Abstract
Background
Obstructive sleep apnea (OSA) is the most prevalent sleep-related breathing disorder which could impair someone's quality of life and is also associated with poor outcomes from many diseases. Currently, the evidence regarding the link between OSA and coronavirus disease 2019 (COVID-19) is still conflicting. This study aims to analyze the relationship between OSA and poor outcomes of COVID-19.Materials and methods
We systematically searched the PubMed and Europe PMC database using specific keywords related to our aims until December 10th, 2020. All articles published on COVID-19 and OSA were retrieved. The quality of the study was assessed using the Newcastle-Ottawa Scale (NOS) tool for observational studies. Statistical analysis was done using Review Manager 5.4 software.Results
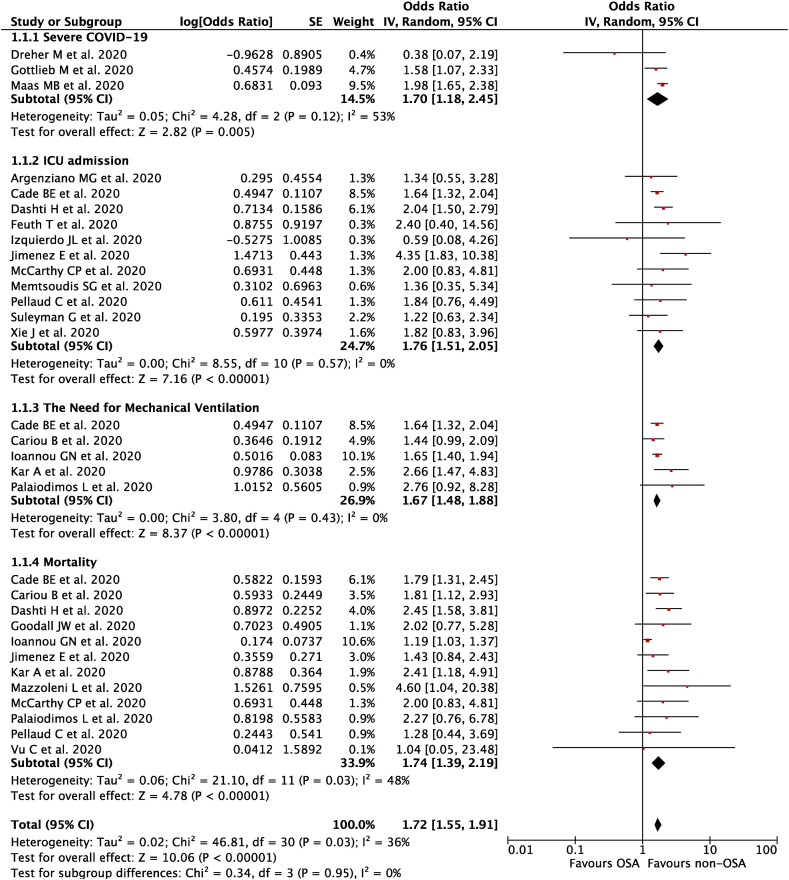
A total of 21 studies with 54,276 COVID-19 patients were included in this meta-analysis. This meta-analysis showed that OSA was associated with composite poor outcome [OR 1.72 (95% CI 1.55-1.91), p < 0.00001, I2 = 36%, random-effect modeling] and its subgroup which comprised of severe COVID-19 [OR 1.70 (95% CI 1.18-2.45), p = 0.005], ICU admissions [OR 1.76 (95% CI 1.51-2.05), p < 0.00001], the need for mechanical ventilation [OR 1.67 (95% CI 1.48-1.88), p < 0.00001], and mortality [OR 1.74 (95% CI 1.39-2.19), p < 0.00001].Conclusions
Extra care and close monitoring should be provided to patients with OSA to minimize the risk of infections. Simple questionnaires such as STOP-Bang questionnaire can be used for screening patients who may be at risk for severe adverse outcomes.Free full text

Obstructive sleep apnea (OSA) and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: a systematic review and meta-analysis
Abstract
Background
Obstructive sleep apnea (OSA) is the most prevalent sleep-related breathing disorder which could impair someone's quality of life and is also associated with poor outcomes from many diseases. Currently, the evidence regarding the link between OSA and coronavirus disease 2019 (COVID-19) is still conflicting. This study aims to analyze the relationship between OSA and poor outcomes of COVID-19.
Materials and methods
We systematically searched the PubMed and Europe PMC database using specific keywords related to our aims until December 10th, 2020. All articles published on COVID-19 and OSA were retrieved. The quality of the study was assessed using the Newcastle–Ottawa Scale (NOS) tool for observational studies. Statistical analysis was done using Review Manager 5.4 software.
Results
A total of 21 studies with 54,276 COVID-19 patients were included in this meta-analysis. This meta-analysis showed that OSA was associated with composite poor outcome [OR 1.72 (95% CI 1.55–1.91), p < 0.00001, I2 = 36%, random-effect modeling] and its subgroup which comprised of severe COVID-19 [OR 1.70 (95% CI 1.18–2.45), p = 0.005], ICU admissions [OR 1.76 (95% CI 1.51–2.05), p < 0.00001], the need for mechanical ventilation [OR 1.67 (95% CI 1.48–1.88), p < 0.00001], and mortality [OR 1.74 (95% CI 1.39–2.19), p < 0.00001].
Conclusions
Extra care and close monitoring should be provided to patients with OSA to minimize the risk of infections. Simple questionnaires such as STOP-Bang questionnaire can be used for screening patients who may be at risk for severe adverse outcomes.
1. Introduction
Coronavirus disease 2019 (COVID-19) can have a wide variety of clinical manifestations, from mild respiratory symptoms such as fever, cough, and anosmia to severe-life threatening conditions such as respiratory distress, arrhythmia, sepsis, shock, and loss of consciousness [1,2]. Previously published meta-analysis studies have identified several comorbidities that were associated with severe outcomes from COVID-19, such as obesity, hypertension, diabetes, thyroid disease, dyslipidemia, cardiovascular disease, and pulmonary disease [[3], [4], [5], [6], [7], [8], [9], [10]]. Among patients with obesity, one of the most prevalent co-existing comorbid conditions was obstructive sleep apnea (OSA). OSA is the most common sleep-related breathing disorder and is highly prevalent, possibly affecting up to 1 billion people around the world [11]. It is characterized by transient increases in upper airway resistance, causing reductions or interruption of the airflow accompanied by increased respiratory effort [12]. This condition can disrupt breathing during sleeping and make someone becomes awake during night time. As a result, people with OSA often experience severe daytime drowsiness, fatigue, irritability, having difficulty concentrating, and may have a higher risk of work-related accidents [13]. During the COVID-19 pandemic, many people experience difficulty in sleeping where their sleep quality has been significantly impaired because of high mental burden, changes in their daily routines, and feelings of helplessness, abandonment, loneliness, and fear [14]. The presence of OSA can make these matters even worse. Unfortunately, until now, the evidence regarding the link between OSA and COVID-19 is still conflicting. Several observational studies showed that the presence of OSA was associated with poor COVID-19 outcomes [[15], [16], [17]], but several others showed that OSA did not alter the outcomes of COVID-19 [18,19]. This article aims to explore and give clear evidence regarding the potential association between OSA and poor outcomes of COVID-19.
2. Materials and methods
2.1. Eligibility criteria
Studies were included in this review if met the following inclusion criteria: representation for clinical questions (Population: positive/confirmed cases of COVID-19; Intervention/Exposure: a group of patients with OSA as their comorbidity; Comparison/Control: a group of patients without OSA; Outcome: composite poor outcomes which comprise of severe COVID-19 and mortality) [20,21], type of study was a randomized control trial, cohort, clinical trial, case-cohort, and cross-over design, and if the full-text article was available. The following types of articles were excluded: articles other than original research (eg, review articles, letters, or commentaries); case reports; articles not in the English language; articles on research in pediatric populations (17 years of age or younger); and articles on research in pregnant women.
2.2. Search strategy and study selection
A systematic search of the literature was conducted on PubMed and Europe PMC using the keywords “sleep apnea syndrome” OR “obstructive sleep apnea” OR “OSA” AND “coronavirus disease 2019” OR “COVID-19,” between 2019 and the present time (December 10th, 2020) with language restricted to English only. The title, abstract, and full text of all articles identified that matched the search criteria were assessed, and those reporting the rate of OSA in COVID-19 patients with a clinically validated definition of each outcomes of interest were included in this meta-analysis. The references of all identified studies were also analyzed (forward and backward citation tracking) to identify other potentially eligible articles. The study was carried out per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.3. Data extraction and quality assessment
Data extraction was performed independently by two authors, we used standardized forms that include author, year, study design, number of participants, age, gender, hypertension, obesity, OSA, body mass index (BMI), and proportion of patients with each outcome of COVID-19.
The outcome of interest was the composite poor outcome that comprised of severe COVID-19, Intensive Care Unit (ICU) admission, the need for mechanical ventilation, and mortality. Severe COVID-19 was defined as patients who had any of the following features at the time of, or after, admission: (1) respiratory distress (≥30 breaths per min); (2) oxygen saturation at rest ≤93%; (3) ratio of the partial pressure of arterial oxygen (PaO2) to a fractional concentration of oxygen inspired air (fiO2) ≤300 mmHg; or (4) critical complication (respiratory failure, septic shock, and or multiple organ dysfunction/failure) [22]. ICU admission was defined as the patients who were admitted or transferred into the intensive care unit (ICU). The need of mechanical ventilation was defined as the patients who need a mechanical ventilator to assist their breathing. The mortality outcome from COVID-19 was defined as the number of patients who were dead because of COVID-19 infection.
Two investigators independently evaluated the quality of the included cohort and case–control studies using the Newcastle–Ottawa Scale (NOS) [23,24]. The selection, comparability, and exposure of each study were broadly assessed and studies were assigned a score from zero to nine. Studies with scores ≥7 were considered of good quality.
2.4. Statistical analysis
A meta-analysis was performed using Review Manager 5.4 (Cochrane Collaboration) software. We used the Generic Inverse Variance formula with random-effects models to calculate each outcome's risk. The heterogeneity was assessed by using the I2 statistic with a value of <25%, 26–50%, and >50% were considered as low, moderate, and high degrees of heterogeneity, respectively. The effect estimate was reported as odds ratio (OR) along with its 95% confidence intervals (CIs) for dichotomous variables, respectively. P-value was two-tailed, and the statistical significance was set at ≤0.05. Subgroup analysis was performed for each component of composite poor outcomes. We performed Begg's funnel-plot analysis to qualitatively assess the risk of publication bias.
3. Results
3.1. Study selection and characteristics
A total of 255 records were obtained through systematic electronic searches. After the removal of duplicates, 234 records remained. A total of 204 records were excluded after screening the titles/articles because they did not match our inclusion and exclusion criteria. After evaluating 30 full-texts for eligibility, 5 full-text articles were excluded because they do not have the outcome of interest (severe COVID-19, ICU admission, the need for mechanical ventilation, and mortality), 3 full-text articles were excluded because they do not have the control/comparison group, 1 full-text article were excluded because the articles were not in English, and finally, 21 studies [[15], [16], [17], [18], [19],[25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40]] with a total of 54,276 COVID-19 patients and 4640 patients with OSA were included in the meta-analysis (Fig. 1 ). Of a total of 21 included studies, 19 were retrospective cohort, 1 study was a prospective cohort, while the remaining 1 study was a case–control study. The diagnosis of COVID-19 was confirmed by using Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR) from respiratory tract swabs in all of the included studies. The essential characteristics of the included studies are summarized in Table 1 .

PRISMA diagram of the detailed process of selection of studies for inclusion in the systematic review and meta-analysis.
Table 1
Characteristics of included studies.
| Study | Sample size | Design | Overall age mean ± SD | Male n (%) | Hypertension n (%) | Obesity n (%) | OSA n (%) | BMI | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Argenziano MG et al. [25] 2020 | 1000 | Retrospective cohort | 62.6 ± 18.5 | 596 (59.6%) | 601 (60.1%) | 352 (35.2%) | 24 (2.4%) | 28.9 ± 5.8 | ICU admission |
| Cade BE et al. [15] 2020 | 4668 | Retrospective cohort | 55.9 ± 22.6 | 2072 (44.4%) | N/A | N/A | 443 (9.5%) | 29.1 ± 5.7 | ICU admission, mechanical ventilation, mortality |
| Cariou B et al. [26] 2020 | 1317 | Retrospective cohort | 69.8 ± 13 | 855 (64.9%) | 1003 (76.1%) | 428 (38.3%) | 144 (10.9%) | 28.7 ± 5.7 | Mechanical ventilation, mortality |
| Dashti H et al. [27] 2020 (pre-print) | 4140 | Retrospective cohort | 51 ± 21.4 | 1863 (45%) | 910 (21.9%) | 1135 (27.4%) | 259 (6.2%) | 30 ± 5.7 | ICU admission, mortality |
| Dreher M et al. [28] 2020 | 50 | Retrospective cohort | 66.3 ± 13.3 | 33 (66%) | 35 (70%) | 17 (34%) | 7 (14%) | 28.3 ± 4.4 | Severe COVID-19 (respiratory distress) |
| Feuth T et al. [18] 2020 | 28 | Retrospective cohort | 58.3 ± 18.5 | 15 (54%) | 12 (43%) | 10 (35.7%) | 8 (29%) | 28.1 ± 4.9 | ICU admission |
| Goodall JW et al. [29] 2020 | 981 | Retrospective cohort | 68.3 ± 17.7 | 631 (64.3%) | 486 (49.6%) | N/A | 17 (1.7%) | N/A | Mortality |
| Gottlieb M et al. [30] 2020 | 8673 | Case-control | 41.3 ± 18.5 | 4045 (46.6%) | 1917 (22.1%) | 1290 (14.8%) | 288 (3.3%) | 27.2 ± 6.5 | Severe COVID-19 (respiratory distress) |
| Ioannou GN et al. [31] 2020 | 10,131 | Retrospective cohort | 63.6 ± 16.2 | 9221 (91%) | 6294 (62.1%) | 4542 (44.8%) | 2270 (26.8%) | N/A | Mechanical ventilation, mortality |
| Izquierdo JL et al. [32] 2020 | 10,504 | Retrospective cohort | 58.2 ± 19.7 | 5519 (52.5%) | 3527 (33.6%) | 936 (8.9%) | 212 (2%) | N/A | ICU admission |
| Jimenez E et al. [33] 2020 | 1549 | Retrospective cohort | 68.3 ± 19.2 | 890 (57.5%) | 851 (55%) | 240 (15.7%) | 79 (8.4%) | N/A | ICU admission, mortality |
| Kar A et al. [16] 2020 (pre-print) | 213 | Prospective cohort | 54.3 ± 14.8 | 144 (68%) | 94 (44%) | N/A | 140 (67%) | 26.6 ± 4.3 | Mechanical ventilation, mortality |
| Maas MB et al. [17] 2020 | 9405 | Retrospective cohort | 48.3 ± 17.1 | 4317 (45.9%) | N/A | N/A | 592 (6.3%) | N/A | Severe COVID-19 (respiratory failure) |
| Mazzoleni L et al. [34] 2020 | 40 | Retrospective cohort | 75.3 ± 11.1 | 23 (57.5%) | 37 (92.5%) | N/A | 12 (30%) | 29.7 ± 5.4 | Mortality |
| McCarthy CP et al. [35] 2020 | 247 | Retrospective cohort | 62.3 ± 19.2 | 143 (57.9%) | 128 (51.8%) | 108 (43.7%) | 23 (9.3%) | 28.8 ± 5.8 | ICU admission, mortality |
| Memtsoudis SG et al. [19] 2020 | 124 | Retrospective cohort | 61.5 ± 9.8 | 86 (69.3%) | 72 (58%) | 37 (29.8%) | 9 (7.2%) | 28.3 ± 5.3 | ICU admission |
| Palaiodimos L et al. [36] 2020 | 200 | Retrospective cohort | 62.5 ± 17.4 | 98 (49%) | 152 (76%) | 162 (81%) | N/A | 30.3 ± 6.6 | Mechanical ventilation, mortality |
| Pellaud C et al. [37] 2020 | 196 | Retrospective cohort | 70 ± 14.8 | 119 (61%) | 118 (60%) | 41 (21%) | 25 (13%) | N/A | ICU admission, mortality |
| Suleyman G et al. [38] 2020 | 463 | Retrospective cohort | 57.5 ± 16.8 | 204 (44.1%) | 295 (63.7%) | 262 (57.6%) | 57 (12.3%) | 33 ± 8.5 | ICU admission |
| Vu C et al. [39] 2020 | 60 | Retrospective cohort | 54 ± 10.1 | 40 (66.7%) | 32 (53.3%) | 35 (58.3%) | 2 (3.3%) | N/A | Mortality |
| Xie J et al. [40] 2020 | 287 | Retrospective cohort | 61.5 ± 15.2 | 124 (43.2%) | 230 (80.1%) | 187 (65.2%) | 29 (10.1%) | 33.8 ± 8.5 | ICU admission |
3.2. Quality of study assessment
Studies with various study designs including cohort and case–control were included in this review and assessed accordingly with the appropriate scale or tool. Newcastle–Ottawa Scales (NOS) were used to assess the cohort and case–control studies (Table 2 ). All included studies were rated ‘good’. In conclusion, all studies were seemed fit to be included in the meta-analysis.
Table 2
Newcastle–Ottawa quality assessment of observational studies.
| First author, year | Study design | Selection | Comparability | Outcome | Total score | Result |
|---|---|---|---|---|---|---|
| Argenziano MG et al. [25] 2020 | Cohort |    |   |    | 8 | Good |
| Cade BE et al. [15] 2020 | Cohort |   |   |    | 7 | Good |
| Cariou B et al. [26] 2020 | Cohort |     |   |    | 9 | Good |
| Dashti HT et al. [27] 2020 | Cohort |   |   |    | 7 | Good |
| Dreher M et al. [28] 2020 | Cohort |    |   |    | 8 | Good |
| Feuth T et al. [18] 2020 | Cohort |    |   |    | 8 | Good |
| Goodall JW et al. [29] 2020 | Cohort |    |   |    | 8 | Good |
| Gottlieb M et al. [30] 2020 | Case-control |    |   |    | 8 | Good |
| Ioannou GN et al. [31] 2020 | Cohort |    |   |     | 9 | Good |
| Izquierdo JL et al. [32] 2020 | Cohort |    |   |    | 8 | Good |
| Jimenez E et al. [33] 2020 | Cohort |    |   |    | 8 | Good |
| Kar A et al. [16] 2020 | Cohort |    |   |    | 8 | Good |
| Maas MB et al. [17] 2020 | Cohort |    |   |   | 7 | Good |
| Mazzoleni L et al. [34] 2020 | Cohort |    |   |    | 8 | Good |
| McCarthy CP et al. [35] 2020 | Cohort |     |   |    | 9 | Good |
| Memtsoudis SG et al. [19] 2020 | Cohort |   |   |    | 7 | Good |
| Palaiodimos L et al. [36] 2020 | Cohort |    |   |    | 8 | Good |
| Pellaud C et al. [37] 2020 | Cohort |    |   |    | 8 | Good |
| Suleyman G et al. [38] 2020 | Cohort |    |   |    | 8 | Good |
| Vu C et al. [39] 2020 | Cohort |    |   |    | 8 | Good |
| Xie J et al. [40] 2020 | Cohort |    |   |    | 8 | Good |

 means score 2,
means score 2, 

 means score 3,
means score 3, 


 means score 4.
means score 4.
3.3. Obstructive sleep apnea (OSA) and outcomes
Our pooled analysis showed that OSA was associated with composite poor outcome [OR 1.72 (95% CI 1.55–1.91), p < 0.00001, I 2 = 36%, random-effect modeling] (Fig. 2 ). Subgroup analysis showed that OSA was associated with severe COVID-19 [OR 1.70 (95% CI 1.18–2.45), p = 0.005, I 2 = 53%, random-effect modeling], ICU admissions [OR 1.76 (95% CI 1.51–2.05), p < 0.00001, I 2 = 0%, random-effect modeling], the need for mechanical ventilation [OR 1.67 (95% CI 1.48–1.88), p < 0.00001, I 2 = 0%, random-effect modeling], and mortality from COVID-19 [OR 1.74 (95% CI 1.39–2.19), p < 0.00001, I 2 = 48%, random-effect modeling].
3.4. Publication bias
The funnel-plot analysis showed a qualitatively symmetrical inverted funnel-plot for the association between OSA and composite poor outcome (Fig. 3 ), showing no indication of publication bias.
4. Discussion
Based on our pooled analysis of available data, obstructive sleep apnea (OSA) seems to be associated with an enhanced risk of severe COVID-19, ICU admission, the need for mechanical ventilation, and mortality from COVID-19 infection. Several reasons can be proposed to explain this result. First, most of the patients with obstructive sleep apnea (OSA) were overweight or obese and OSA itself is strongly associated with other comorbid conditions such as hypertension, cardiovascular disease, and diabetes [41]. As we know, obesity, hypertension, cardiovascular disease, and diabetes is also associated with severe COVID-19 and mortality outcomes from COVID-19 [3,6,10]. Second, repetitive episodes of complete/partial cessation of airflow or breathing during sleep which is frequently observed in patients with OSA, is associated with dysregulation of the renin–angiotensin system (RAS) pathway [42]. A published meta-analysis study has shown that higher levels of angiotensin II and aldosterone were observed in patients with OSA, especially in patients with pre-existing hypertension [43]. Another study has also shown that patients with untreated OSA, regardless of the presence of hypertension, waere associated with an increased angiotensin-converting enzyme (ACE) activity [44]. In addition, obesity, significant comorbidity in OSA patients, also influences the RAS [41]. On the other side, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19, uses the angiotensin-converting enzyme 2 (ACE2) as their entry receptor into host cells. ACE2 is the nonclassical pathway of RAS, thus, RAS itself is involved in the pathogenesis of COVID-19 [42]. The dysregulation of RAS which is happened in patients with OSA can promote SARS-CoV-2 entry into host cells, increasing its viral load and infectivity, and finally resulting in the severe outcome and mortality of the disease. The dysregulation of RAS may also contribute to the increased prevalence of cardiovascular and cerebrovascular disease reported in OSA patients where all of those are associated with severity and mortality outcomes from COVID-19 [42,44]. Third, patients with OSA often have sleep deprivation because of frequent awakening during sleeping time. Sleep deprivation itself will increase the interleukin-6 (IL-6), interleukin-17 (IL-17), and tumor necrosis factor-α (TNF-α) which promote the inflammatory activity in neutrophils [45,46]. Elevation in the IL-6 and TNF-α levels have been implicated in severe COVID-19 cases, thus sleep deprivation in OSA could exacerbate the cytokine storm in COVID-19 which can cause acute respiratory distress syndrome (ARDS) and multiorgan failure [47]. Finally, repetitive airway collapse which happened in OSA not only causes dysregulation in RAS but also causes hypoxia during sleep. This resulting hypoxia from OSA could potentially contribute to worsening of the hypoxia in COVID-19 pneumonia and increase the likelihood of developing poor outcomes from COVID-19 [41].
This study has several limitations. First, data on the definition, duration, grading, and type of treatment for OSA patients were lacking in the included studies, hence, cannot be analyzed. Second, we include some pre-print studies to minimize the risk of publication bias, however, the authors have made exhaustive efforts to ensure that only sound studies were included and we expect that most of those studies currently available in pre-print form will eventually be published and that we will identify them through ongoing electronic literature surveillances. We hope that this study can give further insight into OSA in COVID-19 patients.
5. Conclusions
Patients with obstructive sleep apnea (OSA) should be given extra care and monitoring to minimize exposure to the virus. Physicians can use the telemedicine-based practice to provide care and evaluations for OSA patients to minimize their visits to the hospitals. Physicians and caregivers should also be engaged in close monitoring of OSA patients with suspected COVID-19, for early diagnosis and treatment to avoid severe infections. Physicians must also consider the diagnosis of OSA in COVID-19 patients where a simple questionnaire can be used without the need for a polysomnography study. STOP-Bang questionnaire which consists of four subjective (STOP: Snoring, Tiredness, Observed apnea, and high blood Pressure), and four demographics items (BANG: BMI, Age, Neck circumference, Gender) provides good results for screening patients with suspected OSA and could identify patients at risk for adverse outcomes [48,49]. Finally, OSA should be considered as an important factor in future risk stratification models for COVID-19.
Author statement
Timotius Ivan Hariyanto: Conceptualization; Data curation; Methodology; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing. Andree Kurniawan: Conceptualization; Validation; Resources; Writing – original draft; Writing – review & editing; Supervision.
Footnotes
The ICMJE Uniform Disclosure Form for Potential Conflicts of Interest associated with this article can be viewed by clicking on the following link: https://doi.org/10.1016/j.sleep.2021.03.029.
Conflict of interest
The following is the supplementary data related to this article:
References
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/103135363
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1016/j.sleep.2021.03.029
Article citations
Impact of Obstructive Sleep Apnea on Health Outcomes in Veterans Hospitalized with COVID-19 Infection.
Ann Am Thorac Soc, 21(7):1106-1111, 01 Jul 2024
Cited by: 1 article | PMID: 38578801 | PMCID: PMC11284328
Obstructive Sleep Apnea and Acute Lower Respiratory Tract Infections: A Narrative Literature Review.
Antibiotics (Basel), 13(6):532, 06 Jun 2024
Cited by: 0 articles | PMID: 38927198
Review
Pre-existing sleep disturbances and risk of COVID-19: a meta-analysis.
EClinicalMedicine, 74:102719, 05 Jul 2024
Cited by: 1 article | PMID: 39070174 | PMCID: PMC11276919
Acromegaly and COVID-19, lessons, and new opportunities.
Pituitary, 31 May 2024
Cited by: 0 articles | PMID: 38819618
Review
Fibrinaloid Microclots and Atrial Fibrillation.
Biomedicines, 12(4):891, 17 Apr 2024
Cited by: 2 articles | PMID: 38672245 | PMCID: PMC11048249
Review Free full text in Europe PMC
Go to all (45) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Dementia and outcomes from coronavirus disease 2019 (COVID-19) pneumonia: A systematic review and meta-analysis.
Arch Gerontol Geriatr, 93:104299, 19 Nov 2020
Cited by: 76 articles | PMID: 33285424 | PMCID: PMC7674980
Review Free full text in Europe PMC
Parkinson's disease may worsen outcomes from coronavirus disease 2019 (COVID-19) pneumonia in hospitalized patients: A systematic review, meta-analysis, and meta-regression.
Parkinsonism Relat Disord, 87:155-161, 24 Apr 2021
Cited by: 44 articles | PMID: 33931304 | PMCID: PMC8065236
Review Free full text in Europe PMC
Use and Performance of the STOP-Bang Questionnaire for Obstructive Sleep Apnea Screening Across Geographic Regions: A Systematic Review and Meta-Analysis.
JAMA Netw Open, 4(3):e211009, 01 Mar 2021
Cited by: 40 articles | PMID: 33683333 | PMCID: PMC7941199
Review Free full text in Europe PMC