Abstract
Free full text

LTB4-Driven Inflammation and Increased Expression of ALOX5/ACE2 During Severe COVID-19 in Individuals With Diabetes
Abstract
Diabetes is a known risk factor for severe coronavirus disease 2019 (COVID-19), the disease caused by the new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, there is a lack of knowledge about the mechanisms involved in the evolution of COVID-19 in individuals with diabetes. We aimed to evaluate whether the chronic low-grade inflammation of diabetes could play a role in the development of severe COVID-19. We collected clinical data and blood samples of patients with and without diabetes hospitalized for COVID-19. Plasma samples were used to measure inflammatory mediators and peripheral blood mononuclear cells, for gene expression analysis of the SARS-CoV-2 main receptor system (ACE2/TMPRSS2), and for the main molecule of the leukotriene B4 (LTB4) pathway (ALOX5). We found that diabetes activates the LTB4 pathway and that during COVID-19 it increases ACE2/TMPRSS2 as well as ALOX5 expression. Diabetes was also associated with COVID-19–related disorders, such as reduced oxygen saturation as measured by pulse oximetry/fraction of inspired oxygen (FiO2) and arterial partial pressure of oxygen/FiO2 levels, and increased disease duration. In addition, the expressions of ACE2 and ALOX5 are positively correlated, with increased expression in patients with diabetes and COVID-19 requiring intensive care assistance. We confirmed these molecular results at the protein level, where plasma LTB4 is significantly increased in individuals with diabetes. In addition, IL-6 serum levels are increased only in individuals with diabetes requiring intensive care assistance. Together, these results indicate that LTB4 and IL-6 systemic levels, as well as ACE2/ALOX5 blood expression, could be early markers of severe COVID-19 in individuals with diabetes.
Introduction
As of 17 May 2021, >162 million confirmed cases of coronavirus disease 19 (COVID-19) and >3.3 million deaths worldwide from the pandemic had been recorded (1). The disease is caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that emerged in China and rapidly spread around the world (2). Estimates indicate that ~80% of infected individuals are asymptomatic or develop mild symptoms. The other 20% can develop moderate to severe disease, occasionally requiring medical assistance due to acute respiratory disease and pneumonia, burdening health care systems (3,4). Risk factors in developing severe COVID-19 include, among others, hypertension, age, obesity, and diabetes (5–9). Individuals with diabetes are at high risk of developing severe COVID-19 as accounted for by their high rates of intensive care unit (ICU) admission and death (7).
Considering that 463 million people live with diabetes worldwide (10) and that COVID-19 is a highly transmissible disease, the need for identification of mechanisms that prevent infection in this population is urgent (6,7). As seen in multiple infectious diseases, including COVID-19, infection-induced inflammatory response can result in a cytokine storm, recruiting cells to infected tissues and establishing a proinflammatory feedback loop. This uncontrolled inflammation causes multiorgan damage, especially of the heart, liver, and kidneys, with high risk of death (11). Although several reports have described cytokines and chemokines involved in the inflammatory storm during COVID-19 (11,12), studies on lipid mediators of inflammation and their roles in this new disease are scarce.
Eicosanoids are potent lipid mediators produced by arachidonic acid metabolism, found in cell surface, that signals many biological processes, including inflammation and immune responses (13). Some classes of eicosanoids, especially leukotrienes, have been associated with the pathogenesis of respiratory disease (14,15). We and others have already shown increased levels of leukotriene B4 (LTB4) in diabetes, which is associated with inflammation, compromised wound healing, insulin resistance, and susceptibility to infections (16–20). LTB4 is a product of the action of 5-lipoxygenase (5-LO) (encoded by the arachidonate 5-lipoxygenase [ALOX5] gene) and its activating protein FLAP (encoded by ALOX5AP gene) that are rapidly produced after several stimuli, mainly by neutrophils and monocytes/macrophages. After its release, LTB4 can be signaled in an autocrine or paracrine manner by different cell types through the leukotriene receptor (encoded by the LTB4R gene), triggering an increase in chemotaxis and inflammatory exacerbation (18,21–23). In the current study, we sought to evaluate whether LTB4 plays a role in the severity of COVID-19 in individuals with diabetes.
Research Design and Methods
Ethics Statement
This study followed the principles specified in the Declaration of Helsinki. The Institutional Board for Ethics in Human Research at the Gonçalo Moniz Institute (Oswaldo Cruz Foundation) and Irmã Dulce Social Works approved this study (protocol numbers CAAE 36199820.6.0000.0040 and 33366020.5.0000.0047, respectively). Participants gave informed consent previous to any data and sample collection.
Acquisition of Microarray Data Set
Diabetes is considered a risk factor for complicated acute respiratory syndrome caused by SARS-CoV-2 infection (5,7). Given the lack of data on the mechanisms that drive these complications, we sought to analyze public transcriptome data of peripheral blood mononuclear cells (PBMCs) from individuals with diabetes. Microarray analysis was performed from a search of the National Center for Biotechnology Information Gene Expression Omnibus (GEO) database using the terms “diabetes” and “human.” Among the data sets found, we selected the data set with GEO accession number GSE95849 that was done on Phalanx Human lncRNA OneArray v1_mRNA (GPL22448) platform (24). This data set compared six samples of PBMCs from healthy control subjects (individuals with normal glucose tolerance and without a family history of diabetes or chronic diseases) and six samples from individuals with diabetes. The criteria for including individuals in the diabetes group were fasting plasma glucose ≥7
≥7 mmol/L, 2-h plasma glucose after oral glucose tolerance test
mmol/L, 2-h plasma glucose after oral glucose tolerance test ≥11.1
≥11.1 mmol/L, or use of glucose-lowering drugs or physician-diagnosed diabetes. Differentially expressed genes (DEGs) were considered when the fold change ranged from −2.0 to 2.0 with a false discovery rate–adjusted P < 0.05.
mmol/L, or use of glucose-lowering drugs or physician-diagnosed diabetes. Differentially expressed genes (DEGs) were considered when the fold change ranged from −2.0 to 2.0 with a false discovery rate–adjusted P < 0.05.
Detection of Metabolic Network in Diseases and Pathway Enrichment Analysis
Metabolic networks (compound-reaction-enzyme-gene) were found based on the expression of significantly modulated genes in comparisons of healthy control subjects with individuals with diabetes. We used MetDisease version 1.1.0 in Cytoscape 3.7.2 software (Cytoscape Consortium, San Diego, CA) to build disease-based metabolite networks according to the Kyoto Encyclopedia of Genes and Genomes (KEGG). Next, data were further filtered to retain disease Medical Subject Headings terms relevant to reported clinical COVID-19 manifestations, such as pneumonia, respiratory distress syndrome (adult), acute lung injury, and inflammation. Matched metabolites found in these conditions were clustered using a Venn diagram to find common molecules.
The identification of enriched pathways was based on genes and compounds using the integrated KEGG and Edinburgh Human Metabolic Network (EHMN) databases stored at the National Center for Biotechnology Information. Canonical pathways were detected by MetScape version 3.1.3 in the Cytoscape 3.7.2 software using significantly modulated genes between healthy control subjects and individuals with diabetes.
Study Design, Cohort Definition, and Clinical Data
Patients were admitted with confirmed diagnosis of COVID-19 at Ernesto Simões Filho General Hospital, Salvador, Bahia, Brazil. A convenience sample of 53 patients were enrolled in this study (24 without diabetes [the non-DM group: NDM] and 29 with diabetes [the diabetes mellitus group: DM]). This sample size considered a 95% CI (two-sided), and the power estimated for each parameter measured, using Epi Info software, was >80%. All groups were matched for sex, age, and hospitalization type (i.e., clinical beds [CBs], ICU). According to the Brazilian Diabetes Society guidelines, 2019–2020 (25), the diagnosis of diabetes was confirmed by HbA1c levels measured during hospitalization. Patients with HbA1c ≥6.5% (48 mmol/mol) and a medical history of insulin use were considered to have diabetes. The NDM group included individuals with HbA1c ≤6.4% (46 mmol/mol) who were not considered to have diabetes or prediabetes (without the need for insulin during hospitalization). Comorbidity data were collected according to medical records. The study included patients with a positive diagnosis of COVID-19 based on positive molecular test (quantitative real-time PCR), serology or tomography results for or clinical history of COVID-19. Patients who did not agree to sign the free and informed consent, were pregnant, had symptoms for ≥14 days, and had been in the hospital for >48 h were excluded. Clinical data from all patients, obtained from medical records, are shown in Table 1.
Table 1
Characteristics of individuals hospitalized because of complications of COVID-19, Salvador, Bahia, Brazil, 2020 (N = 53)
| NDM | DM | P | |
|---|---|---|---|
| Patients, n | 24 | 29 | |
| Male sex, n (%) | 15 (62.5) | 16 (55) | 0.59 |
| Age (years), median (min–max) | 59 (27–88) | 59 (43–93) | 0.12 |
| HbA1c, median (min–max) | <0.0001 | ||
 % % | 5.6 (4.5–6.3) | 7.9 (6.5–12.9) | |
 mmol/mol mmol/mol | 38 (26–45) | 63 (48–117) | |
| Comorbidities, n/N (%) | |||
 Obesity Obesity | 3/18 (16.6) | 7/21 (33.3) | 0.23 |
 Dyslipidemia Dyslipidemia | 3/13 (23.0) | 3/11 (27.2) | 0.99 |
 Liver disease Liver disease | 1/22 (4.5) | 0/24 (0.0) | 0.47 |
 Kidney disease Kidney disease | 8/24 (33.3) | 5/27 (18.5) | 0.22 |
 COPD COPD | 3/16 (18.7) | 3/14 (21.4) | 0.99 |
 HAS HAS | 9/24 (37.5) | 22/26 (84.6) | 0.001 |
| Symptoms, n/N (%) | |||
 Fever Fever | 12/19 (63.1.0) | 14/21 (66.6) | 0.99 |
 Cough Cough | 16/23 (69.5.5) | 16/22 (72.7) | 0.81 |
 Dyspnea Dyspnea | 13/22 (59.0) | 22/24 (91.6) | 0.01 |
 Expectoration Expectoration | 1/17 (5.8) | 3/16 (18.7) | 0.33 |
| COVID-19 confirmed, n/N (%) | 18/21 (85.7) | 26/27 (96.3) | 0.30 |
HAS, systemic arterial hypertension; min–max, minimum to maximum; n/N positive number/valid number.
Sample Collection
Blood samples from all patients were collected at admission by venipuncture using tubes with heparin. Plasma was separated (to quantify inflammatory mediators), and PBMCs (to analyze gene expression) were purified using Histopaque-1077 (Sigma-Aldrich, St. Louis, MO).
Analysis of Gene Expression in PBMCs
Total RNA was extracted from PBMCs using miRNeasy Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s guidelines. Relative expression of ALOX5 (assay identifier [ID] Hs.PT.56a.28007202.g); ACE2 (assay ID Hs.PT.58.27645939); transmembrane serine protease 2 (TMPRSS2) (assay ID Hs.PT.58.4661363); furin, paired basic amino acid cleaving enzyme (FURIN) (assay ID Hs.PT.58.1294 962), and basigin (CD147) (assay ID Hs.PT.56a.39293590.g) were analyzed. After RNA quantification and quality analysis by spectrophotometry, cDNA synthesis was performed using the SuperScript III Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA). Then, cDNA was amplified by quantitative real-time PCR using the SYBR Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA). Relative gene expression is shown as the fold change between the NDM and DM groups using the 2−ΔΔCT method [ΔΔCt = ΔCt (target DM) – mean ΔCt (target NDM), where ΔCt = Ct (gene of interest) − Ct (housekeeping gene)]. To identify the distribution within the control group (NDM), we applied ΔΔCt = ΔCt (target NDM) – mean ΔCt (target NDM), with ΔCt = Ct (gene of interest) − Ct (housekeeping gene). β-Actin was the housekeeping gene (ACTB) (assay ID Hs.PT.39a.22214847). All primers were purchased from Integrated DNA Technologies (Coralville, IA).
Quantification of Inflammatory Mediators
Based on the inflammatory profile already described in the literature for diabetes and COVID-19 (6,8,26), serum levels of TNF-α, IL-6, and IL-1β cytokines (Invitrogen) were evaluated using sandwich ELISAs. LTB4 levels were determined by Competition ELISA Kit (Cayman Chemical, Ann Arbor, MI), considering the manufacturer’s instructions.
Statistical Analysis
The Benjamini-Hochberg method was used to control false discovery rate in evaluation of DEGs from the GEO transcriptome data set. For variables with normal distribution, we used Student t test (two groups) and one-way ANOVA test followed by Tukey post hoc test (three or more groups). For nonnormal distribution, we used Mann-Whitney test (two groups), Kruskal-Wallis with Dunn posttest (three or more groups), and Spearman test for correlation analysis. Symptom and comorbidity analyses were performed using χ2 or Fisher exact test. All tests were conducted using GraphPad Prism 7 software (GraphPad Software, San Diego, CA). Differences were considered statistically significant when P < 0.05 or adjusted P < 0.05 for DEGs and multiple comparisons.
Data and Resource Availability
The public data set analyzed during the current study is available in GEO under accession number GSE95849 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE95 849). The data sets generated during the current study are not publicly available but can be made available by the corresponding author upon request.
Results
LTB4 Signaling Activated in Individuals With Diabetes Is Similar to That Found in Respiratory Disorders
Initially, we found that 3,585 genes were significantly modulated when comparing cells from individuals with or without diabetes. Of these, 3,405 were upregulated, and 180 were downregulated in individuals with diabetes (Fig. 1A).
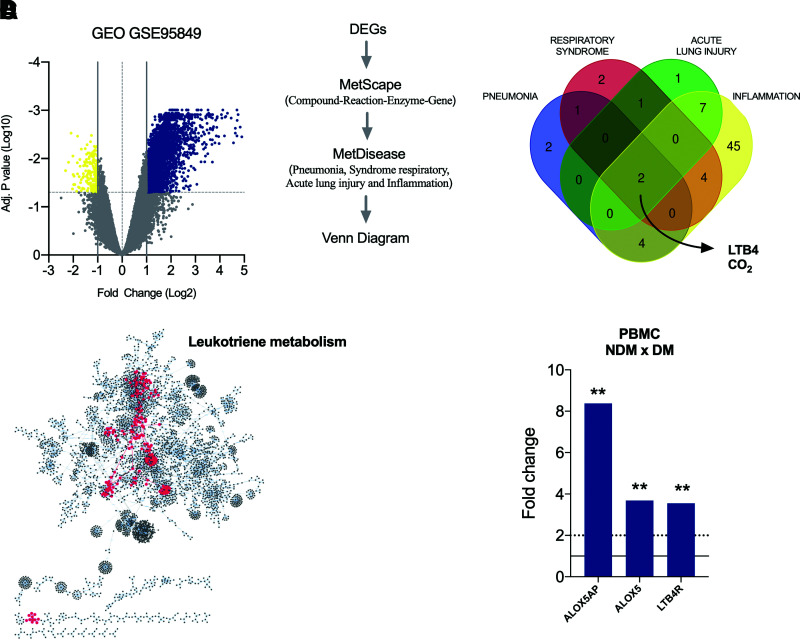
Upregulation of LTB4 signaling in individuals in the DM group. A: Volcano plot with DEGs (blue, upregulated genes; yellow, downregulated genes) in PBMCs from DM patients vs. NDM patients. B: Workflow to identify molecules associated with inflammation and respiratory disorders based on gene expression shown in A and the resulting Venn diagram showing molecules in common among pneumonia, respiratory syndrome, acute lung injury, and inflammation. C: Enriched pathways raised from DEG analyses of PBMCs from DM patients vs. NDM patients, highlighting in red the central position of leukotriene metabolism among pathways. D: Fold change of genes involved with LTB4 production (ALOX5AP and ALOX5) and signaling (LTB4R) in PBMCs of DM vs. NDM patients. Dotted line = cutoff point for a DEG; solid line = average of the control group. Data are medians. **P < 0.01. Adj., adjusted.
Next, we searched for disorders associated with these DEGs by detecting molecule networks. We focused on conditions related to severe COVID-19, such as pneumonia, severe acute respiratory syndrome, and acute lung injury; we also focused on inflammation. Interestingly, we found only two molecules in common among these conditions: carbon dioxide and the lipid mediator LTB4 (Fig. 1B).
We further searched for signaling pathways associated with these DEGs, and among 61 routes found, the LTB4 pathway was at a central position within the network (Fig. 1C). Next, we assessed the expression of molecules crucial for LTB4 production, such as the ALOX5 gene (which encodes the 5-LO enzyme that converts arachidonic acid into leukotrienes), ALOX5AP (the 5-LO–activating protein), and LTB4R (the LTB4 receptor) in this data set. We found increased expression of all evaluated genes in the PBMCs from DM compared with NDM (Fig. 1D). Together, these findings indicate that LTB4 is a potential target to study mechanisms under complicated COVID-19 in individuals with diabetes.
Increased Expression of ALOX5 and ACE2/TMPRSS2 in PBMCs From DM and NDM Patients With COVID-19
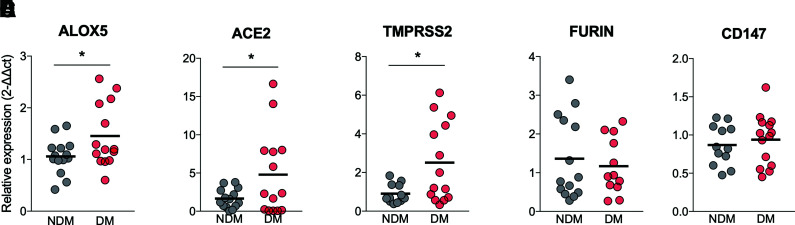
The expression of SARS-CoV-2 receptors (27) and the inflammatory response (11) are related to the complications found in COVID-19. We then assessed the expression of ALOX5 (which encodes for the 5-LO enzyme) and ACE2/TMPRSS2, FURIN, and CD147 (surface molecules used by SARS-CoV-2 to invade human cells). The results showed a significant increase in the expression of ALOX5 (Fig. 2A) and ACE2/TMPRSS2 (Fig. 2B and C) in PBMCs from COVID-19 in DM compared with NDM. The increase in ALOX5, ACE2, and TMPRSS2 was also preliminarily assessed in the tracheal secretion of the NDM and DM groups with COVID-19 under mechanical ventilation. Despite the small sample size, we observed a trend toward increased expression, indicating that blood cells mirror the immune response in the lungs (P = 0.055) (Supplementary Fig. 1). These findings confirm our previous result (from public transcriptome data), showing that ALOX5 expression is increased in diabetes (Fig. 1D). Such findings support the possible role of 5-LO in the chronic low-grade inflammation observed in LTB4 pathway–induced diabetes, rendering individuals with diabetes more prone to infections (19,21). We also found increased expressions of the SARS-CoV-2 main receptor system ACE2 and TMPRSS2 in the PBMCs from individuals in the DM group, suggesting that immune cells that will fight the infection are more prone to viral invasion.
Expression of ALOX5 Correlates With That of ACE2 in PBMCs From DM Patients With COVID-19
ACE2 expression is crucial for cell invasion and progression in COVID-19 (11,27). Therefore, we investigated whether the expression of ALOX could be correlated with ACE2 expression. First, we correlated ALOX5 with ACE2/TMPRSS2 (summarized in the correlation matrix [Fig. 3A]) separately between the DM and NDM groups. We found a positive correlation between ACE2 and TMPRSS2 in both groups since these molecules act together during viral invasion (11) (Fig. 3B and C). However, the correlation between ALOX5 and ACE2 was only present in the DM group (Fig. 3D and Supplementary Fig. 2), suggesting that cells that have high levels of ALOX5 also have increased ACE2 expression in the DM group.
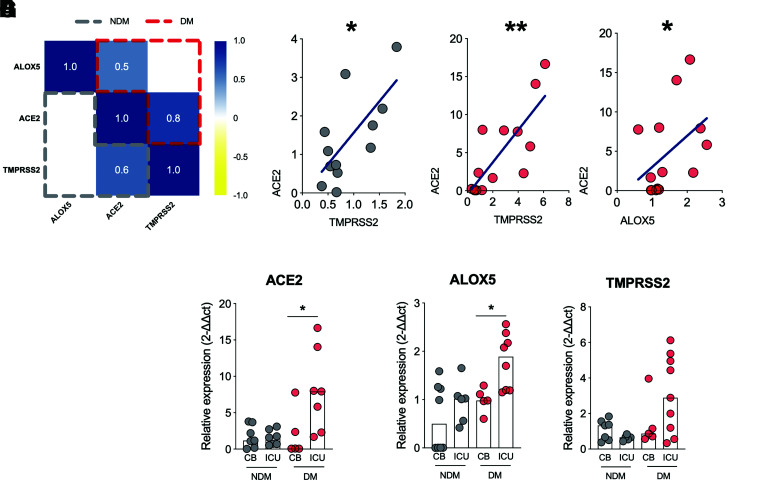
ALOX5 expression positively correlates with ACE2 expression in individuals with diabetes and COVID-19, and this is associated with an increased rate in ICU admissions. A: Correlation matrix between ALOX5 and ACE2/TMPRSS2 expression in PBMCs from DM (red) and NDM (gray) patients. B: Correlation analysis between ACE2 and TMPRSS2 expressions in PBMCs of NDM (B) and DM (C) individuals with COVID-19. D: Correlation analysis between ALOX5 and ACE2 expression in PBMCs from DM patients. E to G: Hospitalization type among DM or NDM individuals with COVID-19 based on the expression of ACE2, ALOX5 and TMPRSS2. Data are medians. Spearman r correlation. *P < 0.05, **P < 0.01.
Next, we evaluated whether ALOX5 and ACE2 expressions are correlated with the clinical evolution of COVID-19. First, we compared the need for ICU admission between the DM and NDM groups stratified by the expression levels of ALOX5 and ACE2. The results showed that individuals in the DM group with higher levels of ACE2 (Fig. 3E) and ALOX5 (Fig. 3F) required ICU care more frequently than those with low expression of these genes, but no difference was found with the gene expression of TMPRSS2 (Fig. 3G). Together, these findings indicate that the increased expressions of ALOX5 and ACE2 in blood cells from individuals with diabetes are associated with more severe conditions of COVID-19, requiring ICU care.
Increased Systemic Levels of LTB4 in DM Patients With COVID-19
The cytokine storm described in COVID-19 is characterized by several inflammatory mediators. However, the role of lipid mediators in this context is still unknown (11). We measured the levels of inflammatory cytokines (IL-6, TNF-α, and IL-1β) and a lipid mediator of inflammation (LTB4) in the plasma of individuals with and without diabetes and COVID-19. The results showed a significant increase of LTB4 levels in the sera of individuals in the DM group (Fig. 4A). No statistical differences in the levels of IL-6 (Fig. 4B), TNF-α (Fig. 4C), or IL-1β (Supplementary Fig. 3) were found in comparisons of the DM and NDM groups. Supplementary Fig. 4 shows the production of these inflammatory mediators individually for each patient in the NDM and DM groups.
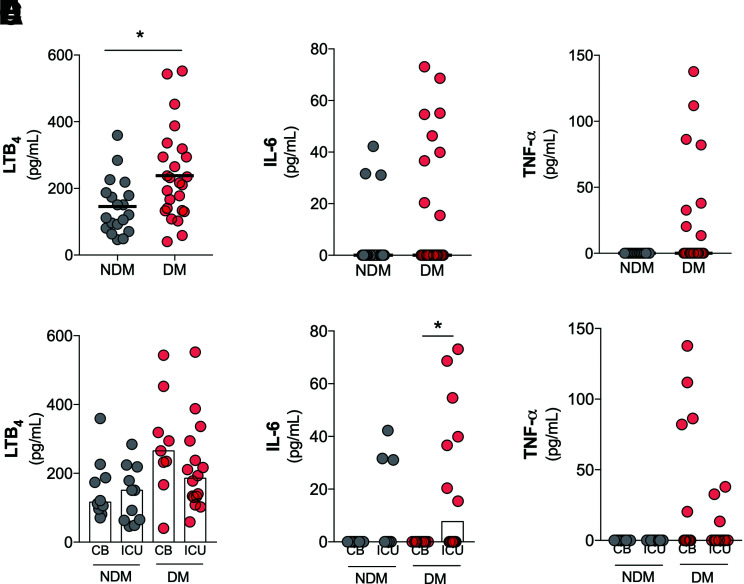
Increased systemic levels of LTB4 in patients in the DM group, with COVID-19. Levels of LTB4 (A), IL-6 (B), and TNF-α (C) in plasma samples from DM and NDM patients affected by COVID-19. Plasma levels of LTB4 (D), IL-6 (E), and TNF-α (F) in NDM and DM patients with COVID-19 categorized by hospitalization type: clinical beds (CB) or intensive care units (ICU). Data are means. *P < 0.05.
We further detailed the production of LTB4, IL-6, and TNF-α between the NDM and DM groups based on the hospitalization type. No differences were found for LTB4 and TNF-α production (Fig. 4D and F). With regard to IL-6 production, in the DM group, there was a significant increase in ICU admissions compared with CB admissions (Fig. 4E). Together, these findings indicate the predominance of LTB4 production in the DM group compared with the NDM group. Moreover, IL-6 production seems to be an indicator for COVID-19 severity (hospitalization type) in the DM group.
ALOX5 Expression, Involved in LTB4 Synthesis, Was Correlated With Clinical Outcomes of COVID-19 in the DM Group
Despite studies reporting diabetes as a risk factor for COVID-19, few explored the mechanisms related to these patients’ worse prognosis (7,28,29). We compared LTB4 signaling in patients with different clinical outcomes associated with COVID-19. In an analysis of days spent in the hospital (Fig. 5A) and death rate (Fig. 5B), we found no difference between the NDM and DM groups. However, there was a significantly longer disease duration (the period between symptom onset and disease outcome [death or hospital discharge]) in the DM group (Fig. 5C). These data suggest that individuals with diabetes develop COVID-19 symptoms for prolonged periods, possibly due to the low-grade inflammation already present in these individuals even in the absence of an infectious agent. Furthermore, the pulmonary condition in the DM group was more severe than in the NDM group, measured by oxygen saturation by pulse oximetry (SpO2)–to–fraction of inspired oxygen (FiO2) ratio (Fig. 5D), arterial partial pressure of oxygen (PaO2)–to–FiO2 ratio (Fig. 5E), and O2 saturation (Supplementary Fig. 5) at the moment of admission to the hospital. For both parameters, individuals in the DM group arrived at the hospital in a more critical condition.
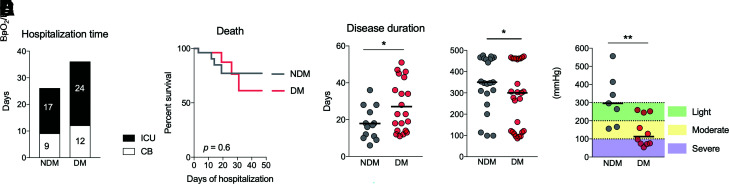
Diabetes induces greater severity of COVID-19. A: Number of days that NDM and DM patients remained hospitalized in CBs or the ICU because of COVID-19. B: Survival curves of NDM and DM patients hospitalized for COVID-19. C: Disease duration measured from the onset of symptoms to hospital discharge for NDM and DM patients with COVID-19. D: O2 saturation of NDM and DM patients with COVID-19. E: Degree of lung injury in NDM and DM patients with COVID-19. Data are medians in A, B, and D and means in C. *P < 0.05, **P < 0.01.
Finally, we correlated these clinical aspects with LTB4 production and ALOX5 and ACE2 expression in all individuals (Fig. 6A). The results show a positive correlation between LTB4 and ALOX5, as expected, since the 5-LO enzyme produces LTB4 (r = 0.5) (Supplementary Fig. 6A). We found that ALOX5 negatively correlates with the worse pulmonary condition, such as SpO2-to-FiO2 ratio (r = −0.6) and PaO2-to-FiO2 ratio (r = −0.9) (Fig. 6B and C). In addition, we found that patients with a low SpO2-to-FiO2 ratio and increased production of IL-6 had a longer hospital stay for COVID-19 (Fig. 6D).
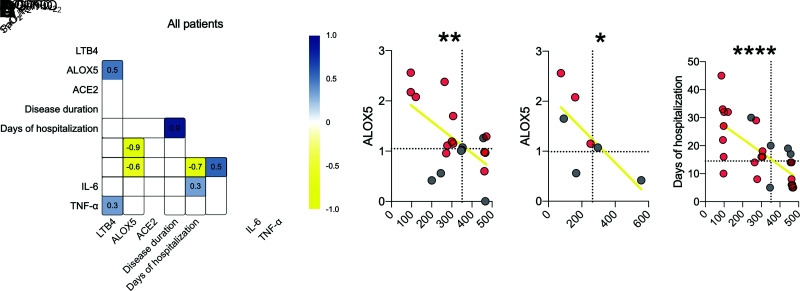
ALOX5 plays a role in the severity of COVID-19 in individuals with diabetes. A: Correlation matrix among genes, inflammatory parameters, and clinical outcome changes found in all patients with COVID-19. B and C: Dispersion of values with all patients between the correlation of ALOX5 with SpO2-to-FiO2 and PaO2-to-FiO2 ratios. D: Correlation between oxygen saturation and days of hospitalization. Dotted lines = median of the NDM group. Spearman r correlation. *P < 0.05, **P < 0.01, ****P < 0.0001.
Taken together, these results show that patients with COVID-19 and diabetes develop a more pronounced systemic inflammatory response with the predominance of LTB4 and increased expression of SARS-CoV-2 receptor system ACE2/TMPRSS2. These individuals more frequently require critical care due to lung injury, suggesting that LTB4 signaling could be a mediator produced by individuals with diabetes that increases the risk for severe COVID-19.
Discussion
As SARS-CoV-2 emerged and spread globally, identifying mechanisms involved in severe COVID-19 and its risk factors became crucial for improving disease management. Diabetes is considered a risk factor for severe COVID-19 (5,7,28), but the mechanisms under these complications remain unknown. Inflammation associates with severe COVID-19 (18,21,22), and LTB4 drives the chronic low-grade inflammation observed in experimental models of diabetes, while its role is not fully elucidated in humans with diabetes (17–19,21,30–32). The current study shows that individuals with diabetes and COVID-19 have increased expression of genes from the LTB4 pathway in blood cells. During COVID-19, the expression of ACE2 and TMPRSS2, which encode the main receptor system for SARS-CoV-2 cell invasion, are also increased in PBMCs of individuals with diabetes. Moreover, the increased expression of ALOX5 correlates with ACE2, which was present in patients with critical conditions requiring intensive care.
As revealed by pathway analysis, LTB4 is critical in several physiological disorders (observed in severe COVID-19), including inflammation and respiratory complications such as pneumonia, respiratory distress syndrome, and acute lung injury (11,28). LTB4 is also an essential molecule in diabetes pathogenesis. Several studies with experimental models have indicated that LTB4 dictates the chronic low-grade inflammation in diabetes, rendering mice more prone to infections (17,19,33). Our group previously showed that increased production of LTB4 induced by diabetes alters the outcome of cutaneous leishmaniasis (17). Another study showed that LTB4 is associated with pulmonary complications, such as pneumonia, acute lung injury, acute respiratory distress syndrome (ARDS), and respiratory failure (15,34,35).
The interaction between SARS-CoV-2 and host cells involves several molecules, such as ACE2 and TMPRSS2 that interact with the viral spike protein (11,36,37). High glucose concentrations increase the expression of ACE2 and SARS-CoV-2 viral load in human monocytes (27). A meta-analysis revealed an increase of ACE2 expression in the lungs of patients with comorbidities, including diabetes (5), and another study showed an increase in the ACE2 protein in the lungs of individuals with diabetes (38). Besides the expression of ACE2 in the lungs, monocytes and lymphocytes are crucial for the COVID-19 immunopathogenesis (5,11,12,27,36). Our data show that ACE2 and TMPRSS2 expression are increased in PBMCs of individuals with diabetes and COVID-19, which can be related to a greater susceptibility to SARS-CoV-2 infection (27,38).
Additionally, ALOX5 expression positively correlates with ACE2, and ICU admission is associated with increased ALOX5/ACE2 expression in patients with diabetes and COVID-19. The interaction between the LTB4 and ACE2 pathways is still unknown, but the positive independent regulation of these genes in monocytes can influence the process of inflammation and infection, respectively (21,27). During SARS-CoV-2 infection, mononuclear cells are recruited to the lung tissue, where they probably contribute to the control of infection and the healing process but also cause cause tissue damage (11).
In the current study, individuals in the DM group with COVID-19, age and sex matched to individuals in the NDM group with COVID-19, had a higher frequency of dyspnea, which is in agreement with data from Wuhan, China (7). Hypertension is more frequent in patients with diabetes and patients with COVID-19 and is a known risk factor for severe COVID-19 (7,38). According to previous studies, diabetes and hypertension are frequent in patients with COVID-19 and may play a role in increased death rates (6,7,39). In our study, mortality rates were similar between patients with COVID-19 with or without diabetes, but the disease severity is more pronounced in those with diabetes. Although our cohort shows no difference in obese individuals between the NDM and DM groups, the influence of weight differences between the groups should not be excluded, since obesity was determined only by medical observation.
The cytokine storm contributes to mortality in ~28% of fatal COVID-19 cases (11). This condition encompasses several cytokines and chemokines, such as IL-1β, IL-6, IFN-γ, MCP-1, CCL2, CXCL10, and TNF-α (11,28). The IL-6 cytokine is one of the most related to the severity of COVID-19, and as in previous studies, our findings demonstrate this association in the context of COVID-19 in individuals with diabetes (6,8,26). However, knowledge is lacking about the implications of lipid mediators in the inflammatory response during COVID-19. LTB4 is a potent inducer of inflammatory cytokines, including those of the cytokine storm, which may drive COVID-19 severity (16,21). Bronchoalveolar lavage fluid exhibited high levels of LTB4 in an experimental model of acute lung injury (34). LTB4 plays a significant role in the chronic obstructive pulmonary disease (COPD), and individuals with severe COPD have high levels of LTB4 in exhaled air; such levels correlate with disease severity (14). LTB4 levels better correlate with lung injury severity and clinical outcomes in ARDS than several other eicosanoids (35).
The number of patients with severe COVID-19 who require ICU care is a challenge for health care systems worldwide. Individuals with ARDS exhibit three to five times more LTB4 levels than control subjects (40). The role of LTB4 in the outcome of lung diseases is associated with neutrophil tissue infiltration, a condition present in COVID-19 (12). Our group has recently shown that LTB4 is involved in the activation of pathogen-induced inflammasomes (18). A recent preliminary study associated the activation of inflammasomes in the lungs of patients with COVID-19 with a worse disease prognosis (41).
The Randomized Evaluation of COVID-19 Therapy (RECOVERY) study showed that dexamethasone slightly reduced death rates among patients with COVID-19 requiring invasive mechanical ventilation or oxygen therapy (42). Additionally, montelukast, a leukotriene antagonist, is proposed for the prophylaxis of COVID-19 symptoms (43). Together, these studies suggested strategies to treat COVID-19 that, directly or indirectly, act through eicosanoids. Our results confirm that LTB4 signaling is a crucial branch of the inflammatory response observed in COVID-19 and reinforces the possibility of its inhibition in clinical practice.
Several studies reported the association of diabetes and increased COVID-19 death rates (4,5,19,22), whereas others did not find such an association with disease severity (4,5,33). We have not found a direct association between diabetes and mortality rates in our cohort. The participants in the DM group in this study developed severe forms of COVID-19, requiring ICU hospitalization, but their disease evolution seemed similar to that of patients in the NDM group. On the other hand, we found a significantly longer disease duration in DM patients with COVID-19. The disease duration refers to the period between the onset of symptoms until the patient’s discharge or death, indicating that patients with diabetes experience COVID-19 symptoms for prolonged periods.
Although we have not found a direct association between systemic levels of LTB4 and a worse COVID-19 prognosis in individuals with diabetes, our findings show that patients with COVID-19 and diabetes more frequently present reduced SpO2-to-FiO2 and PaO2-to-FiO2 ratios that correlate with ALOX5 expression in the blood. The dissociation between the expression of the ALOX5 gene and its metabolic product may be due to different sources of LTB4 detected in the bloodstream. Different immune cell types are able to produce LTB4, such as neutrophils (14), a cell type not represented in our sample of mononuclear cells. LTB4 is also locally produced at the site of infection caused by different agents (17–19,44) and has been associated with increased lung injury in experimental models (34). Our results add a new player to the inflammation panorama of COVID-19, suggesting that circulating mononuclear cells already present a proinflammatory profile that, once recruited to the lung, may amplify local inflammation and tissue injury. Further studies are necessary to confirm pulmonary production of LTB4 and its role in COVID-19 outcomes.
In summary, our findings show that diabetes induces a proinflammatory profile on circulating immune cells with increased expression of ACE2 and ALOX5 genes, rendering these cells more prone to SARS-CoV-2 invasion. Together, our data reveal a potential role of LTB4 in COVID-19, which is poorly explored, and open new ways to study implications and applications of this mediator in SARS-CoV-2 infection. Furthermore, we found that IL-6, a known cytokine for COVID-19 severity, is also a potential indicator in individuals with diabetes in need of intensive care.
Article Information
Acknowledgments. The authors thank the developers of the MetScape and MetDisease software for making it possible to analyze the data in a more integrated way, Dr. Manuela da Silva Solcà (Federal University of Bahia) for help in the construction of the table, and the health professionals who participated directly and indirectly in the care of patients.
Funding. This work was supported by the Inova Fiocruz/Fundação Oswaldo Cruz to N.M.T. (VPPCB-005-FIO-20-2-75), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brazil (CAPES) under Finance Code 001 to I.B.-S., S.N., and J.S., Conselho Nacional de Desenvolvimento Científico e Tecnológico–Brazil (CNPq) (to I.B.-S., S.N., A.B., C.B., and M.B.-N.), and National Institutes of Health grants R01HL124159-01, DK122147-01A1 and AI149207A (to C.H.S.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. I.B.-S., A.F.A.M., T.C.-S., S.N., and H.S contributed to the acquisition of the data or the analysis and interpretation of information. I.B.-S., T.C.-S., S.N., R.L.S., A.B., P.R.S.O., R.K., C.B., M.B.-N., V.B., and N.M.T. contributed to the writing of the manuscript or had substantial involvement in its revision before submission. I.B.-S., S.N., and J.S. conducted the processing of biological samples in the laboratory. I.B.-S., V.B., and N.M.T. were involved in the conception, hypotheses delineation, and design of the study. A.F.A.M., M.R.S.C., and J.R.C. conducted the medical care of the research participants. N.M.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
V.B. and N.M.T. contributed equally to this work.
This article contains supplementary material online at https://doi.org/10.2337/figshare.14770662.
This article is part of a special article collection available at https://diabetes.diabetesjournals.org/collection/diabetes-and-COVID19-articles.
References
Articles from Diabetes are provided here courtesy of American Diabetes Association
Full text links
Read article at publisher's site: https://doi.org/10.2337/db20-1260
Read article for free, from open access legal sources, via Unpaywall:
https://diabetesjournals.org/diabetes/article-pdf/70/9/2120/627443/db201260.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/107905410
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.2337/db20-1260
Article citations
BCR, not TCR, repertoire diversity is associated with favorable COVID-19 prognosis.
Front Immunol, 15:1405013, 28 Oct 2024
Cited by: 0 articles | PMID: 39530088 | PMCID: PMC11550956
YTHDF1-regulated ALOX5 in retinal pigment epithelial cells under hypoxia enhances VEGF expression and promotes viability, migration, and angiogenesis of vascular endothelial cells.
Sci Rep, 14(1):23226, 05 Oct 2024
Cited by: 0 articles | PMID: 39369033 | PMCID: PMC11455921
Potential Effects of Hyperglycemia on SARS-CoV-2 Entry Mechanisms in Pancreatic Beta Cells.
Viruses, 16(8):1243, 02 Aug 2024
Cited by: 0 articles | PMID: 39205219 | PMCID: PMC11358987
Review Free full text in Europe PMC
Neutrophil swarming: Is a good offense the best defense?
iScience, 26(9):107655, 17 Aug 2023
Cited by: 1 article | PMID: 37670784 | PMCID: PMC10475518
Review Free full text in Europe PMC
Sex Differences in Fatty Acid Metabolism and Blood Pressure Response to Dietary Salt in Humans.
Cardiogenetics, 13(1):33-46, 03 Mar 2023
Cited by: 0 articles | PMID: 38605973 | PMCID: PMC11008634
Go to all (13) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
GEO - Gene Expression Omnibus (3)
- (2 citations) GEO - GSE95849
- (1 citation) GEO - GSE95
- (1 citation) GEO - GPL22448
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Nasopharyngeal Expression of Angiotensin-Converting Enzyme 2 and Transmembrane Serine Protease 2 in Children within SARS-CoV-2-Infected Family Clusters.
Microbiol Spectr, 9(3):e0078321, 03 Nov 2021
Cited by: 9 articles | PMID: 34730438 | PMCID: PMC8567246
Leukotriene B4-mediated sterile inflammation promotes susceptibility to sepsis in a mouse model of type 1 diabetes.
Sci Signal, 8(361):ra10, 27 Jan 2015
Cited by: 36 articles | PMID: 25628460 | PMCID: PMC4356178
Glycated ACE2 receptor in diabetes: open door for SARS-COV-2 entry in cardiomyocyte.
Cardiovasc Diabetol, 20(1):99, 07 May 2021
Cited by: 63 articles | PMID: 33962629 | PMCID: PMC8104461
Interactions of renin-angiotensin system and COVID-19: the importance of daily rhythms in ACE2, ADAM17 and TMPRSS2 expression.
Physiol Res, 70(s2):S177-S194, 01 Dec 2021
Cited by: 10 articles | PMID: 34913351 | PMCID: PMC8884363
Review Free full text in Europe PMC
Funding
Funders who supported this work.
NHLBI NIH HHS (1)
Grant ID: R01 HL124159
NIAID NIH HHS (1)
Grant ID: R21 AI149207
NIDDK NIH HHS (1)
Grant ID: R01 DK122147