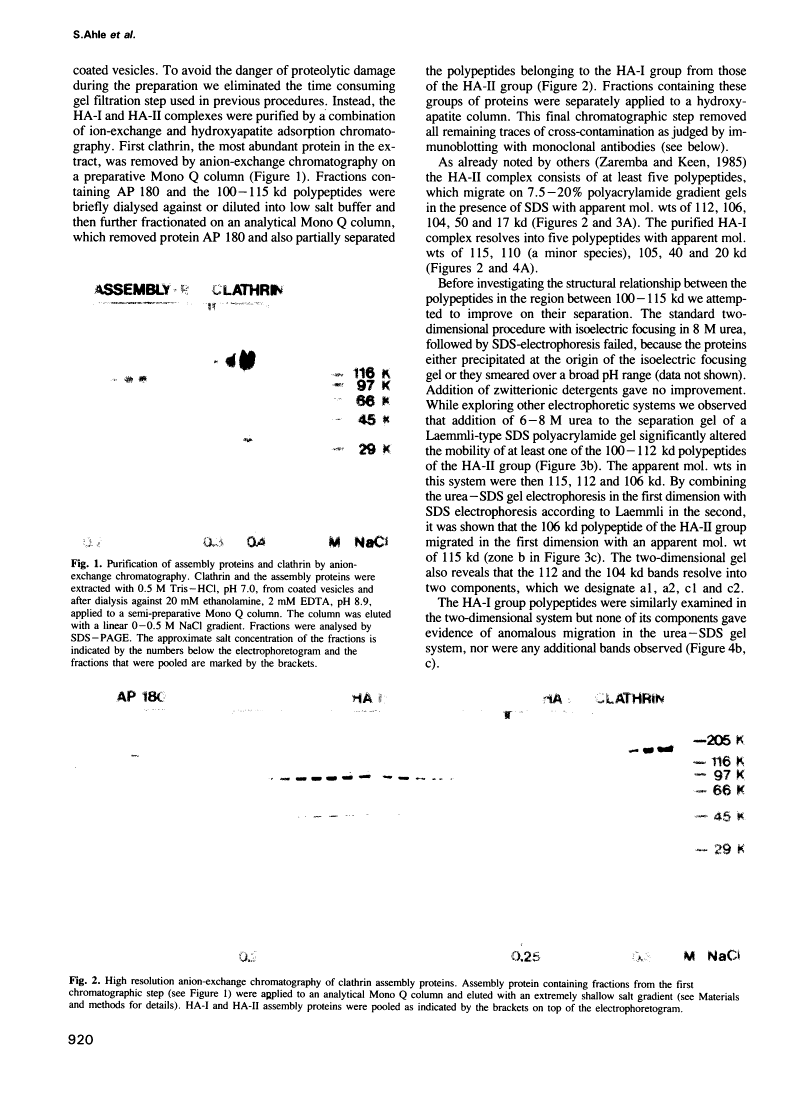
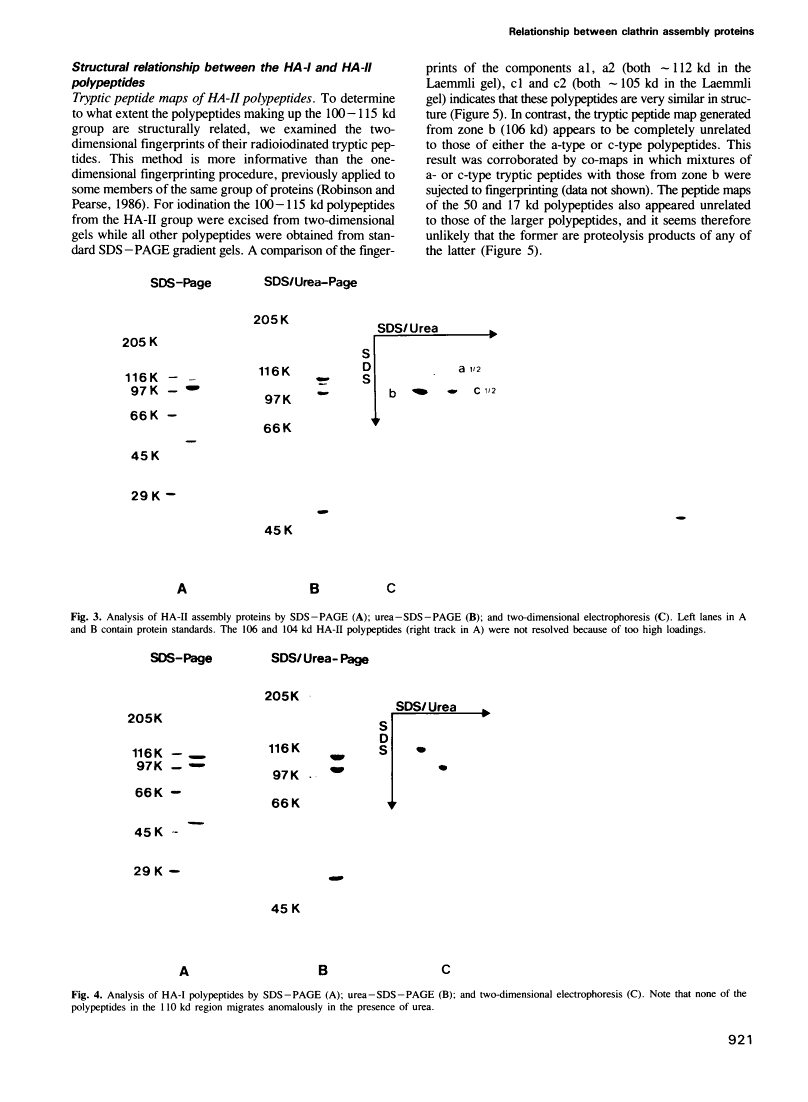
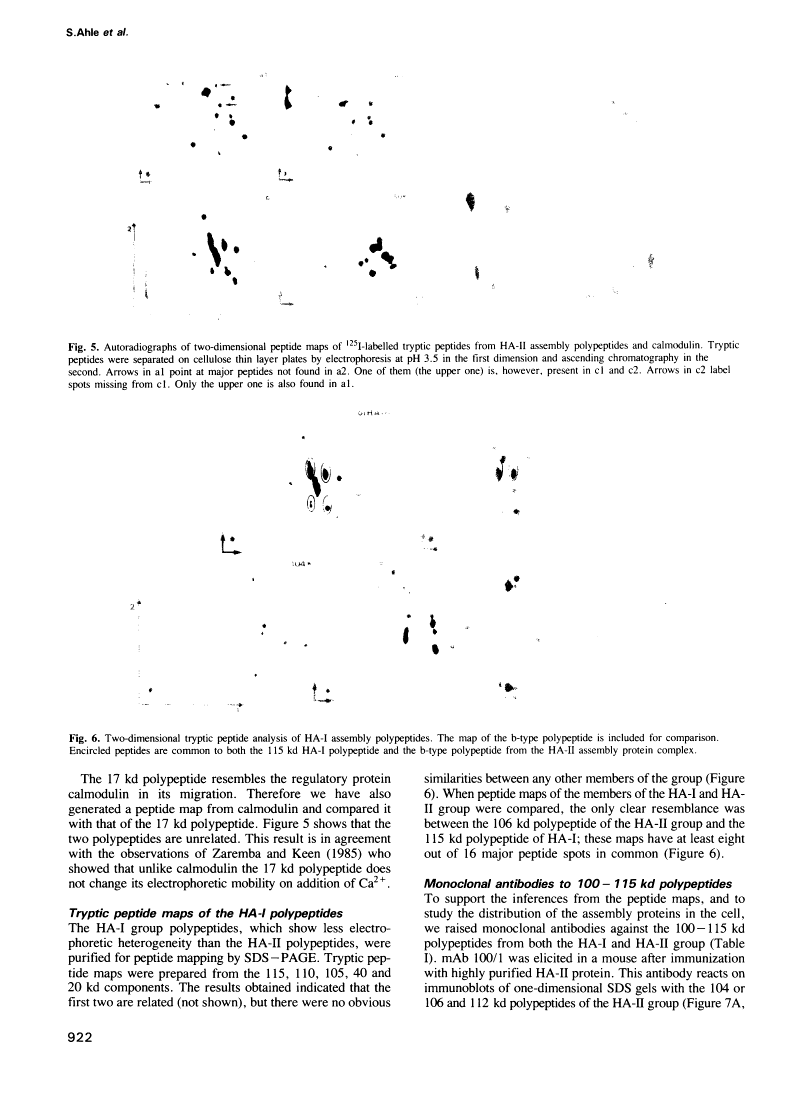
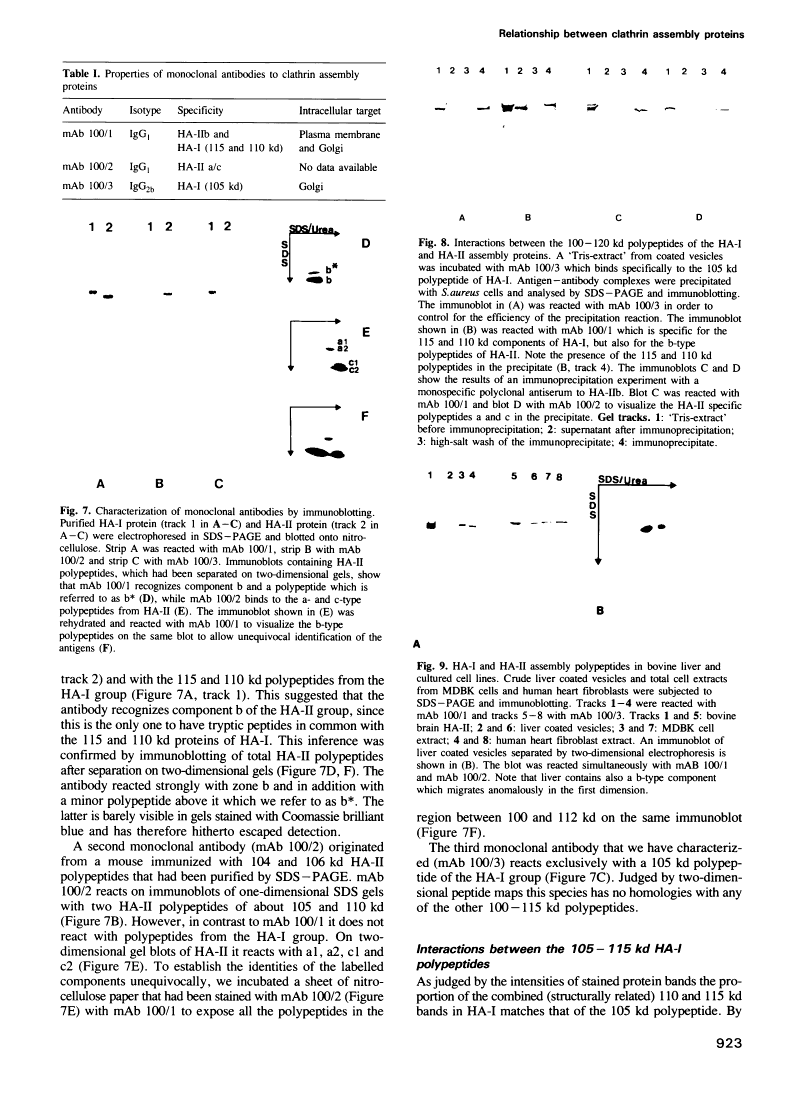
Abstract
Free full text

Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane.
Abstract
We have established by peptide mapping and immunochemical analysis of purified clathrin assembly protein preparations from bovine brain, that the cluster of components of mol. wt 100-120 kd fall into four classes, which we term alpha, beta, beta' and gamma. The beta and beta' proteins are immunologically related and generate a series of common tryptic peptides. The same criteria reveal no such homologies between the alpha, beta(beta') and gamma polypeptides. The so-called HA-II assembly protein group contains equimolar amounts of alpha and beta class polypeptides, which are shown to interact with each other. In the HA-I group assembly protein complex gamma and beta' class polypeptides form a stoichiometric complex. Immunofluorescence microscopy reveals that the HA-I complex is specifically associated with clathrin-coated membranes in the Golgi region of cultured cells, whereas the HA-II complex appears to be restricted to coated pits on the plasma membrane. The data lead to the tentative conclusion that the clathrin assembly proteins are involved in the recognition of the intracellular targets by uncoated vesicles.
Full text
Full text is available as a scanned copy of the original print version. Get a printable copy (PDF file) of the complete article (2.7M), or click on a page image below to browse page by page. Links to PubMed are also available for Selected References.
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahle S, Ungewickell E. Purification and properties of a new clathrin assembly protein. EMBO J. 1986 Dec 1;5(12):3143–3149. [Europe PMC free article] [Abstract] [Google Scholar]
- Anderson RG, Vasile E, Mello RJ, Brown MS, Goldstein JL. Immunocytochemical visualization of coated pits and vesicles in human fibroblasts: relation to low density lipoprotein receptor distribution. Cell. 1978 Nov;15(3):919–933. [Abstract] [Google Scholar]
- Bar-Zvi D, Branton D. Clathrin-coated vesicles contain two protein kinase activities. Phosphorylation of clathrin beta-light chain by casein kinase II. J Biol Chem. 1986 Jul 25;261(21):9614–9621. [Abstract] [Google Scholar]
- Bretscher A, Weber K. Tropomyosin from bovine brain contains two polypeptide chains of slightly different molecular weights. FEBS Lett. 1978 Jan 1;85(1):145–148. [Abstract] [Google Scholar]
- Campbell C, Squicciarini J, Shia M, Pilch PF, Fine RE. Identification of a protein kinase as an intrinsic component of rat liver coated vesicles. Biochemistry. 1984 Sep 11;23(19):4420–4426. [Abstract] [Google Scholar]
- Cantournet B, Creuzet C, Komano O, Loeb J. Clathrin beta-light chain of rat liver coated vesicles is phosphorylated in vitro and in vivo. FEBS Lett. 1987 Aug 10;220(1):143–148. [Abstract] [Google Scholar]
- Debus E, Weber K, Osborn M. Monoclonal antibodies to desmin, the muscle-specific intermediate filament protein. EMBO J. 1983;2(12):2305–2312. [Europe PMC free article] [Abstract] [Google Scholar]
- Elder JH, Pickett RA, 2nd, Hampton J, Lerner RA. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [Abstract] [Google Scholar]
- Fenner C, Traut RR, Mason DT, Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. [Abstract] [Google Scholar]
- Goldstein JL, Brown MS, Anderson RG, Russell DW, Schneider WJ. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. [Abstract] [Google Scholar]
- Hiller G, Weber K. Golgi detection in mitotic and interphase cells by antibodies to secreted galactosyltransferase. Exp Cell Res. 1982 Nov;142(1):85–94. [Abstract] [Google Scholar]
- Keen JH, Black MM. The phosphorylation of coated membrane proteins in intact neurons. J Cell Biol. 1986 Apr;102(4):1325–1333. [Europe PMC free article] [Abstract] [Google Scholar]
- Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. [Abstract] [Google Scholar]
- Ketis NV. Analysis of 100 kDa polypeptides from coated vesicles of bovine brain: two-dimensional tryptic and chymotryptic maps. Biochim Biophys Acta. 1987 May 19;924(2):303–311. [Abstract] [Google Scholar]
- Lisanti MP, Shapiro LS, Moskowitz N, Hua EL, Puszkin S, Schook W. Isolation and preliminary characterization of clathrin-associated proteins. Eur J Biochem. 1982 Jul;125(2):463–470. [Abstract] [Google Scholar]
- Manfredi JJ, Bazari WL. Purification and characterization of two distinct complexes of assembly polypeptides from calf brain coated vesicles that differ in their polypeptide composition and kinase activities. J Biol Chem. 1987 Sep 5;262(25):12182–12188. [Abstract] [Google Scholar]
- Nermut MV. The 'cell monolayer technique' in membrane research. Eur J Cell Biol. 1982 Aug;28(1):160–172. [Abstract] [Google Scholar]
- Olmsted JB. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [Abstract] [Google Scholar]
- Orci L, Ravazzola M, Amherdt M, Louvard D, Perrelet A. Clathrin-immunoreactive sites in the Golgi apparatus are concentrated at the trans pole in polypeptide hormone-secreting cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5385–5389. [Europe PMC free article] [Abstract] [Google Scholar]
- Osborn M, Weber K. Immunofluorescence and immunocytochemical procedures with affinity purified antibodies: tubulin-containing structures. Methods Cell Biol. 1982;24:97–132. [Abstract] [Google Scholar]
- Pearse BM. On the structural and functional components of coated vesicles. J Mol Biol. 1978 Dec 25;126(4):803–812. [Abstract] [Google Scholar]
- Pearse BM. Assembly of the mannose-6-phosphate receptor into reconstituted clathrin coats. EMBO J. 1985 Oct;4(10):2457–2460. [Europe PMC free article] [Abstract] [Google Scholar]
- Pearse BM, Bretscher MS. Membrane recycling by coated vesicles. Annu Rev Biochem. 1981;50:85–101. [Abstract] [Google Scholar]
- Pearse BM, Robinson MS. Purification and properties of 100-kd proteins from coated vesicles and their reconstitution with clathrin. EMBO J. 1984 Sep;3(9):1951–1957. [Europe PMC free article] [Abstract] [Google Scholar]
- Pfeffer SR, Rothman JE. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. [Abstract] [Google Scholar]
- Robinson MS. 100-kD coated vesicle proteins: molecular heterogeneity and intracellular distribution studied with monoclonal antibodies. J Cell Biol. 1987 Apr;104(4):887–895. [Europe PMC free article] [Abstract] [Google Scholar]
- Robinson MS, Pearse BM. Immunofluorescent localization of 100K coated vesicle proteins. J Cell Biol. 1986 Jan;102(1):48–54. [Europe PMC free article] [Abstract] [Google Scholar]
- Schlossman DM, Schmid SL, Braell WA, Rothman JE. An enzyme that removes clathrin coats: purification of an uncoating ATPase. J Cell Biol. 1984 Aug;99(2):723–733. [Europe PMC free article] [Abstract] [Google Scholar]
- Schook WJ, Parker C, Silva WI, Kohtz DS, Puszkin S. Phosphorylation characteristics of brain clathrin-coated vesicle endogenous proteins. J Neurochem. 1987 Aug;49(2):434–441. [Abstract] [Google Scholar]
- Schook W, Puszkin S, Bloom W, Ores C, Kochwa S. Mechanochemical properties of brain clathrin: interactions with actin and alpha-actinin and polymerization into basketlike structures or filaments. Proc Natl Acad Sci U S A. 1979 Jan;76(1):116–120. [Europe PMC free article] [Abstract] [Google Scholar]
- Unanue ER, Ungewickell E, Branton D. The binding of clathrin triskelions to membranes from coated vesicles. Cell. 1981 Nov;26(3 Pt 1):439–446. [Abstract] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. [Europe PMC free article] [Abstract] [Google Scholar]
- Ungewickell E, Branton D. Assembly units of clathrin coats. Nature. 1981 Jan 29;289(5796):420–422. [Abstract] [Google Scholar]
- Vigers GP, Crowther RA, Pearse BM. Location of the 100 kd-50 kd accessory proteins in clathrin coats. EMBO J. 1986 Sep;5(9):2079–2085. [Europe PMC free article] [Abstract] [Google Scholar]
- Woodward MP, Roth TF. Coated vesicles: characterization, selective dissociation, and reassembly. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4394–4398. [Europe PMC free article] [Abstract] [Google Scholar]
- Zaremba S, Keen JH. Limited proteolytic digestion of coated vesicle assembly polypeptides abolishes reassembly activity. J Cell Biochem. 1985;28(1):47–58. [Abstract] [Google Scholar]
Associated Data
Articles from The EMBO Journal are provided here courtesy of Nature Publishing Group
Full text links
Read article at publisher's site: https://doi.org/10.1002/j.1460-2075.1988.tb02897.x
Read article for free, from open access legal sources, via Unpaywall:
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1002/j.1460-2075.1988.tb02897.x
Citations & impact
Impact metrics
Citations of article over time
Article citations
Insights of Endocytosis Signaling in Health and Disease.
Int J Mol Sci, 24(3):2971, 03 Feb 2023
Cited by: 9 articles | PMID: 36769293 | PMCID: PMC9918140
Review Free full text in Europe PMC
Direct trafficking pathways from the Golgi apparatus to the plasma membrane.
Semin Cell Dev Biol, 107:112-125, 13 Apr 2020
Cited by: 56 articles | PMID: 32317144 | PMCID: PMC7152905
Review Free full text in Europe PMC
A Quantitative Genetic Interaction Map of HIV Infection.
Mol Cell, 78(2):197-209.e7, 20 Feb 2020
Cited by: 14 articles | PMID: 32084337 | PMCID: PMC7462049
HHV-7 U21 exploits Golgi quality control carriers to reroute class I MHC molecules to lysosomes.
Mol Biol Cell, 31(3):196-208, 18 Dec 2019
Cited by: 4 articles | PMID: 31851583 | PMCID: PMC7001482
The AP2 adaptor enhances clathrin coat stiffness.
FEBS J, 286(20):4074-4085, 03 Jul 2019
Cited by: 8 articles | PMID: 31199077 | PMCID: PMC6852553
Go to all (201) article citations
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Subunit interaction and function of clathrin-coated vesicle adaptors from the Golgi and the plasma membrane.
J Biol Chem, 266(12):7910-7918, 01 Apr 1991
Cited by: 36 articles | PMID: 1902231
Purification and properties of a new clathrin assembly protein.
EMBO J, 5(12):3143-3149, 01 Dec 1986
Cited by: 110 articles | PMID: 3816757 | PMCID: PMC1167304
100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties.
J Cell Biol, 117(6):1171-1179, 01 Jun 1992
Cited by: 70 articles | PMID: 1607381 | PMCID: PMC2289504
Assembly of clathrin-coated pits onto purified plasma membranes.
Science, 236(4801):558-563, 01 May 1987
Cited by: 75 articles | PMID: 2883727
Review