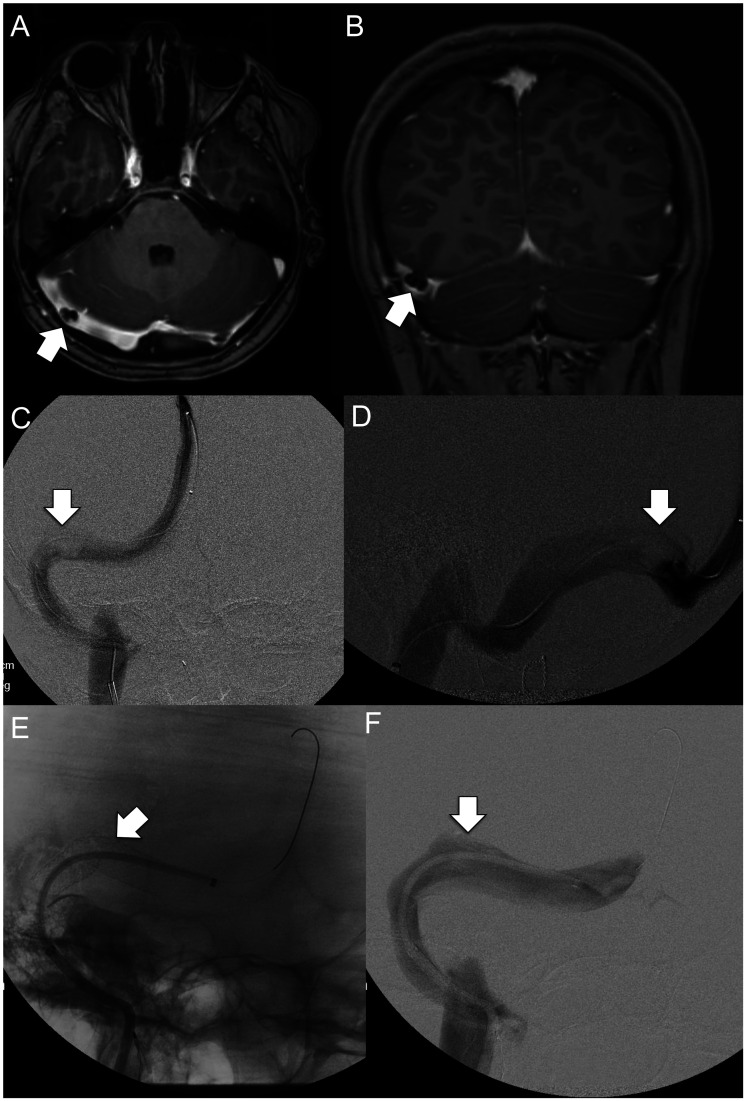
Axial (a) and coronal plane (b) contrast-enhanced MRV images demonstrate focal short segment intrinsic stenosis of the right distal transverse sinus related to underlying arachnoid granulation. Intrinsic stenosis is again appreciated on frontal (c) and lateral (d) projections during catheter venography. Following stent placement (e), the previously seeeffects of intrinsic stenosis have resolved with normalization of sinus caliber (F).
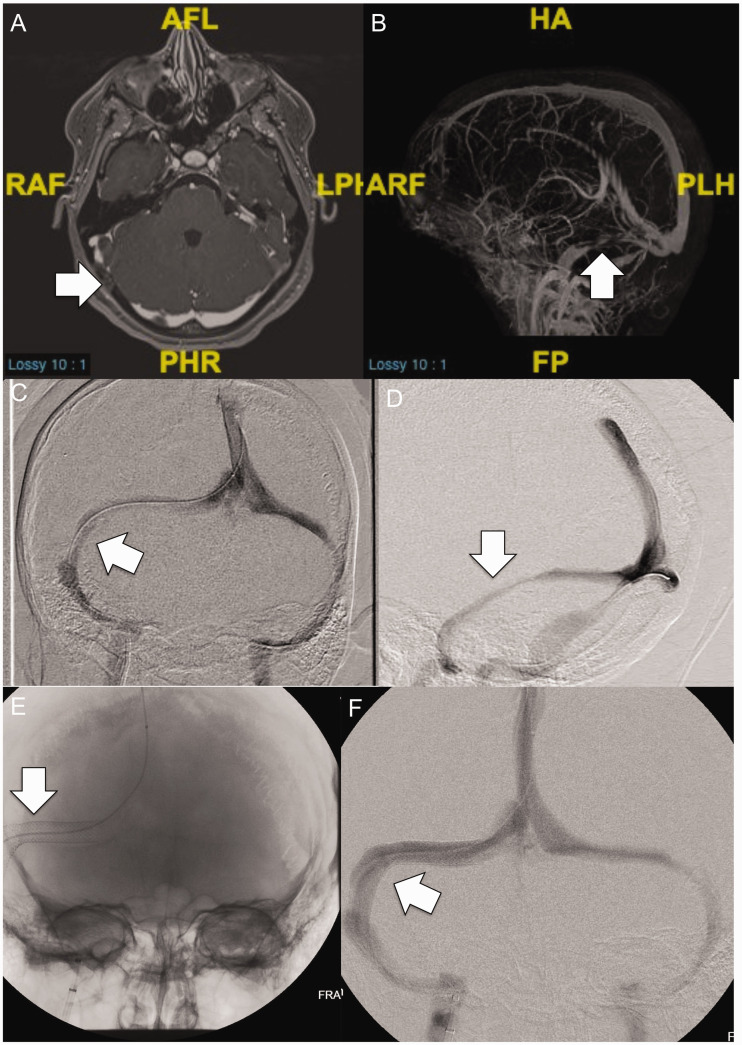
Axial (a) and 3 D sagittal plane (b) contrast-enhanced MRV images demonstrate long segment extrinsic stenosis of the right transverse sinus related to overlying brain parenchyma. Extrinsic stenosis is again appreciated on frontal (c) and oblique (d) projections during catheter venography. Following stent placement (e), the previously seen effects of extrinsic stenosis have resolved with normalization of sinus caliber (f).
D sagittal plane (b) contrast-enhanced MRV images demonstrate long segment extrinsic stenosis of the right transverse sinus related to overlying brain parenchyma. Extrinsic stenosis is again appreciated on frontal (c) and oblique (d) projections during catheter venography. Following stent placement (e), the previously seen effects of extrinsic stenosis have resolved with normalization of sinus caliber (f).
Post-stent symptoms
There was an average follow-up of 21 months post-stenting (range: 6–42 months), as seen in Table 1. All 8 patients had severe headaches pre-VSS and 7 patients had a significant subjective improvement with resolution of headaches post-VSS in 6 patients (Table 2). Patient 5 had improved but still mild headaches post-stenting. Patient 1 had no significant change in headache symptomatology and ultimately underwent placement of a ventriculoperitoneal shunt 3 months following VSS and a repeat suboccipital decompression and duraplasty 14 months after VSS. Notably, the patient’s symptoms did not improve following any of his procedures. The 3 patients with tinnitus pre-stent had complete resolution post-procedure.
Post-stent neuro-ophthalmologic data
All of the 4 patients with pre-stenting papilledema had complete resolution of optic disc edema post-stenting. Of the 6 patients who were taking ACTZ pre-VSS, 4 patients were tapered completely off of their ACTZ. Patient 2 reduced her ACTZ from 1.5 g/day to 1.0
g/day to 1.0 g/day, while patient 8 went from ACTZ 4.0
g/day, while patient 8 went from ACTZ 4.0 g/day to topiramate (TPM) 100
g/day to topiramate (TPM) 100 mg/day. The 2 remaining patients who were not on ACTZ or TPM prior to VSS did not start it post-VSS.
mg/day. The 2 remaining patients who were not on ACTZ or TPM prior to VSS did not start it post-VSS.
Post-stent opening pressure (OP)
Five of the 8 patients had an LP before and after the VSS with an average reduction in opening pressure of 28.3 cm of water (range: 5.5–54
cm of water (range: 5.5–54 cm of water) with an average percent reduction in opening of 52% (range: 14–69%), as seen in Table 3. The two patients who refused post-stent LP had complete resolution of symptoms post-stenting (Patients 6, 8).
cm of water) with an average percent reduction in opening of 52% (range: 14–69%), as seen in Table 3. The two patients who refused post-stent LP had complete resolution of symptoms post-stenting (Patients 6, 8).
Adverse events
There were no procedural or peri-procedural complications. In one patient, there was decreased flow in the vein of Labbe post-procedure which was treated with anticoagulation for 14 days (in addition to dual anti-platelet therapy) according to our previously described protocol.16 There were no associated symptoms. Follow-up MRV showed patent vein of Labbe with no cerebral parenchymal changes. There was no neurological morbidity or mortality.
days (in addition to dual anti-platelet therapy) according to our previously described protocol.16 There were no associated symptoms. Follow-up MRV showed patent vein of Labbe with no cerebral parenchymal changes. There was no neurological morbidity or mortality.
Re-treatment
Two out of 8 patients underwent repeat venous sinus stenting. Patient 2 (Table 3) had recurrence of her initial symptoms (headache, diplopia) 24 months after the initial VSS with an LP demonstrating an elevated opening pressure of 30 cm of water and venography showing a TSG of 17
cm of water and venography showing a TSG of 17 mm Hg with severe venous sinus stenosis in the transverse sinus proximal to her previously placed stent. She underwent stenting of the region of re-stenosis with complete resolution of her symptoms 27 months after her initial VSS. She has not had recurrence of her symptoms for nearly 2 years.
mm Hg with severe venous sinus stenosis in the transverse sinus proximal to her previously placed stent. She underwent stenting of the region of re-stenosis with complete resolution of her symptoms 27 months after her initial VSS. She has not had recurrence of her symptoms for nearly 2 years.
Patient 1 (Table 3) underwent repeat venous sinus stenting after having no symptom relief after his first VSS. Of note, the patient’s family refused LP prior to his first VSS. An LP 4 months post stenting showed an OP of 27 cm of water and MRV and venography demonstrated venous stenosis proximal to the previously placed stent with a TSG of 7
cm of water and MRV and venography demonstrated venous stenosis proximal to the previously placed stent with a TSG of 7 mm Hg. Due to the child’s age, both the OP and TSG measurements were performed under general anesthesia. The patient’s symptoms persisted despite stenting the region of new stenosis. The headaches persisted and he then had a ventriculoperitoneal shunt placed 3 months after his second stenting and a repeat suboccipital decompression and duraplasty 14 months after re-stenting. Neither procedure had an effect on the patient’s debilitating headaches.
mm Hg. Due to the child’s age, both the OP and TSG measurements were performed under general anesthesia. The patient’s symptoms persisted despite stenting the region of new stenosis. The headaches persisted and he then had a ventriculoperitoneal shunt placed 3 months after his second stenting and a repeat suboccipital decompression and duraplasty 14 months after re-stenting. Neither procedure had an effect on the patient’s debilitating headaches.
Discussion
Venous sinus stenting is an effective minimally invasive treatment option for adults with IIH and LSS, but its safety and efficacy are not well documented in the pediatric population. There are very limited data in the literature about the utility of VSS in pediatric IIH patients. To date, only few case reports and a very recent single case series of 14 patients have been published.17–20 Our institutional experience of pediatric IIH patients with venous sinus stenosis who failed or did not tolerate medical management, or had fulminant IIH demonstrates that venous sinus stenting may be an effective and safe treatment option for carefully selected pediatric IIH patients and should be considered as an alternative to other surgical treatments for IIH.
Venous sinus stenting is feasible in the pediatric population using the same technique that has been previously described in the adult IIH population.4 For children aged five years or older, the size of peripheral vasculature, including the femoral vein and arteries, and the cerebral vessels is similar to the adults.21–24 This allows for the use of the same stent delivery systems and stent sizing in the adult and pediatric population. Nonetheless, caution must be exercised in treating each individual patient, particularly those younger than 5 years or those with a particularly short stature or low body weight.
Despite the small number of patients, our series shows some differences in the patient’s characteristics compared to the adult patients; half of the patients are male, for example, which is expected in the pediatric population. Interestingly, the majority (6/8) had intrinsic stenosis, which is a higher rate than we see in the adult population.
Our venous sinus stenting protocol stipulates dual antiplatelet therapy for 1 month post procedure, followed by 1 year of aspirin alone. Antiplatelet therapy has been shown to be a relatively common and safe practice in the pediatric interventional cardiology literature.25–28 Nonetheless, due to the inherent risks while on antiplatelet medications, parents and children must be appropriately counseled prior to its initiation, particularly in regard to avoiding activities with the potential for falls or contact. We did not have any complications in our series pertaining to antiplatelet therapy.
Procedural safety is of paramount importance. The overall rate of major neurologic complications like intracranial bleeding, venous sinus thrombosis or venous infarction following VSS in adult IIH patients is reported to be very low, less than 2%.29 We had no peri- or postprocedural complications in our pediatric VSS population. Of particular concern in VSS is impaired drainage of the vein of Labbe following stent placement. The incidence of this phenomenon in our adult series was approximately 13%, with appropriate management consisting of anticoagulation for a period of 2 weeks postoperatively to prevent secondary cortical vein thrombosis.16 Recognition and treatment of impaired drainage through the vein of Labbe is critical because it can result in cerebral edema, venous infarction, or cerebral hemorrhage if not addressed.30 One of our pediatric patients was found to have slow flow through the vein of Labbe following stent placement. The patient was continued on anticoagulation for 2
weeks postoperatively to prevent secondary cortical vein thrombosis.16 Recognition and treatment of impaired drainage through the vein of Labbe is critical because it can result in cerebral edema, venous infarction, or cerebral hemorrhage if not addressed.30 One of our pediatric patients was found to have slow flow through the vein of Labbe following stent placement. The patient was continued on anticoagulation for 2 weeks post-procedurally and did not have any adverse neurologic sequelae. Stent adjacent stenosis (SAS) is the main long-term complication that results in VSS failure and the need for re-stenting. It is observed to occur in 14% of adult patients.29 Two (25%) of our patients had SAS and underwent repeat stenting which resulted in durable improvement in 1 patient while the other did not respond. In the recently published case series of VSS in pediatric IIH patients with mean follow-up of 1.7 years, 4 (29%) out of 14 patients required re-treatment for persistent symptoms, 2 of them were re-stented and all 4 patients required shunting.20 In the same case series, no neurologic complications were observed but access site complications occurred in 4 out of 14 patients.20 None of our 8 patients experienced access site complications.
weeks post-procedurally and did not have any adverse neurologic sequelae. Stent adjacent stenosis (SAS) is the main long-term complication that results in VSS failure and the need for re-stenting. It is observed to occur in 14% of adult patients.29 Two (25%) of our patients had SAS and underwent repeat stenting which resulted in durable improvement in 1 patient while the other did not respond. In the recently published case series of VSS in pediatric IIH patients with mean follow-up of 1.7 years, 4 (29%) out of 14 patients required re-treatment for persistent symptoms, 2 of them were re-stented and all 4 patients required shunting.20 In the same case series, no neurologic complications were observed but access site complications occurred in 4 out of 14 patients.20 None of our 8 patients experienced access site complications.
The overall effectiveness of VSS is dependent upon appropriate patient selection. This requires a multidisciplinary approach to patient evaluation that includes neurologists, neuro-ophthalmologists, neurosurgeons, and neurointerventionalists. Patients being considered for VSS must have appropriate symptomatology, but IIH symptoms are non-specific and can be difficult to elicit in the pediatric population. Objective measures, such as LP opening pressure and trans-stenotic pressure gradients, are essential to confirm a clinically and hemodynamically significant venous stenosis that would benefit from stenting. LP opening pressure and TSG are most reliably determined in an awake or minimally sedated patient.31 This poses a significant problem in the pediatric population as many young patients cannot tolerate these procedures without deep sedation or general anesthesia. Sedation and general anesthesia have the potential to change intracranial venous pressures and the TSG. Even though there is some evidence that certain anesthetic agents increase intracranial venous pressure, larger studies showed that the pressures and gradients are generally decreased under general anesthesia. With these in mind we considered a TSG of 6 mm Hg acceptable for patients evaluated under general anesthesia.
mm Hg acceptable for patients evaluated under general anesthesia.
In our series, VSS was considered as an alternative to CSF diversion surgery. Although effective in reducing ICP and alleviating symptoms, CSF diversion surgeries are invasive and have high rates of revision in the first 12 months, exceeding 40% in some studies.30 Furthermore, the need for hospitalizations and shunt revisions lead to significantly higher cost of CSF diversion surgery compared to VSS.32 The majority of the patients treated in our pediatric IIH series had a durable and significant improvement in symptoms. The single patient who did not have a response to VSS did not meet all of the objective criteria that we typically use for VSS evaluation. The patient did not have a pre-stenting LP due to parental refusal and had venous manometry performed under general anesthesia. Venous manometry demonstrated a TSG of 7 mm Hg, which is the threshold that we use to proceed to VSS. The patient was ultimately stented with consensus agreement amongst the multidisciplinary team with the decision based heavily on the convincing symptomatology explained by the patient’s parents. This highlights the importance of strict adherence to objective measures while deciding to perform VSS, particularly in the pediatric population. It also argues for using a potentially higher trans-stenotic gradient threshold for venous manometry performed under general anesthesia. Importantly, the patient who did not improve following VSS subsequently underwent a ventriculoperitoneal shunt and repeat suboccipital craniectomy and duraplasty, neither of which significantly improved his symptoms suggesting an alternative pathophysiology for his symptomatology.
mm Hg, which is the threshold that we use to proceed to VSS. The patient was ultimately stented with consensus agreement amongst the multidisciplinary team with the decision based heavily on the convincing symptomatology explained by the patient’s parents. This highlights the importance of strict adherence to objective measures while deciding to perform VSS, particularly in the pediatric population. It also argues for using a potentially higher trans-stenotic gradient threshold for venous manometry performed under general anesthesia. Importantly, the patient who did not improve following VSS subsequently underwent a ventriculoperitoneal shunt and repeat suboccipital craniectomy and duraplasty, neither of which significantly improved his symptoms suggesting an alternative pathophysiology for his symptomatology.
Our pediatric IIH series demonstrates that VSS may be an effective option in carefully selected patients at improving symptoms and objective measures, such as LP opening pressure and papilledema. VSS may allow pediatric IIH patients who are intolerant to medication, fail conservative management, or present with fulminant disease.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Justin Schwarz https://orcid.org/0000-0002-2772-3896
Ali Al Balushi https://orcid.org/0000-0002-8759-2576
Athos Patsalides https://orcid.org/0000-0002-3035-4331
References
Articles from Interventional Neuroradiology are provided here courtesy of SAGE Publications
Full text links
Read article at publisher's site: https://doi.org/10.1177/1591019920976234
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8050535
Citations & impact
Impact metrics
Article citations
A systematic review of surgical and interventional radiology procedures for pediatric idiopathic intracranial hypertension.
Front Pediatr, 12:1466688, 30 Oct 2024
Cited by: 0 articles | PMID: 39539766 | PMCID: PMC11557315
Review Free full text in Europe PMC
Combined surgical repair and venous sinus stenting for patients with skull base encephaloceles secondary to dural venous sinus stenosis.
Acta Neurochir (Wien), 165(8):2283-2292, 21 Jun 2023
Cited by: 1 article | PMID: 37344735
Integrated understanding of hydrocephalus - a practical approach for a complex disease.
Childs Nerv Syst, 37(11):3313-3324, 10 Jun 2021
Cited by: 11 articles | PMID: 34114082 | PMCID: PMC8578093
Review Free full text in Europe PMC
Casper Versus Precise Stent for the Treatment of Patients with Idiopathic Intracranial Hypertension.
Clin Neuroradiol, 31(3):853-862, 18 May 2021
Cited by: 1 article | PMID: 34003319 | PMCID: PMC8463398
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Superior Ophthalmic Vein Flow Patterns as a Marker of Venous Sinus Stenosis and Hypertension in Idiopathic Intracranial Hypertension: A Case of Emergent Transverse Sinus Stenting as Treatment of Fulminant Idiopathic Intracranial Hypertension.
World Neurosurg, 161:170-178, 02 Jul 2021
Cited by: 0 articles | PMID: 34224883
Idiopathic Intracranial Venous Hypertension: Toward a Better Understanding of Venous Stenosis and the Role of Stenting in Idiopathic Intracranial Hypertension.
J Neuroophthalmol, 43(4):451-463, 05 Jul 2023
Cited by: 2 articles | PMID: 37410913
Venous Sinus Stenting in Idiopathic Intracranial Hypertension: Results of a Prospective Trial.
J Neuroophthalmol, 37(2):113-121, 01 Jun 2017
Cited by: 45 articles | PMID: 27556959
Life-threatening idiopathic intracranial hypertension: the role of venous sinus stenting.
Childs Nerv Syst, 38(8):1433-1443, 10 Jun 2022
Cited by: 2 articles | PMID: 35687167
Review





