Abstract
Free full text

The NOP Receptor System in Neurological and Psychiatric Disorders: Discrepancies, Peculiarities and Clinical Progress in Developing Targeted Therapies
Abstract
The nociceptin opioid peptide (NOP) receptor and its endogenous ligand nociceptin/orphanin FQ (N/OFQ) are the fourth members of the opioid receptor and opioid peptide families. Although they have considerable sequence homology to the other family members, they are not considered opioid per se because they do not have pharmacological profiles similar to the other family members. The number of NOP receptors in the brain is higher than the other family members, and NOP receptors can be found throughout the brain. Because of the widespread distribution of NOP receptors, N/OFQ and other peptide and small molecule agonists and antagonists have extensive CNS activities. Originally thought to be anti-opioid, NOP receptor agonists block some opioid activities, potentiate others, and modulate other activities not affected by traditional opiates. Because the effect of receptor activation can be dependent upon site of administration, state of the animal, and other variables, the study of NOP receptors has been fraught with contradictions and inconsistencies. In this article, the actions and controversies pertaining to NOP receptor activation and inhibition are discussed with respect to CNS disorders including pain (acute, chronic, and migraine), drug abuse, anxiety and depression. In addition, progress towards clinical use of NOP receptor-directed compounds is discussed.
1. Introduction
The NOP (Nociceptin Opioid Peptide) receptor, formerly called ORL1 [1], LC132 [2], XOR1 [3], kappa 3 [4], ROR-C [5], and C3 [6], is a G protein-coupled receptor (GPCR) that is considered to be the fourth member of the opioid receptor family[7, 8]. This receptor was cloned separately by so many investigators, based upon its high homology to the other opioid receptors. Despite amino acid and genetic similarity to the other receptors in this family (mu, delta, kappa), NOP is not considered an opioid receptor because it generally does not have high affinity for the normal opioid alkaloids or peptides. Furthermore, NOP receptor activity is not inhibited by the antagonist naloxone, which has often been used as the definitive test for opioid-mediated events. The NOP receptor [9], like each receptor in this family [10–12], has been crystalized and so the 3D structure and binding pocket are well defined, as are the amino acids within the binding pocket that preclude opioid peptides from binding with high affinity.
Shortly after the identification of the NOP receptor, the endogenous ligand was purified and sequenced simultaneously by groups from Le Centre National de la Recherche Scientifique (CNRS) in France and Hoffman La Roche from rat brain and bovine pituitary, respectively [13, 14]. The peptide was identified by using cells transfected with the NOP receptor, as inhibition of cyclic adenosine monophosphate (cAMP) accumulation could be used as a bioassay. This is considered the first example of “reverse pharmacology” in which an orphan receptor was used to identify the endogenous ligand. The substance identified turned out to be a 17-amino-acid peptide (Phe-Gly-Gly-Phe-Thr-Gly-Ala-Arg-Lys-Ser-Ala-Arg-Lys-Leu-Ala-Asn-Gln), called nociceptin [13], and Orphanin FQ [14] from the two groups, which has sequence similarity to the opioid peptides, particularly dynorphin. The one important difference is that sequence of nociceptin/orphanin FQ (now called N/OFQ), is Phe-Gly-Gly-Phe rather than the opioid sequence of Tyr-Gly-Gly-Phe. The lack of the hydroxyl on the N-terminal Phe precludes N/OFQ from having high affinity at the opioid receptors [15]. N/OFQ is also a highly basic amino acid, with 4 Arg or Lys residues, similar to dynorphin. Furthermore, the preproN/OFQ gene (PNOC) has high primary sequence homology to the opioid peptide genes [16].
Among the initial studies undertaken after the identification of both the NOP receptor and N/OFQ was to determine the location of the protein and the mRNA. The most definitive studies were carried out by Watson and colleagues who identified NOP receptors by in vitro autoradiography and in situ hybridization [17], and N/OFQ by immunohistochemistry and in situ hybridization [18], though there were many additional less comprehensive studies [19–22]. Although antibodies to NOP receptors have been produced, none have been validated to be absent in NOP receptor knockout (KO) mice and this has hampered localization studies. This was remedied by the production of knock-in mice that contain enhanced green fluorescent protein (eGFP) attached to the carboxy terminal (C-terminal) of the NOP receptor [23]. These mice have been useful to identify NOP receptors in different brain regions, identify changes induced by chronic pain, and colocalize NOP receptors with other cell-specific markers [23, 24].
Both receptor and peptide are found throughout the brain, as well as in the spinal cord, dorsal root ganglia (DRG), and a variety of peripheral tissues, which is consistent with the array of behavioral actions that are mediated by N/OFQ and small molecule agonists. NOP receptors are found in high numbers in brain regions involved in nociceptive processing, including both the ascending and descending pain pathways including the somatosensory cortex, periaqueductal grey (PAG), rostral ventral medulla (RVM), spinal cord, and DRG, as well as in regions involved in cephalic pain, trigeminal ganglia (TG) and trigeminal nucleus caudalis (TNC). NOP receptor levels are also high in regions involved in the affective component of pain, including the anterior cingulate cortex (ACC) and the amygdala. It was the high levels of NOP receptors in the amygdala, a region involved in emotional responses, that led Hoffman LaRoche to examine their first small molecule agonist (Ro 64–6198) as an anxiolytic [25]. Although there has been some controversy, NOP agonists are generally considered to possess anxiolytic activity. High levels of NOP receptors have also been detected in hypothalamus, hippocampal formations, as well as regions involved in reinforcement and reward. These brain regions are consistent with many of the physiological actions identified in both the endogenous ligand, subsequent to intracerebroventricular (i.c.v.) administration, and after systemic administration of small molecule agonists. Location of NOP receptors with relevance to central nervous system (CNS) disorders is shown in Fig 1. NOP receptors are Gi/o [1, 26] coupled and except for a very recent publication indicating some excitatory activity [27], NOP receptor activation uniformly leads to membrane hyperpolarization and a decrease in neuronal activity. It is almost certainly this inhibitory activity induced by N/OFQ and small molecule agonists that leads to known physiological actions induced by NOP receptor activation. These actions, including modulation of pain, anxiety, depression, sleep, and reward, as well as more detailed descriptions of neuronal localization, will be discussed below.

Some regions of high expression and of particular relevance to CNS disorders are shown here. ACC (Anterior Cingulate Cortex), PAG (Periaqueductal Gray), RVM (Rostral Ventromedial Medulla) are involved in pain; NAcc (Nucleus Accumbens) and VTA (Ventral Tegmental Are), are involved in drug reward; AMG (amygdala) Anxiety; Hy (hypothalamus), sleep; DRN (Dorsal Raphe Nucleus), depression; TNC (Trigeminal Nucleus Caudalis), head pain.
2. Pain
2.1. Acute Pain.
The most extensive, and most confusing application of NOP receptor-active compounds pertains to potential use as analgesics. The initial studies after the identification of N/OFQ demonstrated that i.c.v. administration into mice lead to a decrease in hot plate and tail flick latency [13, 14]. Naturally, this is where the name nociceptin came from, as the peptide appeared to be nociceptive in mice. There was some confusion since N/OFQ was not nociceptive in rats, when delivered i.c.v. through an implanted guide cannula [28]. This was solved by Grandy and colleagues who determined that N/OFQ did not appear to actually be nociceptive per se, but rather it blocked the stress-induced analgesia that was induced by the i.c.v. injection in mice [29]. Additional studies by Grandy demonstrated that N/OFQ blocked analgesia induced by mu, kappa, and delta analgesics [30]. Therefore, with respect to pain, N/OFQ had anti-opiate activity. However, even this notion was quickly dismissed as subsequently, N/OFQ was determined to be analgesic and potentiated opiate analgesia when administered intrathecally [31–33].
Based upon the original observations that N/OFQ was nociceptive or anti-opiate, the original hypothesis was that NOP receptor antagonists might have antinociceptive activity. This in itself is controversial. The first antagonists were peptides based upon the N/OFQ sequence, produced by Calo, Guerrini and colleagues. Some of these high affinity and very selective antagonists, such as UFP-101, have potent antinociceptive activity when administered i.c.v. [34]. However, the first selective non-peptide antagonist J-113397 inhibited N/OFQ hyperalgesia, but was devoid of antinociceptive activity per se after systemic administration [35, 36]. The highest affinity and most selective antagonist, SB-612111, demonstrated a similar profile [37, 38]. To add to the confusion, the slightly less selective antagonist JTC-801 has potent analgesic activity in both acute and chronic pain models and this is not reversed by naloxone [39, 40]. Currently, it is unclear why peptide antagonists have antinociceptive activity per se while the most selective non-peptide (alkaloid) antagonists don’t, perhaps it has to do with the fact that the peptides were administered i.c.v. and alkaloids were always administered systemically. It is also unclear how JTC-801 produces analgesic activity not reversed by naloxone, while other more selective non-peptide antagonists do not.
Based upon the unusual profile of N/OFQ (blocks opiate analgesic activity i.c.v. but is analgesic intrathecally (i.t.), the potential antinociceptive activity of a small molecule given systemically was not clear. The first high affinity and selective non-peptide agonist reported was Ro 64–6198, from Hoffman La Roche. This compound was shown to have reasonable anxiolytic activity, but was ineffective in the tail flick test, when given systemically [25, 41]. In fact, this and other NOP receptor agonists can block opioid analgesic activity when given systemically in the tail flick assay. However, Ro 64–6198 did show modest antinociceptive activity in hot plate test, and there is also evidence that systemic administration of small molecule agonists can be effective to block inflammatory pain, as Ro 65–6570, another high affinity and selective NOP receptor agonist, reduced both Phase I and Phase II of formalin-induced nociceptive behavior [42, 43].
NOP receptors are found throughout the brain, spinal cord, and DRG. In the brain there are high concentrations of NOP receptors in pain-related regions including the PAG, thalamic nuclei, somatosensory cortex, RVM, and lateral parabrachial nucleus [44]. Receptor level is also very high in regions involved in the affective component of pain, including the ACC and the amygdala [44, 45]. The anti-opioid effects of N/OFQ when administered i.c.v. can be explained by a direct action on the descending pain pathway, as NOP receptor activation, by local injection of N/OFQ into the PAG, blocks the actions of opiate analgesics [46, 47]. The actions of N/OFQ in this descending pain pathway was examined in detail by Fields and colleagues. They had previously determined that, in the RVM, mu receptors are on Secondary Cells, which lead to hyperalgesia, while kappa receptors are on Primary Cells [48, 49]. Activation of Primary Cells leads to antinociception, and these cells are disinhibited by mu receptors via inhibitory GABA interneurons. NOP receptors are on both cell types and through the Secondary Cells block cellular activation and therefore mu-mediated antinociception [46]. In opioid naïve animals, NOP receptor activation blocks mu receptor-mediated actions, while after tolerance due to morphine treatment, NOP receptor activation has analgesic properties, similar to morphine [46]. Similarly, in the ventralateral PAG (vlPAG) of rats, mu receptors, which block the descending pain signal, can be found on approximately one third of the neurons, [50, 51], while NOP receptors are found on virtually every cell in the vlPAG [51, 52], and thereby block morphine’s antinociceptive activity.
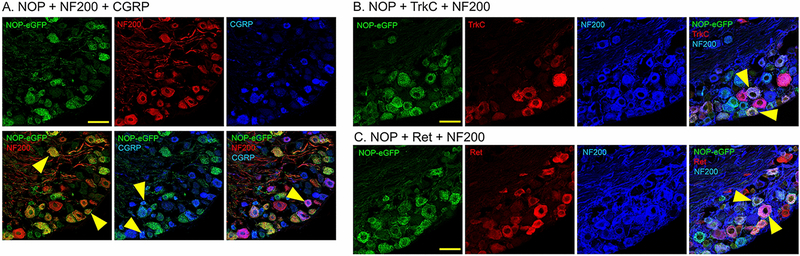
Using knock-in mice with eGFP attached to the C-terminal of the NOP receptor, NOP receptors were found in very high numbers in both DRG and spinal cord. In the spinal cord, receptors are mostly concentrated in laminae I-III, which transmit the signals for both heat (laminae I and II outer) and mechanical (lamina II inner and lamina III) pain, though there are receptors through the more ventral regions of the spinal cord [23]. Approximately 43% of DRG neurons express NOP-eGFP. In the DRG, NOP receptors can be found approximately equally on neurons with small (<400 μm2) and large (>400 μm2) cell bodies. A majority of the NOP+ cells co-express neurofilament 200 (NF200), a marker for neurons with myelinated axons, suggesting a large number of A fibers expressing the receptor [23]. NOP receptors are also co-expressed with calcitonin gene related peptide (CGRP) and mu opioid receptors in a subset of small unmyelinated DRG neurons, which are C nociceptors that are essential to acute heat pain and injury-induced heat hyperlagesia [53]. NOP receptors can also be found on small unmyelinated DRG neurons that bind Isolectin B4 (IB4). These are non-peptidergic DRG neurons, many of which are responsible for acute mechanical pain [53–57]. Furthermore, a small number of medium myelinated DRG neurons that do not express CGRP contain NOP receptors. These neurons are likely to represent low-threshold mechanoreceptors (A LTMRs) that encode touch [58]. Overall, NOP receptors are highly expressed in all pain regions and seem to be involved in various pain modalities from heat to mechanical pain to simple touch.
2.1.1. Non-Human Primates (NHP).
An additional complicating factor is that NOP receptor-mediated activity might be different in rodents and primates. Ko and colleagues have demonstrated that the selective NOP receptor agonist Ro 64–6198 has potent antinociceptive activity in NHPs (rhesus monkeys) in a warm water tail withdrawal test[59, 60]. This result was not confirmed by the Woods group who found no antinociceptive activity of this same compound in the same species [61]. This discrepancy has not been adequately resolved. However, Ko and colleagues also demonstrated that systemic administration of two selective NOP receptor agonists (Ro 64–6198 and SCH 221510) did not attenuate buprenorphine antinociception, but in fact produced synergistic antinociception, again indicating that the activity of NOP agonists is very different in rodents than in NHPs [62]. Considering that work in NHPs has particular relevance for translation to human use, this is an important issue. As discussed below, work continuing on NOP and NOP/mu agonists, using NHPs, have identified novel compounds with particularly favorable profiles.
2.1.2. NOP/Mu Compounds.
The potential of NOP/mu compounds as clinically used analgesics is another important topic. Non-selective opioid agonists and partial agonists have been studied extensively. Mostly, compounds active at mu and kappa receptors have been tested in animal models and some are clinically effective analgesics. In particular, pentazocine, nalbuphine, and butorphanol all have mu and kappa activity and have a long history of use in people. However, due to kappa-mediated side effects, particularly dysphoria in some patients [63, 64], these compounds have not been first-line pain treatments. N/OFQ and other selective NOP receptor agonists appear to block the reward induced by opiates and most every other drug of abuse [65–69]. This will be discussed in more detail below. This can be a useful property if novel compounds are designed to have both NOP and mu activity, as in theory, the NOP agonist activity might block the mu-mediated reward. This was demonstrated with compounds such as SR14150, a mixed partial agonist with analgesic activity and no significant reward in the conditioned place preference (CPP) test [70]. In fact, the level of reward can be titrated with respect to the amount of mu versus NOP activity. As demonstrated by Zaveri, Toll and colleagues, full mu agonists maintain a CPP even in the presence of full NOP receptor activity [70]. This is not necessarily the case with NOP/mu partial agonists, as SR14150 was not rewarding per se [70], while another non-selective partial agonist, BU08028 appeared as rewarding as morphine in mice [71]. However, the analgesic activity of the mu component is reduced by NOP activity, at least in rodents, potentially limiting the effectiveness of such compounds [72]. In NHPs, where NOP and mu antinociceptive activity is synergistic, NOP/mu partial agonists, such as BU08028 [73], AT-121 [74] and BU10038 [75] have very potent analgesic activity, without any apparent rewarding side effects. Cebranopadol (Grunenthol), a full mu/NOP agonist, is very potent in rodent models of acute thermal and inflammatory pain. Interestingly, the antinociceptive activity is reduced by both NOP and mu antagonists in the tail withdrawal test in mice, suggesting both receptors contribute to the analgesic activity, despite the fact that the high affinity and selective NOP full agonist Ro 64–6198 is devoid of antinociceptive activity in this assay [41, 43]. This suggests that something different takes place when both NOP and mu receptors are activated. Cebranopadol is now in Phase III clinical trials [43, 76, 77]. These recent results suggest that selective NOP or NOP/mu agonists have great potential as future clinical drugs to supplement or even replace some use of mu opiates for treatment of pain. A summary of NOP receptor-active compounds on acute pain is given in Table 1.
Table 1
Antinociceptive Activity of NOP Compounds
| Compound | Tail withdrawal | Formalin | Capsaicin | Route of Admin | Species |
|---|---|---|---|---|---|
| N/OFQ | 3 nmol | i.t. | mouse[32] | ||
| UFP-1011 | 10 nmol[34] | 10 nmol[161] | i.c.v. | mouse | |
| JTC-8011 | 0.03 mg/kg2 | 0.1 mg/kg | i.v. | mouse[39] | |
| Ro 64-6198 | 0.002 mg/kg | i.v. | NHP[59] | ||
| Ro 65-6570 | 1 mg/kg | i.v. | mouse [42] | ||
| SR14150 | 3 mg/kg | s.c. | mouse[70] | ||
| Cebranopadol | 0.0056 mg/kg | i.v. | rat [76] | ||
| Cebranopadol | 0.1 mg/kg | 0.030 mg/kg | i.v. | mouse [43] | |
| AT-121 | 0.01 mg/kg | 0.01 mg/kg | s.c. | NHP[74] | |
| BU10038 | 0.002 mg/kg | 0.002 mg | s.c. | NHP[75] |
Values shown represent calculated or approximate ED50 values from indicated publications.
Abbreviations: i.t. intrathecal, i.c.v. intracerebroventricular, i.v. intravenous, s.c. subcutaneous, NHP non-human primate.
2.2. Chronic Pain.
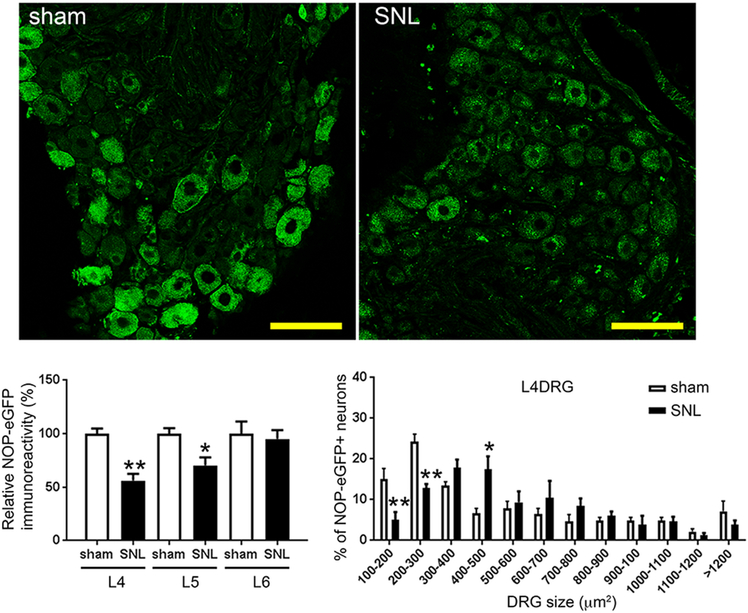
The activity of NOP receptors appears to change in situations of chronic pain, but the exact nature of these changes is not yet clear. Initial studies, using semi-quantitative polymerase chain reaction (PCR), indicated that NOP receptor mRNA was up-regulated in L5-L6 DRG and lumbar spinal cord of rats 7 days after chronic constriction injury (CCI) [78]. Moreover, the number of NOP receptor mRNA positive cells increased, also in the rat PAG and RVM, 7–14 days after CCI [79]. N/OFQ immunoreactivity was found to be increased in rat cingulate cortex, but not in the PAG and RVM, 14 days after CCI [80] and in rat amygdala and PAG 36 days after spinal nerve ligation (SNL) [81]. Both NOP receptor protein and N/OFQ immunoreactivity seemed to be up-regulated in small- and medium-sized L4 DRG neurons in rats 7 and 14 days after partial sciatic nerve transection [20]. These studies used NOP receptor antibodies that were not validated in NOP receptor KO animals. More recent studies using NOP-eGFP mice demonstrated an overall decrease in NOP receptors in both DRG and spinal cord after SNL surgery (see Figure 2) [24]. This was consistent with a decrease in mRNA in both tissues, as determined by quantitative reverse transcription-PCR (RT-PCR). In DRG, NOP receptors were reduced in the smaller DRG cell bodies, less than 300 μm2, but actually increased in cells of 400–500 μm2. In the spinal cord, NOP receptors were primarily decreased in laminae I and II outer, which is consistent with the loss of the small DRG cell bodies. It is not clear if the differences in the various studies are due to different pain models, differences in methods, or non-selective antibodies used for immunohistochemistry.
There have also been inconsistent results with respect to the antiallodynic and antihyperalgesic properties of NOP receptor agonists. Early studies demonstrated that N/OFQ blocked the mechanical allodynia and thermal hyperalgesia in rats after CCI, when administered intrathecally [82]. This is not a surprise, since N/OFQ has the same effect on acute pain after i.t. administration. Systemic administration of small molecule agonists has been more controversial. Although Ro 64–6198 inhibited mechanical and cold allodynia after local peripheral and spinal administration in rats subjected to CCI, it had no effect after systemic administration [83]. More recently, a different picture has emerged. In naïve mice (not in chronic pain), the non-selective NOP agonist SR14150 has naloxone reversible antinociceptive activity in the tail flick, but in the SNL model of chronic neuropathic pain, this compound’s ability to block mechanical allodynia is reversed by SB-612111 and not naloxone [71]. Likewise, the selective NOP agonist, SR16835, is ineffective as an analgesic in the tail flick test, but in the spinal nerve ligated mice, it has potent antiallodynic activity, which is blocked by SB-612111, but not naloxone [71]. This indicates that something changes with respect to the NOP system at least with this one model of chronic pain. In fact, as discussed above, NOP receptor levels change in the spinal cord and DRG in SNL mice, but the change is in the wrong direction. There is a significant decrease in NOP receptors, and it is not clear how this results in increased NOP-mediated antiallodynic activity. This unusual finding is currently being investigated. The effect of NOP-active compounds on chronic pain models is shown in Table 2.
Table 2.
Antinociceptive activity of NOP agonists in chronic pain models.
| Compound | SNL | Diabetic neuropathy | CFA | CCI | Species |
|---|---|---|---|---|---|
| N/OFQ | 10 nmol, i.t. [24] | 5.6 nmol, i.t. [83] | rat [83], mouse [24] | ||
| JTC-8011 | 0.03% in food | rat[152] | |||
| Ro 64-6198 | 23 nmol, i.t. | rat[83] | |||
| Ro 65-6570 | 24 nmol, i.pl. | 0.5 mg/kg, i.v. | rat [162] [163] | ||
| SR14150 | 3 mg/kg, s.c. | mouse [164] | |||
| SR16835 | 10 mg/kg, s.c. | mouse [164] | |||
| Cebranopadol | 2 nmol, i.pl. | 8 mg/kg, i.p. | 2 nmol, i.pl. | rat[163] | |
| Cebranopadol | 0.06 nmol, icv | rat [165] | |||
| SCH221510 | 1 μg, i.t. | mouse [166] | |||
| SCH221510 | 3 μg, i.t | rat [167] |
Values shown represent calculated or approximate ED50 values from indicated publications.
Abbreviations: SNL, spinal nerve ligation; CFA, complete Freund’s adjuvant; CCI, chronic constriction injury; NHP non-human primate; i.t. intrathecal, i.c.v. intracerebroventricular, i.v. intravenous, s.c. subcutaneous i.pl. intraplantar.
2.3. Migraine.
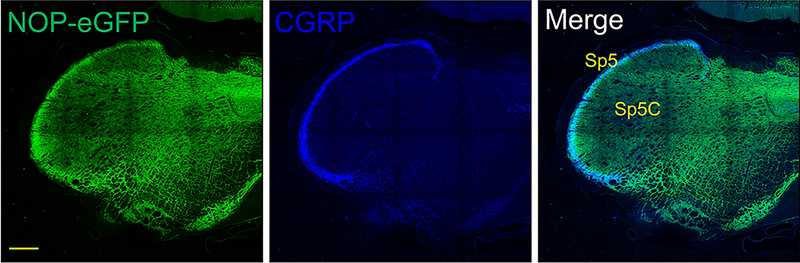
One brain region with particularly high levels of NOP receptors is the TNC as well as the TG cell bodies [21, 84]. Immunostaining of NOP-eGFP was particularly prevalent in the spinal trigeminal tract (sp5), colocalized with CGRP, where peptidergic C-fibers in the TG send their projections (Figure 3). This would be similar to the DRG projections to the spinal cord but mediate cephalic, rather than peripheral pain. A full 72% of TG neurons have NOP receptors [84]. The vast majority of the NOP-eGFP+ cells (88%) are co-stained for NF200, as in the DRG, a marker for neurons with myelinated axons (A-fibers). Therefore, NOP receptors are highly expressed in trigeminal mechanoreceptors (Ret+ NF200+) and proprioceptors (TrkC+ NF200+). NOP-eGFP receptors are present on about one third of the small unmyelinated CGRP-containing TG neurons, which are probably C peptidergic neurons [84], Figure 4. There are additional clues that suggest activation of NOP receptors might be useful for treatment of migraine and other head pain disorders. N/OFQ levels, in the blood, are greatly reduced in migraineurs and further reduced during a migraine [85]. Furthermore, N/OFQ inhibits contractions of smooth muscles [86, 87], including electrically-induced dilation of the middle meningeal artery, in the rat [88]. These observations led to the hypothesis that NOP receptor activation could be useful for treatment of migraine. One standard migraine model is the use of a systemic nitroglycerin injection, which induces allodynia in both the paw and head (periorbital region) that can be quantified using von Frey filaments. Nitroglycerin also produces light sensitivity (photophobia) a typical migraine symptom. All of these symptoms were blocked by Ro 64–6198, suggesting that NOP receptor agonists might be a future treatment for migraine [84].
3. Drug Dependence.
Early CPP studies determined that N/OFQ did not have either rewarding or aversive properties when administered i.c.v. [89]. Due to the “anti-opiate” activity of N/OFQ it was tested to see if it could block the reward induced by opiates and other abused drugs. In fact, when administered i.c.v. prior to the abused drug, N/OFQ blocked the acquisition of a CPP of morphine, cocaine, alcohol, and methamphetamine [65–69]. Concurrently, and consistent with these CPP experiments, it was demonstrated using in vivo microdialysis that N/OFQ could reduce a drug-induced increase in extracellular dopamine in the nucleus accumbens (NAcc), whether administered i.c.v, [90] directly into the ventral tegmental area (VTA) [91], or directly into the NAcc [92]. Additional studies with Ro 64–6198 demonstrated that systemic administration of this compound could block the acquisition and reinstatement, but not the expression of morphine CPP [93]. Rutten et al. demonstrated that the selective NOP receptor agonist Ro 65–6570 could block opioid CPP and that in NOP receptor KO mice, morphine was more effective in displaying a place preference [94]. These results are consistent with those of Murphy and colleagues who demonstrated enhanced CPP to both methamphetamine and alcohol in NOP receptor KO mice [95]. Together these results suggested NOP receptor agonists as potential drug abuse medications.
On the other hand, contrary to these CPP experiments, i.c.v. administration of N/OFQ was unable to reduce morphine self-administration in rats [96]. In fact, the effect of NOP receptor agonists on self-administration is complicated. Some studies have demonstrated that NOP receptor agonists can block self-administration of alcohol, while other results have been negative [97, 98]. Ciccocioppo and colleagues have demonstrated that NOP receptor agonists are effective in Marchigian Sardinian alcohol preferring (msP) rats, an alcohol preferring strain that has an upregulated NOP receptor system [99, 100], or in alcohol post-dependent rats, but not in normal naïve animals [101]. Ciccocioppo also found that buprenorphine (a compound with modest NOP agonist activity) could reduce alcohol self-administration if mu activity was blocked by naloxone, suggesting NOP receptor agonist activity was mediating this effect [102]. The affinity of buprenorphine for NOP receptors is nearly an order of magnitude less than for mu opioid receptors, with quite low efficacy in some studies [103] therefore the mechanism by which this effect is mediated is not perfectly clear. The potent NOP agonist AT-312 can reduce self-administration of cocaine, however this is not a particularly robust effect and seems to result from a drug-induced reduction of the hedonic rather than motivational component of cocaine reinforcement [104]. In contrast, experiments by Rorick-Kehn et al., demonstrated that the selective NOP receptor antagonist LY2940094, rather than agonists, could reduce alcohol self-administration in alcohol preferring rats and reduce the alcohol-induced increase in extracellular dopamine in the nucleus accumbens [105]. Consistent with this observation, the NOP receptor antagonists SB-612111 and LY2817412 were shown to reduce alcohol drinking in the dark, a model for binge alcohol abuse in mice [106]. SB-612111 was also able to reduce both nicotine and alcohol-taking behaviors in rats that concurrently self-administered alcohol and nicotine, an effect consistent in both nicotine dependent and non-dependent rats [107]. Additional studies with NOP antagonists conducted by Cippitelli and colleagues demonstrated that SB-612111 was also able to prevent nicotine-seeking behavior in rats. As shown in Figure 5, doses of 5 and 10 mg/kg SB-612111 completely abolished drug prime-induced reinstatement of nicotine seeking, an experimental measure of nicotine relapse. Finally, when administered directly into the central amygdala, N/OFQ facilitates ethanol self-administration [108]. All of these results are consistent with studies by Ciccocioppo and colleagues who demonstrated that NOP receptor KO rats self-administered cocaine, heroin and ethanol to a lesser extent than wild type rats, and that SB-612111 and LY2817412, two selective NOP receptor antagonists attenuated alcohol self-administration in wild type but not in NOP KO rats [109]. The effect of NOP-active compounds on drug abuse models is shown in Table 3.
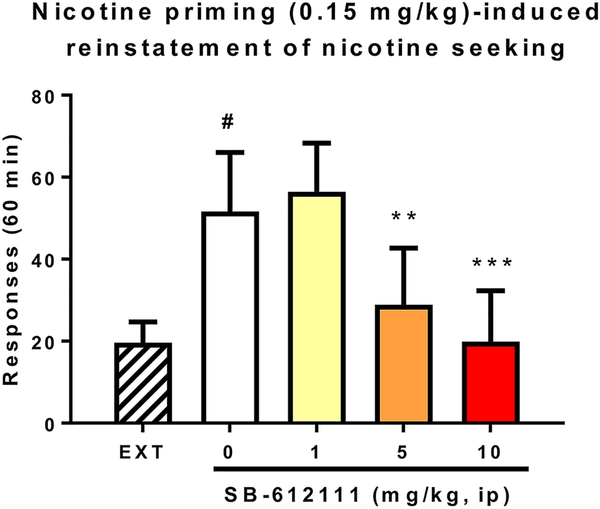
The NOP receptor antagonist SB-612111 blocks reinstatement of nicotine self-administration. N=7 Sprague Dawley rats. **p<0.01, ***p<0.001 difference from vehicle. #p<0.05 difference from extinction (EXT).
Table 3
Ability of NOP-Active Compounds to Attenuate CPP and Self-Administration
| Compound | Drug | CPP | Self-Administration |
|---|---|---|---|
| N/OFQ | Morphine | 500 ng, i.c.v. [1]; 0.6 nmol i.c.v.[2] | |
| N/OFQ | Heroin | Not effective {Walker, 1998 #1578} | |
| N/OFQ | Alcohol | 5 nmol i.c.v. [3] | 0.5 mg/rat i.c.v. msP rats [4] |
| N/OFQ | Cocaine | 0.6 nmol i.c.v.[2] | |
| N/OFQ | Methamphetamine | 10 nmol i.c.v. [5] | |
| Ro 64-6198 | Morphine | 1 mg/kg i.p. [6] | |
| Ro 64-6198 | Ethanol | 0.3 mg/kg, i.p. mouse[3] | 0.3 mg/kg, i.p. rat[7] |
| Ro 64-6198 | Cocaine | 1 mg/kg Wistar rat, more effective in msP rat [8] | |
| AT-312 | Cocaine | 3 mg/kg s.c., mouse[9] | 1 mg/kg i.p., rat [10] |
| AT-312 | Morphine | 3 mg/kg s.c., mouse[9] | |
| AT-312 | Ethanol | 3 mg/kg s.c., mouse [11] | |
| MT-7716 | Ethanol | 1 mg/kg p.o, only in post-dependent rats[12] | |
| LY29400941 | Ethanol | 30 mg/kg p.o., msP rat[13] | |
| SB-6121111 | Ethanol/nicotine2 | 30 mg/kg i.p., rat[14] |
Values shown effective doses from indicated publications.
Abbreviations: CPP conditioned place preference, i.c.v. intracerebroventricular, i.p. intraperitoneal, msP Marchigian Sardinian alcohol preferring, N/OFQ nociceptin/orphanin FQ, NOP nociceptin opioid peptide, p.o. oral administration, s.c. subcutaneous,
Recent positron emission tomography (PET) studies have shed some light on these and other issues. Using [11C]NOP-1A, Narendran et al. showed no difference between alcohol use disorder (AUD) patients and controls, suggesting that alcohol use itself does not seem to modulate the NOP receptor system [110]. Contrary to these results, NOP receptor binding was increased approximately 10% in the midbrain, ventral striatum and cerebellum in individuals with cocaine use disorder (CUD), which could be in response to cocaine-induced changes in N/OFQ or CRF levels [111]. In fact, hydrocortisone treatment of healthy volunteers induced an acute increase in [11C]NOP-1A binding in several brain regions, indicating that stress increases the availability of NOP receptors. Drug abuse-induced changes in NOP receptor function, as well as other disorders modulated by NOP receptor activation, such as post-traumatic stress disorder (PTSD) and sexual violence [112, 113], may be related to stress hormone-induced changes visualized in these PET studies.
The mechanism by which both NOP receptor agonists and antagonists can block aspects of drug reward and addiction is not clear. One possibility that has been suggested is that NOP receptor agonist treatment leads to acute desensitization and reduce or eliminate subsequent receptor activation [105, 114]. It is also not clear why NOP receptor agonists are so effective in blocking CPP but potentially ineffective in blocking operant self-administration. One recent hypothesis is that NOP receptor agonists block acquisition of CPP not because they are blocking the drug-induced reward, but because it blocks learning and memory [27], as there are multiple previous studies demonstrating that N/OFQ blocks long term potentiation and spatial memory [115–117]. In fact, when blocking CPP, one generally measures inhibition of acquisition, while when blocking self-administration, one generally measures inhibition of expression. It is possible these different phases of drug abuse may be affected differently by NOP receptor agonists and antagonists, or perhaps that NOP receptor agonists and antagonists block reward-mediated and stress-mediated aspects of drug abuse respectively. Additional studies that examine the molecular underpinnings of different aspects of addiction should help explain these disparate findings.
New tools are being developed to further investigate the involvement of the N/OFQ-NOP system in motivation and reward. Bruchas and colleagues have developed preproN/OFQ-Cre driver mice, as well as conditional NOP receptor KO animals. They have identified a subset of paranigral VTA neurons enriched in preproN/OFQ that become active when mice stop seeking a natural reward [118]. These cells are required for blocking natural reward, since ablation of these cells increases operant responding, while optogenetic or chemogenetic activation of these cells decreased motivation for rewards.
4. Anxiety
NOP receptors and N/OFQ are both highly expressed in the amygdala, a brain region involved in emotional responses. For this reason, Jenck et al., from Hoffman La Roche, tested N/OFQ in various anxiety models, after i.c.v. administration in both mice and rats and found potent anxiolytic activity [119]. Furthermore, upon synthesis of the first high affinity selective NOP receptor agonist, Ro 64–6198, Jenck and colleagues once again found potent anxiolytic activity [25, 41]. This effect was corroborated with other small molecule NOP receptor agonists [120–122]. In fact, NOP agonists were being developed by Roche as anxiolytics, though ultimately this program was discontinued, and no such compounds were advanced to clinical trials. This however, is not a completely clear picture, as Devine and colleagues found that N/OFQ increased anxiety in an open field test, elevated plus maze, and dark/light test [123]. It also increased circulating concentrations of adrenocorticotrophic hormone and corticosterone in rats, consistent with inducing anxiety [123, 124]. The reason for this discrepancy is not clear, but it was suggested that this has to do with the stress level of the animals when tested. Recent studies by Gavioli and colleagues have demonstrated that stressed mice spend less time in the open arm of an elevated plus maze, indicating an anxiogenic phenotype, and this was reversed by the NOP antagonist SB-612111 rather than a NOP receptor agonist [125]. Perhaps, if the animal is in a high state of stress NOP receptor antagonists are anxiolytic, but in a less stressful situation alleviation of stress requires an agonist. The final answer is unknown, but it is generally accepted in the literature that N/OFQ and small molecule agonists have anxiolytic activity under normal circumstances.
The relationship between NOP receptor activation and anxiety has also been studied in KO animals. Some genetic models are consistent with a reduction in anxiety induced by NOP receptor activation, as KO of either the peptide [126, 127] or the receptor [128] in mice produces an apparent increase in anxiety. In the elevated plus-maze and light-dark box, NOP(−/−) rats and mice displayed increased anxiety-related behavior, consistent with NOP-mediated anxiolytic activity [128, 129]. Conversely, the same study demonstrated that in novelty-suppressed feeding behavior and elevated T-maze, NOP(−/−) mice showed anxiolytic-like phenotype, while no differences were found in the open-field test, hole-board test, marble-burying test, and stress-induced hyperthermia [128]. Contrary to the results of Devine and colleagues, Koster et al, found basal and post-stress plasma corticosterone levels to be elevated in N/OFQ-deficient animals [127]. Altogether, these findings suggest that the N/OFQ-NOP receptor system modulates anxiety-related behavior in a complex manner that may be dependent upon the test employed, the basal anxiety state of the animal, as well as the species.
5. Depression
It’s not uncommon for receptors that are involved in anxiety to be also involved in depressive behavior. It is uncommon that an agonist of a particular receptor appears effective to treat one disorder but an antagonist can treat the other. That is the case with NOP receptors, as NOP receptor antagonists appear to be effective in animal models of depression. The initial study demonstrated that both the peptide antagonist [Nphe1]-N/OFQ (1–13)-NH2, and the small molecule antagonist J-113397 displayed antidepressant-like effects in the forced swim test in mice [130]. Calo and Gavioli proceeded to study various antagonists in several different antidepressant paradigms in mice and rats and they uniformly demonstrated antidepressant behaviors, in these rodent models [131, 132], while agonists exacerbated the depressive behavior [133]. Likewise, KO mice [134] and rats [131] uniformly exhibit an antidepressive-like phenotype and are more resistant to stressors [135]. These studies demonstrate that in contrast to the effect of NOP receptor activation on anxiety, the effect on depressant like behavior is consistent, with a reduction in receptor activation leading to an antidepressant behavior, at least in the standard animal models. Similar results are emerging when examining the effect of the NOP receptor system on stress disorders, such as PTSD. Stressors increase N/OFQ release [136] and in various models of PTSD-like behaviors, NOP receptor antagonists appear to reduce behavioral freezing and other fear/stress responses [137, 138]. However, this is not settled, as older studies indicated functional antagonism between corticotropin-releasing factor (CRF) and the N/OFQ system in behaviors such as feeding and stress-induced anxiety [139, 140].
The mechanism by which NOP receptor antagonists have antidepressant activity is not clear. NOP receptors are present on serotonergic cells in the dorsal raphe nucleus [141, 142], and N/OFQ has been demonstrated to reduce 5-HT release from these cells by microdialysis, but at very high concentrations [143]. This would be consistent with antidepressant activity of NOP receptor antagonists. However, using lower concentrations of N/OFQ, Le Maitre found the opposite, an increase in extracellular 5-HT, but even this required 1 μM N/OFQ, far above active concentrations [142]. Nevertheless, in both cases, the N/OFQ effect on extracellular 5-HT was blocked by NOP receptor antagonists. Being Gi coupled, one would expect N/OFQ to block release of 5-HT, as demonstrated by Tao et al. [143], so Le Maitre suggested N/OFQ might be acting on GABAergic interneurons, thereby disinhibiting the serotonergic neurons to increase 5-HT. To further complicate matters, NOP receptor agonists prevent the antidepressant-like effects of nortriptyline and fluoxetine but not R-ketamine [144]. This may demonstrate that ketamine mediates antidepressant activity by a different mechanism than the selective serotonin reuptake inhibitors (SSRIs). In fact, this is not surprising since ketamine appears to work immediately in humans, as opposed to the well-known need for multiple weeks for SSRIs to have efficacy in people.
6. Clinical Progress
Although there are no NOP receptor-active compounds that have been approved for use in humans, several compounds have been in clinical trials, for a number of disorders. Despite the emphasis of this receptor on its neurologic and psychiatric functions, the initial clinical trials pertained to the renal and cardiovascular functions of the NOP receptor system. Based upon initial studies in rodents demonstrating an effect on the micturition reflex [145], N/OFQ itself was tested after intravesical implantation for patients with an overactive bladder [146–148]. After intravesical administration N/OFQ significantly reduced urine leakage episodes and increased urodynamic bladder capacity in overactive bladder patients but not in normal subjects. These trials are continuing with a longer-lasting high affinity peptide NOP receptor agonist, UFP-112 (also called Rec 0438, Recordati Group) [149]. In addition, the partial agonist peptide Ac-RYYRW-NH2 [150] was stabilized with a polylysine tail to make Ac-RYYRWKKKKKKK-NH2. This compound was tested and found to be moderately effective in patients with isolated systolic hypertension [151].
More important for this review, a few NOP-active small molecules have also been tested in people for pain and other CNS-mediated disorders. The NOP antagonist JTC-801 (Japan Tobacco), which showed antinociceptive activity in models of acute and chronic pain in rodents [39, 152], was taken into Phase II clinical trials in Japan and the UK, as an injectable and oral formulation for the treatment of neuropathic and postoperative pain, but was ultimately discontinued. As mentioned above, the selective NOP receptor antagonist LY2940094 (Eli Lilly and later licensed by Blackthorn Therapeutics and called BTRX-246040) was selected for clinical trials as a treatment for depression and alcohol dependence [153, 154]. In the alcohol study, LY2940094 did not significantly decrease the number of nondrinking days compared to placebo, but did produce a significant reduction in heavy drinking days and in the percent days abstinent [153]. In a clinical trial in patients with major depressive disorder (MDD), LY2940094 showed some signs of a positive effect, leaving the door open for additional trials [154]. Further Phase IIa trials on MDD patients by Blackthorn Therapeutics failed to meet primary or secondary endpoints, and the compound has been deprioritized. The selective NOP receptor agonist SCH486757 (Schering-Plough) was also taken to clinical trials for cough. Although there did appear to be some antitussive activity, the maximum clinical dose was limited by its tendency to produce sleep [155]. In fact, the sedative activity of NOP receptor agonists is well established [8, 156], but the examination of the effect on sleep per se, is fairly recent. This was formalized by Byford et al, who demonstrated that two NOP receptor agonists (Ro 65–6570 and Org 26383) produced a relatively long-lasting loss of righting reflex at very low doses after intravenous administration, which was reversed by a NOP receptor antagonist, but not by naloxone [42]. EEG demonstrated that this was due to hypnotic activity of the NOP receptor agonists. One mechanism by which N/OFQ could induce sleep is by inhibition of wake-promoting neuropeptide hypocretin/orexin containing neurons in the lateral hypothalamus [157]. This sedation, or hypnotic activity does not appear to be uniform, since some NOP/mu agonists, such as cebranopadol and BU10038 clearly activate NOP receptors with no reported sedative effects [75, 158]. The reasons for these differences are not clear, but might have to do with signaling bias, as cebranopadol has been demonstrated to be biased towards G protein rather than β-arrestin coupling [43]. Or it may simply be due to significant mu activity, which leads to an increase in locomotor activity in mice. Regardless of the mechanism, V117957 (Imbrium Therapeutics) a NOP receptor partial agonist that was designed to treat insomnia by promoting sleep onset and maintenance, has shown satisfactory safety in first-in-man studies [159].
The NOP-active compound farthest along is the high affinity non-selective NOP/mu agonist cebranopadol. Cebranopadol is currently in multiple Phase III clinical trials for various pain modalities, after demonstrating efficacy and safety in Phase II [77, 160]. Interestingly, it also showed a beneficial effect on sleep. Cebranopadol, although considered a mu/NOP full agonist, actually has high affinity for all four receptors in the opioid family, which somewhat complicates the mechanism of action [43, 76]. Nevertheless, Cebranopadol does not appear to be sedative, and Grunenthal reports reduced abuse liability, which is presumably due to activation of the NOP receptor. This type of compound, along with NOP/mu partial agonists such as AT-121 (Astraea Therapeutics) and BU10038 (recently licensed to Phoenix PharmaLabs and renamed PPL-138) provide considerable hope for the future of NOP-active compounds and potential for opioid-type analgesics with reduced side effects and abuse liability.
Footnotes
Conflicts of Interest. Authors declare no conflicts of interest
Declarations
Ethics Approval. All procedures used on animals were preapproved by the Institutional Animal Care and Use Committee
Consent to participate. Not applicable
Consent for publication. Not applicable.
Availability of data and material. Not applicable
Code availability. Not applicable
References
Full text links
Read article at publisher's site: https://doi.org/10.1007/s40263-021-00821-0
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8279133
Citations & impact
Impact metrics
Citations of article over time
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1007/s40263-021-00821-0
Article citations
Targeting Nociceptin/Orphanin FQ receptor to rescue cognitive symptoms in a mouse neuroendocrine model of chronic stress.
Mol Psychiatry, 29(3):718-729, 20 Dec 2023
Cited by: 1 article | PMID: 38123728
Naturally Inspired Molecules for Neuropathic Pain Inhibition-Effect of Mirogabalin and Cebranopadol on Mechanical and Thermal Nociceptive Threshold in Mice.
Molecules, 28(23):7862, 30 Nov 2023
Cited by: 1 article | PMID: 38067591 | PMCID: PMC10708129
Neuromodulation in Parkinson's disease targeting opioid and cannabinoid receptors, understanding the role of NLRP3 pathway: a novel therapeutic approach.
Inflammopharmacology, 31(4):1605-1627, 15 Jun 2023
Cited by: 0 articles | PMID: 37318694
Review
Involvement of the Opioid Peptide Family in Cancer Progression.
Biomedicines, 11(7):1993, 14 Jul 2023
Cited by: 1 article | PMID: 37509632 | PMCID: PMC10377280
Review Free full text in Europe PMC
PPL-138 (BU10038): A bifunctional NOP/mu partial agonist that reduces cocaine self-administration in rats.
Neuropharmacology, 211:109045, 01 Apr 2022
Cited by: 4 articles | PMID: 35378170 | PMCID: PMC9074796
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.
Pharmacol Ther, 141(3):283-299, 01 Nov 2013
Cited by: 109 articles | PMID: 24189487 | PMCID: PMC5098338
Review Free full text in Europe PMC
The use of bifunctional NOP/mu and NOP receptor selective compounds for the treatment of pain, drug abuse, and psychiatric disorders.
Curr Pharm Des, 19(42):7451-7460, 01 Jan 2013
Cited by: 17 articles | PMID: 23448477
Review
Nociceptin/Orphanin FQ Receptor Structure, Signaling, Ligands, Functions, and Interactions with Opioid Systems.
Pharmacol Rev, 68(2):419-457, 08 Mar 2016
Cited by: 152 articles | PMID: 26956246 | PMCID: PMC4813427
Review Free full text in Europe PMC
Cellular mechanisms of nociceptin/orphanin FQ (N/OFQ) peptide (NOP) receptor regulation and heterologous regulation by N/OFQ.
Mol Pharmacol, 83(5):907-918, 08 Feb 2013
Cited by: 26 articles | PMID: 23395957 | PMCID: PMC3629824
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NIDA NIH HHS (1)
Grant ID: R01 DA023281
National Institute on Drug Abuse (1)
Grant ID: DA023281