Abstract
Background
The decision to pursue chronic mechanical ventilation involves a complex mix of clinical and social considerations. Understanding the medical indications to pursue tracheostomy would reduce the ambiguity for both providers and families and facilitate focus on appropriate clinical goals.Objective
To describe potential indications to pursue tracheostomy and chronic mechanical ventilation in infants with severe BPD (sBPD).Study design
We surveyed centers participating in the Children's Hospitals Neonatal Consortium to describe their approach to proceed with tracheostomy in infants with sBPD. We requested a single representative response per institution. Question types were fixed form and free text responses.Results
The response rate was high (31/34, 91%). Tracheostomy was strongly considered when: airway malacia was present, PCO2 ≥ 76-85 mmHg, FiO2 ≥ 0.60, PEEP ≥ 9-11 cm H2O, respiratory rate ≥ 61-70 breaths/min, PMA ≥ 44 weeks, and weight <10th %ile at 44 weeks PMA.Conclusions
Understanding the range of indications utilized by high level NICUs around the country to pursue a tracheostomy in an infant with sBPD is one step toward standardizing consensus indications for tracheostomy in the future.Free full text

Qualitative indications for tracheostomy and chronic mechanical ventilation in patients with severe bronchopulmonary dysplasia
Associated Data
Abstract
Background
The decision to pursue chronic mechanical ventilation involves a complex mix of clinical and social considerations. Understanding the medical indications to pursue tracheostomy would reduce the ambiguity for both providers and families and facilitate focus on appropriate clinical goals.
Objective
To describe potential indications to pursue tracheostomy and chronic mechanical ventilation in infants with severe BPD (sBPD).
Study design
We surveyed centers participating in the Children’s Hospitals Neonatal Consortium to describe their approach to proceed with tracheostomy in infants with sBPD. We requested a single representative response per institution. Question types were fixed form and free text responses.
Results
The response rate was high (31/34, 91%). Tracheostomy was strongly considered when: airway malacia was present, PCO2 ≥
≥ 76–85
76–85 mmHg, FiO2
mmHg, FiO2 ≥
≥ 0.60, PEEP
0.60, PEEP ≥
≥ 9–11
9–11 cm H2O, respiratory rate
cm H2O, respiratory rate ≥
≥ 61–70 breaths/min, PMA
61–70 breaths/min, PMA ≥
≥ 44 weeks, and weight <10th %ile at 44 weeks PMA.
44 weeks, and weight <10th %ile at 44 weeks PMA.
Conclusions
Understanding the range of indications utilized by high level NICUs around the country to pursue a tracheostomy in an infant with sBPD is one step toward standardizing consensus indications for tracheostomy in the future.
Introduction
Infants with severe bronchopulmonary dysplasia (sBPD) have higher rates of cardiopulmonary and neurodevelopmental sequelae, growth failure, multiple medication exposures and the need for long-term ventilation that prompts a decision to perform a tracheostomy compared to preterm infants without sBPD [1–3]. Our prior study showed that although clinical variables were associated with and predictive of death or tracheostomy [4], there were wide (2–46%) risk-adjusted rates of death or tracheostomy in 22 regional Neonatal Intensive Care Units (NICUs). This work raised questions about the variable and uncertain clinical indications to pursue tracheostomy and chronic mechanical ventilation in infants affected by sBPD.
Improved growth and developmental trajectory after tracheostomy and chronic mechanical ventilation are reported in infants with sBPD [5]. In addition, a recent study demonstrated that a tracheostomy, specifically, does not have negative effects on language or cognitive development in infants with sBPD [6]. For infants with chronic respiratory failure, establishing safe mechanical ventilation can begin a path to be discharged from the NICU [7]. However, this clinical path is associated with multiple risks including uncertain duration of the need for this support (often years), perioperative risks at the time of tracheostomy, serial procedures such as bronchoscopies, final decannulation with airway reconstruction, and the low, but life-threatening risk of accidental decannulation or obstruction at home [7, 8]. In addition, social considerations include the technological burden of maintaining a tracheostomy in areas of the country that lack home ventilation programs [9–11]. Home health provider shortages are a glaring problem for families with long-term medical needs. Children who are technology-dependent require lengthy hours of home health nursing. Limited availability of home health resources contributes to delays in care including family training, lack of equipment, and risk of readmission [9–11]. There is considerable regional variation. Rasooly et. al shows the probability of receiving home nursing across states ranged from 3.4 to 19.2%, after adjusting for patient characteristics. Among those children who received home nursing the adjusted median days of care received ranged from 6.6 to 24.5 days [12].
Thus, the decision to pursue chronic mechanical ventilation involves a complex mix of clinical and social considerations [13]. Understanding the medical indications to pursue tracheostomy would reduce the ambiguity for both providers and families and facilitate focus on appropriate clinical goals.
Our objective was to identify provider-defined indications prompting the decision to pursue tracheostomy in infants with sBPD at regional NICUs in the Children’s Hospitals Neonatal Consortium (CHNC). We hypothesized that although the indications for tracheostomy and chronic ventilatory support vary across institutions there are some indications common to a majority of institutions that could be used to inform eventual consensus guidelines.
Materials and methods
Participating hospitals in the CHNC were identified [14, 15]. Targeted responders were prospectively identified by direct contact: they included exclusively neonatology faculty from the 34 participating hospitals who were particularly invested in patients with sBPD at their institution and agreed to represent their group’s practice regarding infants with sBPD. Each institution’s targeted responder was surveyed using an online tool (RedCAP), and the survey was designed to receive one representative response per center. Responders were instructed to have group meetings and to respond based on their institutional, and not their individual, preferences. To account for variability amongst providers within an institution, we allowed free text and narrative responses to offer alternative approaches, ideas and thoughts. The authors were not blinded to each responder’s institution affiliation.
The primary aim of this survey was to understand centers’ indications for chronic ventilator support via tracheostomy. The secondary aim was to describe the prevalence of dedicated BPD clinical teams and their role in clinical decision making in the NICU for these patients.
The survey was presented in four sections with a total of 102 questions. The first section focused on patient inclusion/entry into BPD programs, if present, at each institution. The second section probed descriptive characteristics of the services provided to patients with sBPD at each institution. The third section requested clinical thresholds that prompted the decision for tracheostomy in affected infants. Factors that were specifically explored included respiratory support, interventions, and patients’ clinical status: carbon dioxide thresholds (PCO2), fraction of inspired oxygen (FiO2), positive end expiratory pressure (PEEP), echocardiographic findings, and timing of tracheostomy. The final section contained open-ended questions that asked participants to describe clinical scenarios which may or may not prompt consideration of tracheostomy. These questions were:
Please describe a clinical situation wherein everyone at your institution would NOT decide to move forward with a tracheostomy in an infant with sBPD?
Please describe a clinical situation wherein everyone at your institution would decide to move forward with a tracheostomy in an infant with sBPD?
This study was approved by the institutional review board at UT Southwestern
The survey included questions that required dichotomous (Yes/No) responses, modified Likert scales, multiple choice, and narrative answers. The survey also included space for responders to elaborate and share institutional guidelines. Not every question was answered by each site, and responses occurred over a 9-month period in 2018. No compensation was provided for survey completion. Please see supplementary material for survey.
All replies were reviewed by the PI (SGY) and narrative responses were reviewed first by the PI, then by two co-authors (KM, JML) and a summary was presented to all authors to identify consistent themes. Reviewing authors were not blinded to the institution. Quotations selected for analysis were done by group consensus to illustrate conceptual themes (e.g., timing, severity of illness, co-morbidities) and to minimize repetition. Summative qualitative analysis was performed on open-ended responses to questions listed above, identifying codes, and calculating their frequency in order to identify patterns in the data and contextualize codes [16]. Descriptive results and qualitative analyses are reported.
Results
Of the 34 CHNC sites surveyed, 31 sites responded to the survey (91%). Thirteen centers (42%) had a self-identified BPD program. Among all centers, when asked about age at enrollment, the most common response (10/13 =
= 77%) was for infants to be enrolled into the BPD program at 36 weeks post-menstrual age (PMA), and the second most common enrolled at 40–44 weeks PMA if infants still required respiratory support [17]. In addition, in descending frequency, time points for enrollment into BPD clinical programs were: At the time of tracheostomy, post-tracheostomy, survival without chronic ventilation or tracheostomy. No program enrolled infants prior to 32 weeks PMA into their BPD program. Centers reported the following disciplines were integral members of the sBPD team when making the decision for tracheostomy placement: respiratory therapy (45%), nursing (39%), advanced practice nurses (39%), physical & occupational rehabilitation (35%), speech therapy (35%), social work (35%), pulmonary hypertension service (32%), physiatry (19%), pediatric critical care (10%), and cardiology (10%).
77%) was for infants to be enrolled into the BPD program at 36 weeks post-menstrual age (PMA), and the second most common enrolled at 40–44 weeks PMA if infants still required respiratory support [17]. In addition, in descending frequency, time points for enrollment into BPD clinical programs were: At the time of tracheostomy, post-tracheostomy, survival without chronic ventilation or tracheostomy. No program enrolled infants prior to 32 weeks PMA into their BPD program. Centers reported the following disciplines were integral members of the sBPD team when making the decision for tracheostomy placement: respiratory therapy (45%), nursing (39%), advanced practice nurses (39%), physical & occupational rehabilitation (35%), speech therapy (35%), social work (35%), pulmonary hypertension service (32%), physiatry (19%), pediatric critical care (10%), and cardiology (10%).
Centers were asked if there was an institution-specific consensus for the indication for tracheostomy for chronic ventilation that was used for patients with sBPD. Four centers (13%) reported that they had such criteria and 26 (87%) did not. The four centers who reported criteria did not elaborate further in the survey.
The specific indications for tracheostomy at each center, regardless of a dedicated BPD team, were then explored. There were no significant differences in responses between centers with and without a self-identified BPD team. Nearly all (93%) of responders reported that infants with sBPD were commonly referred for respiratory failure. Most responders (80%) noted that patients were specifically referred for tracheostomy, the decision having been strongly recommended or made at the referring hospital. The population of infants with sBPD least commonly referred to CHNC centers were those who already had a tracheostomy, and mainly referred for pulmonary management and transition to home (20% of responders). Among 29 responders, the most common criteria that contributed to the decision for tracheostomy included: Airway and ventilation (total 32%), subcategorized as airway malacia (13%), need for higher PEEP (8%), and endotracheal positive pressure (11%). Failure to thrive on noninvasive support (11%), multiple courses of corticosteroid therapy (11%), pulmonary hypertension (16%), and poor growth and feeding (10%) (Fig. 1). The least common indications for tracheostomy included: PMA (9%), social considerations (3%) and tolerance, avoidance or thriving with developmental care (8%).
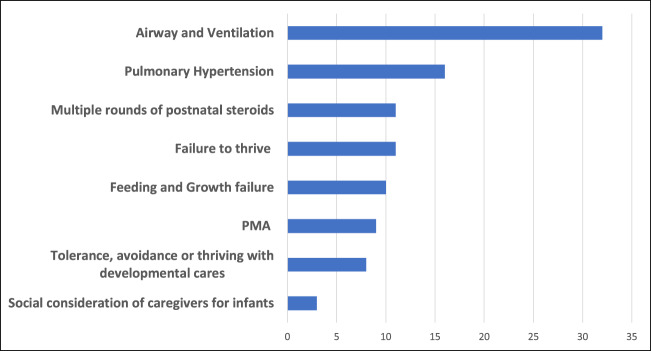
Indications for tracheostomy, divided by reported level of importance (%). N =
= 29 responders.
29 responders.
Table 1 describes the topics that were routinely considered when making the decision to recommend a tracheostomy with answers provided in a dichotomous manner. Findings on echocardiogram (right ventricular dilation, dysfunction, and hypertrophy) were important considerations (60%, 80%, 50% respectively), among 30 responding centers. Systemic corticosteroid exposure was also an important consideration (69%), among 29 responding centers. While growth parameters contributed to the decision, they were given a lower priority, including appropriate linear growth (41%) and head growth (35%), among 29 responding centers. Of note, none of the responders mentioned utilization of CT, bronchoscopy and/or infant pulmonary function testing in their decision-making algorithm.
Table 1
Parameters considered when making a decision for tracheostomy (Yes/No or N/A).
| Parameter #centers, (% total) | Yes | No | N/A | Notes |
|---|---|---|---|---|
Appropriate linear growth (n | 12 (41%) | 6 (21%) | 12 (41%) | 0.8–1 cm/week cm/week |
Appropriate head growth (n = = 29 responders) 29 responders) | 10 (35%) | 5 (17%) | 14 (48%) | 0.5–1 cm/week cm/week |
Right ventricular (RV) hypertrophy on echo (n | 15 (50%) | 10 (33%) | 5 (17%) | No additional information reported |
RV dilation on echo (n = = 30 responders) 30 responders) | 18 (60%) | 7 (23%) | 5 (17%) | No additional information reported |
RV dysfunction on echo (n = = 30 responders) 30 responders) | 24 (80%) | 3 (10%) | 3 (10%) | No additional information reported |
Systemic corticosteroid exposure (n | 20 (69%) | 4 (14%) | 5 (17%) | Most reported >2 courses |
Notes are written for most common ranges (>50% of hospitals) provided by institutions.
Responders used Likert scales to rate the level of importance of possible indications for tracheostomy and chronic ventilation (Table 2). Expressed as a percent of the total number of responding centers (n =
= 28) and reported in decreasing frequency, PMA
28) and reported in decreasing frequency, PMA >
> 44 weeks’ (82%), the need for increased PEEP (62%), pulmonary arterial hypertension (PAH) by echocardiography (55%), multiple postnatal corticosteroid courses (52%), multiple failed planned extubation attempts after 36 weeks’ PMA (48%), physiologic parameters (PCO2 and FiO2: 45%), failure to thrive with noninvasive support (45%), projected concerns for abnormal future neurocognitive development (45%), continued endotracheal positive pressure support (38%), and social circumstances of the family (35%) were the most prevalent indications cited by responders. Feeding intolerance was of lower importance in the decision to recommend tracheostomy (52% minimal/none).
44 weeks’ (82%), the need for increased PEEP (62%), pulmonary arterial hypertension (PAH) by echocardiography (55%), multiple postnatal corticosteroid courses (52%), multiple failed planned extubation attempts after 36 weeks’ PMA (48%), physiologic parameters (PCO2 and FiO2: 45%), failure to thrive with noninvasive support (45%), projected concerns for abnormal future neurocognitive development (45%), continued endotracheal positive pressure support (38%), and social circumstances of the family (35%) were the most prevalent indications cited by responders. Feeding intolerance was of lower importance in the decision to recommend tracheostomy (52% minimal/none).
Table 2
Center reported ratings of the relative importance of indications in the decision to recommend tracheostomy.
| Measure | Importance | |||
|---|---|---|---|---|
| Minimal/none; n, % | Moderate; n, % | High; n, % | Virtually always; n, % | |
| Airway and ventilation | ||||
| Specific physiologic parameters (i.e., high pCO2, FiO2) | 1 (3%) | 3 (11%) | 12 (41%) | 13 (45%) |
| Tolerance, avoidance or not thriving with developmental cares | 2 (7%) | 8 (27%) | 13 (45%) | 6 (21%) |
| Multiple failed, planned extubation attempts >36 weeks’ PMA | 2 (7%) | 1 (3%) | 14 (48%) | 12 (42%) |
| Multiple rounds of postnatal corticosteroid courses (defined >5 days) to ameliorate the severity of BPD? | 2 (7%) | 5 (17%) | 15 (52%) | 7 (24%) |
| Endotracheal Positive pressure support | 4 (14%) | 4 (14%) | 11 (38%) | 10 (34%) |
| Airway malacia | 1 (3%) | 7 (24%) | 15 (52%) | 6 (21%) |
| Need for higher PEEP | 1 (3%) | 8 (28%) | 18 (62%) | 2 (7%) |
| Post-menstrual age (PMA) | 1 (3%) | 4 (14%) | 12 (41%) | 12 (41%) |
| Pulmonary arterial hypertension (PAH) | ||||
 PAH by echocardiogram PAH by echocardiogram | 0 (0%) | 8 (28%) | 16 (55%) | 5 (17%) |
 PAH by cardiac catheterization PAH by cardiac catheterization | 8 (28%) | 10 (35%) | 7 (24%) | 4 (14%) |
 PAH on both echo PAH on both echo + + cath cath | 5 (17%) | 9 (31%) | 9 (31%) | 6 (21%) |
| Feeding & growth | ||||
| Growth (weight, height, head circumference) | 1 (3%) | 13 (45%) | 10 (35%) | 5 (17%) |
| Failure to thrive on noninvasive support | 3 (10%) | 5 (17%) | 13 (45%) | 8 (28%) |
| Feeding intolerance | 15 (52%) | 9 (31%) | 4 (14%) | 1 (3%) |
| Social consideration of caregivers for infant | 6 (20%) | 8 (28%) | 10 (35%) | 5 (17%) |
N =
= 28 responders.
28 responders.
The next section surveyed the ranges of parameters that would prompt discussion of tracheostomy as an intervention, if all therapies affecting each parameter were optimized. Among 29 responders, the most common range for PCO2 was 76–85 mmHg, FiO2
mmHg, FiO2 >
> 0.60, PEEP 9–11
0.60, PEEP 9–11 cm H2O, respiratory rate 61–70 breaths/min consistently, PMA
cm H2O, respiratory rate 61–70 breaths/min consistently, PMA >
> 44 weeks, and weight <10th percentile at 44 weeks PMA (Table 3).
44 weeks, and weight <10th percentile at 44 weeks PMA (Table 3).
Table 3
Reported clinical indications for tracheostomy.
| Parameter | Most commonly reported range |
|---|---|
| PCO2 | 76–85 |
| FiO2 | >0.60 |
| PEEP requirement | 9–11 |
| Respiratory rate | 61–70 |
| PMA | >44 weeks |
| Weight % at PMA | <10th |
N =
= 29 responders.
29 responders.
Using qualitative analysis to analyze open-ended responses, we found that clinicians were generally less likely to agree on offering tracheostomy if the infant was demonstrating weaning of respiratory support, was on non-invasive ventilation (NIV), FiO2 lower than 0.4, or PMA <44 weeks, even if on a ventilator or PMA up to 52 weeks if on NIV. Social considerations were only mentioned by four responders. Three were in the context of “the family does not want a tracheostomy”; one responder mentioned poor social support as a deciding factor. On the other hand, most clinicians seemed to agree that a tracheostomy is clearly indicated if the patient is of certain PMA and still intubated or has failed extubation. However, centers reported heterogeneity regarding which other clinical criteria, such as ventilator settings, use of corticosteroids, especially more than one course, were weighted and which combination. In addition, even when the same criteria were mentioned centers revealed heterogeneity; for example, some centers reported PMA of 44 weeks, others >48 weeks. The data suggests, however, that the decision to recommend a tracheostomy was generally agreed upon when PMA, ventilator, and medical therapies were exceeding what is “normal” (as defined by each center). Interestingly, none mentioned potential social or financial burdens on the affected families. A summary of our findings is available in Table 4. Sixteen centers responded. Dark rectangles represent each clinical criterion reported by the center. If the center was specific about the criterion, it is reported in white within the dark rectangles.
Table. 4
Qualitative analysis of open ended responses.
 |
PEEP =
= positive end expiratory pressure; MAP
positive end expiratory pressure; MAP =
= mean airway pressure, PMA
mean airway pressure, PMA =
= post menstrual age; CENTER
post menstrual age; CENTER =
= CHNC institution identifier.
CHNC institution identifier.
Discussion
Severe BPD (sBPD) is a disease that primarily affects infants born <32 weeks’ gestation, with a reported incidence of 42% among infants 501–750 grams birth weight and 25% among those with 751–1000
grams birth weight and 25% among those with 751–1000 grams birth weight [18, 19]. Chronic mechanical ventilation with tracheostomy that serves to transition infants and their families from the NICU to the outpatient setting can be a viable management strategy but is associated with significant complications and morbidities including poor wound healing, air leak, stoma problems, accidental decannulation, and mucus plugs [20–22]. As infants grow older there are common reports of tracheal lesions such as fistulas, stenosis, collapse and peristomal granulation [23]. The decision to pursue tracheostomy and chronic home ventilation is often favored when the perceived alternatives are prolonged or uncertain duration of hospitalization, and/or mortality. This uncertainty of deciding to pursue tracheostomy is balanced against the potential hope that the infant will improve and will require a lower level of invasive support as well as decrease variation in institutional practices of decannulation [24, 25]. Taking all of this into consideration, this study qualitatively captures the perceived indications that prompt the decision to pursue tracheostomy and describes the variability in both the selected criteria and their relative importance across multiple Level IV NICUs in North America.
grams birth weight [18, 19]. Chronic mechanical ventilation with tracheostomy that serves to transition infants and their families from the NICU to the outpatient setting can be a viable management strategy but is associated with significant complications and morbidities including poor wound healing, air leak, stoma problems, accidental decannulation, and mucus plugs [20–22]. As infants grow older there are common reports of tracheal lesions such as fistulas, stenosis, collapse and peristomal granulation [23]. The decision to pursue tracheostomy and chronic home ventilation is often favored when the perceived alternatives are prolonged or uncertain duration of hospitalization, and/or mortality. This uncertainty of deciding to pursue tracheostomy is balanced against the potential hope that the infant will improve and will require a lower level of invasive support as well as decrease variation in institutional practices of decannulation [24, 25]. Taking all of this into consideration, this study qualitatively captures the perceived indications that prompt the decision to pursue tracheostomy and describes the variability in both the selected criteria and their relative importance across multiple Level IV NICUs in North America.
Home chronic ventilation places a large burden on affected infants and families. This was first brought to the forefront of the American public by President Ronald Reagan in the early 1980s, followed by a funding waiver program established by the Department of Health and Human services, to assist families with ventilator dependent children at home [26, 27]. The impact on families cannot be underscored enough as they deal with life-threatening events felt to be related to equipment malfunction and/or tracheostomy occlusion, frequent re-hospitalizations and mortality [13, 28]. In fact, through the course of this survey, we learned that many centers cite a 5–10% risk of adverse events after NICU discharge. Having a child that requires chronic home ventilation can burden caregivers, have significant effects on siblings and family relationships, restricts parental employment opportunities and adversely affects maternal health [29–33]. Families will spend a great deal of time on lengthy education to care for their child at home prior to discharge, and stable transition to appropriate home ventilator devices may be delayed significantly while awaiting home nursing care [28]. Often caregiver needs cannot be met with available home health nursing, even when approved by third-party payers. In 2016, The American Thoracic Society published guidelines regarding chronic home ventilation in pediatric patients [34, 35]. In their recommendations, among other important items, they suggest an awake, trained caregiver be in the family’s home at all times, at least two trained family caregivers prepared to care for the child at home, ongoing education for all caregivers, and updating and maintaining all durable medical equipment (DME) regularly. While these recommendations are necessary and prudent, the burden on the family as well as availability of home health care services is paramount [34].
PMA and specific physiologic parameters were the most common indications for tracheostomy. Responders also cited that airway malacia was an important parameter, followed by failure to thrive on noninvasive support, multiple courses of postnatal corticosteroids and endotracheal positive pressure. Though failure to thrive is subjectively determined and postnatal corticosteroids also have varied indications, our results suggest that a potential consensus definition of therapeutic failure would include PCO2 of 76–85, FiO2 of >0.60, PEEP 9-11, respiratory rate of 61–70, PMA >44 weeks and weight <10th percentile at this PMA. Most centers did agree that appropriate linear and head growth, corticosteroid exposure, and right heart insufficiency on echocardiogram were very important indications when considering tracheostomy. Although there was significant variability in approaches to tracheostomies across centers, it is interesting that there was some degree of agreement on which of the most severe scenarios would prompt consideration of tracheostomy.
The strengths of this survey include the high response rate (91%) and the candid, detailed answers provided as one collective opinion per institution. On the other hand, although each responder replied on behalf of their institutional practice, this limited our ability to ascertain variability within the same institution. We included space for narration to allow responders to offer the nuances of their group’s practices. Our survey may not have ideally delineated those infants receiving tracheostomy for airway obstruction, though in preterm infants with sBPD, lung disease was most likely the predominant respiratory issue. Further, infants at these NICUs are largely referral-based (>90%) and biases that govern referral to these hospitals may not be generalizable to NICUs that care for mostly inborn populations. Referral timing, indications, and parental circumstances vary and, correspondingly, interventions to reduce the likelihood and/or severity of chronic respiratory failure are often instituted variably at referring hospitals.
In conclusion, our data demonstrate that reported indications for tracheostomy differ widely across regional NICUs. However, despite these differences between regional NICUs, there are indications that could allow the development of standardized indications in future studies. The development of standardized indications for tracheostomy and chronic ventilation techniques in infants with sBPD are needed to develop the evidence for best practices for the subset of infants with sBPD that require long-term respiratory support. Diagnostic certainty is needed to determine which infants require these interventions to achieve medical readiness for a safe, discharge home in a defined period of time, while achieving optimal long-term outcomes. Although clinical markers, genetic predisposition, assessments of the intrauterine environment, and biomarker validation hold promise to understand infants’ prognoses more accurately, clearly the ability to provide evidence-based practices to these infants should also impact their long-term prognoses.
Author contributions
SY, RCS, JL, WT, NP and KM contributed to the design and implementation of the survey, as well as writing and reviewing the paper. SMB provided key qualitative research input and reviewed the paper. LN, HZ, SVR contributed to the design of the survey as well as editing the paper. RD, GN, EW, BH, JM, KP contributed to writing and editing of this paper.
Funding
None. RCS holds the William Buchanan Chair in Pediatrics at UT Southwestern.
Competing interests
Dr KM is Chair and Board Member of CHNC, a 501(c)3 organization.
Footnotes
*Members of the Children’s Hospital Neonatal Consortium severe bronchopulmonary dysplasia subgroup are listed below Funding.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41372-021-01165-9.
References
Full text links
Read article at publisher's site: https://doi.org/10.1038/s41372-021-01165-9
Read article for free, from open access legal sources, via Unpaywall:
https://www.nature.com/articles/s41372-021-01165-9.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/111228289
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1038/s41372-021-01165-9
Article citations
The NICU tracheostomy team: multidisciplinary collaboration for improvement in survival of complex patients.
J Perinatol, 24 Jun 2024
Cited by: 0 articles | PMID: 38914748
Managing established bronchopulmonary dysplasia without using routine blood gas measurements.
J Perinatol, 44(7):995-1000, 23 Apr 2024
Cited by: 0 articles | PMID: 38654082 | PMCID: PMC11226395
Identifying barriers and facilitators to care for infants with bronchopulmonary dysplasia after NICU discharge: a prospective study of parents and clinical stakeholders.
J Perinatol, 44(7):970-978, 26 Jan 2024
Cited by: 2 articles | PMID: 38278963
Tracheostomy in infants with severe bronchopulmonary dysplasia: A review.
Front Pediatr, 10:1066367, 12 Jan 2023
Cited by: 2 articles | PMID: 36714650 | PMCID: PMC9878282
Review Free full text in Europe PMC
Hyperinflation and its association with successful transition to home ventilator devices in infants with chronic respiratory failure and severe bronchopulmonary dysplasia.
J Perinatol, 43(3):332-336, 13 Dec 2022
Cited by: 0 articles | PMID: 36513765
Go to all (9) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Predicting death or tracheostomy placement in infants with severe bronchopulmonary dysplasia.
J Perinatol, 34(7):543-548, 20 Mar 2014
Cited by: 33 articles | PMID: 24651732
Predischarge death or lung transplantation in tracheostomy and ventilator dependent grade 3 bronchopulmonary dysplasia.
Pediatr Pulmonol, 59(4):867-873, 02 Jan 2024
Cited by: 0 articles | PMID: 38165155
Invasive mechanical ventilation at 36 weeks post-menstrual age, adverse outcomes with a comparison of recent definitions of bronchopulmonary dysplasia.
J Perinatol, 41(8):1936-1942, 25 May 2021
Cited by: 18 articles | PMID: 34035454 | PMCID: PMC8342304
Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia.
Neonatology, 107(4):358-359, 05 Jun 2015
Cited by: 15 articles | PMID: 26044104
Review

 1
1 


