Abstract
Free full text

Neutrophil swarming: Is a good offense the best defense?
Summary
The phenomenon of swarming has long been observed in nature as a strategic event that serves as a good offense toward prey and predators. Imaging studies have uncovered that neutrophils employ this swarm-like tactic within infected and inflamed tissues as part of the innate immune response. Much of our understanding of neutrophil swarming builds from observations during sterile inflammation and various bacterial, fungal, and parasitic infections of the skin. However, the architecture and function of the skin differ significantly from vital organs where highly specialized microenvironments carry out critical functions. Therefore, the detrimental extent this perturbation may have on organ function remains unclear. In this review, we examine organ-specific swarming within the skin, liver, and lungs, with a detailed focus on swarming within microvascular environments. In addition, we examine potential “swarmulants” that initiate both transient and persistent swarms that have been implicated in disease.
Swarming: A ubiquitous biological phenomenon
Neutrophil behaviors have been observed for over a century with the use of intravital microscopy, most notably when Elie Metchnikoff first described neutrophil functions after inserting thorns into translucent invertebrates.1 Since then, it has been widely accepted that neutrophils are extremely important to innate immunity. Within their short lifespan, neutrophils mobilize from the bone marrow and infiltrate inflamed tissues from the blood, forming the first line of defense against infection by employing an array of host resistant processes harmful to neutralize invading pathogens.1,2 Over the preceding decades, intravital microscopy techniques have enabled researchers to uncover a new type of neutrophil behavior that utilizes the unique phenomenon of swarming that is typically observed macroscopically in nature.3 Swarming is a phenomenon observed across biology. For instance, a flock of starling birds form swarming murmurations to provide “safety in numbers” which increases protection against predatory birds or environmental perturbations.4 In a similar fashion, honeybees (Apis mellifera) release a potent blend of alarm pheromones that recruit nearby bees and initiate swarming to tactically overwhelm and kill invading hornets by thermo-balling.3,5,6 Furthermore, studies into locust decimation of crops have revealed the importance of pheromones as chemoattractants that initiate large gregarious swarms of locusts.7 Moreover, inappropriate release of powerful chemoattractants from a single lost army ant permits trailing ants to follow in a continuous and exhaustive circling swarm known as a death spiral.8 Interestingly, directional cues are also used on a microscopic level by soil-dwelling amoeba Dictyostelium discoideum, which secrete cyclic adenosine monophosphate that creates chemotactic concentration gradients, leading other Dictyostelium to form aggregated clusters around the pioneering cells.9 Regarding immune cells, upon injury or infection, circulating neutrophils are recruited via hierarchies of chemoattractants and adhesion molecules that guide them to the foci within tissue. Once at the inflamed site, pioneering neutrophils initiate a swarm-like behavior, which involves individual neutrophils migrating toward, and encircling, their target as part of a larger coordinated group.10,11 Multiple neutrophils then form dense clusters where their effector functions can collectively influence immune defense by preventing pathogen escape and clearance, and promoting wound healing and repair processes.8,9,11,12,13,14,15,16 Despite several years of active research in neutrophil swarming, important mechanistic information remains undiscovered. In this review, we will compare factors that drive neutrophil swarming within specific tissue microenvironments during disease states both to promote host resistance, defense and resolution, but also as a driver of inflammatory immunopathology and tissue damage.
Neutrophil migration niches
Upon infection or injury, neutrophils must be recruited to the site of inflammation before participating in swarming behavior. The leukocyte recruitment cascade has classically been described within the intravascular space of the murine cremaster muscle where neutrophils exit the microvasculature through the venous system and into inflamed tissue. This cascade has been reviewed in greater detail elsewhere.17,18 However, neutrophil recruitment and migration is a highly context-driven process with non-canonical cascades being exhibited in several different organ tissues.17,19,20,21,22,23 For example, sterile liver injury and infection have separate hierarchies of chemoattractants that alter neutrophil entry into hepatic tissue. During sterile liver injury, McDonald et al. described neutrophil adhesion to sinusoidal microvascular endothelium via Mac-1 and intercellular adhesion molecule-1 (ICAM-1) dependent interactions. However, the systemic introduction of circulating Escherichia coli (E. coli), or their endotoxic lipopolysaccharide (LPS), changes the hierarchical order of adhesion molecules for neutrophils; switching them from the classical Mac1-ICAM-1-mediated adhesion to CD44-hyaluronan-dependent adhesion.12,14,24 Furthermore, neutrophil recruitment to the microvasculature of the lungs remains unaffected by selectins, as demonstrated in mice deficient of E- and P-selectins during a pneumonia model of Streptococcus pneumoniae.21 Instead, CD11b-dependent adhesion is required to facilitate neutrophil recruitment within the pulmonary circulation.25 Therefore, neutrophils do not always follow the “classical” rolling and adhesion cascade observed in other organs and tissues, indicating that the type of inflammatory stimuli in a specific tissue alters cell surface adhesion molecule expression when transiting through different organs and tissue microvasculature. Therefore, neutrophil migration to the site of inflammation is highly context-driven and may affect how neutrophils respond and swarm at the inflamed site.
Defining the swarm
A consensus definition of swarming currently does not exist. However, the initial conceptual framework of swarming came when Chtanova et al. first reported dynamic neutrophil cooperative behaviors in vivo during intracellular parasitic Toxoplasma gondii infection within the subcapsular sinus of lymph nodes. Here, pioneering neutrophils respond to parasitic egress and amplify signals to late-arriving neutrophils from >70 μm away from the swarm center and induce rapid migration of up to 11 μm/min to the site of infection.3 Since then, neutrophil swarming has been observed with intravital microscopy in several types of inflamed, infected, and sterilely wounded tissue (Table 1). The first quantitative definition of neutrophil swarms characterized two types of behaviors: diffuse transient swarms consisting of 20–150 neutrophils (<4 × 104 μm3) which can quickly mobilize or fuse into large persistent swarms of up to 300 neutrophils (>6 × 105 μm3) within 10–40 min, lasting up to 1 h before dissipating.3,26,27 However, multiple transient swarms can compete for additional neutrophil support and remain a small cluster of cells that can quickly dissipate and reform elsewhere.3,27 These early models characterized a rapid 23-min, 3-step cascade where neutrophils scout for injury and then amplify recruitment of other neutrophils to the injured site where retention of neutrophils is then stabilized.28 Further adaptations of this cascade now include (1) initiation, (2) nascent clustering, (3) amplification through intercellular signal relays, (4) aggregation and tissue remodeling, and lastly (5) termination and resolution (Figure 1).27,29 Much of our molecular understanding of neutrophil swarming is derived from murine models of skin infection and sterile inflammation.11,30 Indeed, the first essential molecular requirements of swarming were elucidated by Lämmermann et al. where leukotriene B4 (LTB4) was identified as an essential swarm initiator following laser-induced sterile inflammation in the dermis of the ear.11 Subsequently, sterile necrotic hepatic tissue injury revealed that neutrophils follow CXCR2 ligands IL-8 and macrophage inflammatory protein-2 (MIP-2, murine homolog of IL-8) from >600 μm away from the foci of damage. Then, once at the border of the necrotic injury, neutrophils switch to formyl peptide receptor-1 (FPR1)-dependent guidance through the release of necrotic cell mitochondrial formyl-Met-Leu-Phe (fMLP), while becoming desensitized to their former CXCR2 cues. Together, these concerted chemokine gradients facilitate guidance into the final ~150 μm of the lesion.14 Deletion of FPR1 prevents neutrophils from entering the proximal 150 μm injury site, yet neutrophils continue to swarm and cluster at the necrotic border.14 Thus, our current definitions of swarming are based on visualization, quantification of speed and size of neutrophil aggregates, as well as molecular cues and signals. However, further work is needed to better define and characterize the swarming behavior.
Table 1
Neutrophil swarmulants
| Tissue type | Type of inflammatory stimuli | Location of swarming | Swarming-associated molecules, kinases, and receptors | Reference |
|---|---|---|---|---|
| Lungs | Candida albicans | Intravascular | Zymosan, C5a, LTB4 | Lee et al.13 |
| Ischemia reperfusion injury | Intravascular | Kreisel et al.31 | ||
| LPS | Intravascular | Carestia et al.32 | ||
| E. coli | Extravascular | Kreisel et al.31 | ||
| Aspergillus fumigatus | Intravascular | Bruns et al.33 | ||
| Aspergillus fumigatus | Intravascular | Zymosan, LXA4 | Podstawka et a.34 | |
| Liver | Sterile injury | Extravascular | αMβ2-ICAM1 | McDonald et al.14, Dal-Secco et al.35, Slaba et al.36 |
| E. coli, LPS | Intravascular | CD44-hyaluronan | McDonald et al.14 | |
| Sterile injury | Extravascular | β2 integrin, LTB4, CXCL2, GRK2, Pertussis-toxin sensitive signals, cyclic ADP ribose | Lämmermann et al.11, McDonald et al.14, Ng et al.28, Peters et al.30, Kienle et al.37, Park et al.38, Shannon et al.39, Cho et al.40, Baik et al.41, Roth et al.42 | |
| Yersinia pestis | Extravascular | Shannon et al.39 | ||
| Leishmania major | Extravascular | Peters et al.30 | ||
| Staphylococcus aureus | Extravascular | Complement, IL-1 | Liese et al.43 | |
| Carotid Artery | Neointima | Intravascular | CRAMP, FPR2 | Soehnlein et al.44 |
| Brain | Sterile injury | Extravascular | Baik et al.41, Roth et al.42 | |
| Lymph Node | Toxoplasma gondii | Extravascular | Chtanova et al.3 | |
| Ovalbumin | Extravascular | Ng et al.28 | ||
| Staphylococcus aureus | Extravascular | Kamenyeva et al.45 | ||
| Pseudomonas aeruginosa | Extravascular | LTB4, GRK2 | Kienle et al.37 | |
| Salmonella typhimurium | Extravascular | LTB4, CXCL2, GRK2 | Kienle et al.37 | |
| Micropattern | Heat killed Staphylococcus aureus | Ex vivo | LTB4, CXCL2, GRK2 | Kienle et al.37 |
| Zymosan | Ex vivo | LTB4, Serum (unknown), CXCL7, CXCL8, LTB4, galectin-3, lipocalin-2, LXA2, pentraxin-3, PGD2, PGE2, NDP1/PD1, nidogen-1, TSP-1, BLT1/2 | Reátegui et al.46 | |
| Staphylococcus aureus bioparticles | Ex vivo | Walters et al.47 | ||
| Staphylococcus aureus, E. coli | Ex vivo | Galectin-3 | Walters et al.48 | |
| Imaging chamber | Caenorhabditis elegans | Ex vivo | GRK2 | Chtanova et al.3 |
| Trichomonas vaginalis | Ex vivo | Serine proteases | Mercer et al.49 | |
| Cryptococcus neoformans | Ex vivo | LTB4, C3, C5a, CD11b | Sun et al.50 | |
| Aspergillus fumigatus | Ex vivo | LTB4, fMLP, IL-8 | Jones et al.51 | |
| Zebrafish | Sterile injury | Extravascular | Calcium, ATP, Connexin 43, LTB4 | Poplimont et al.29 |
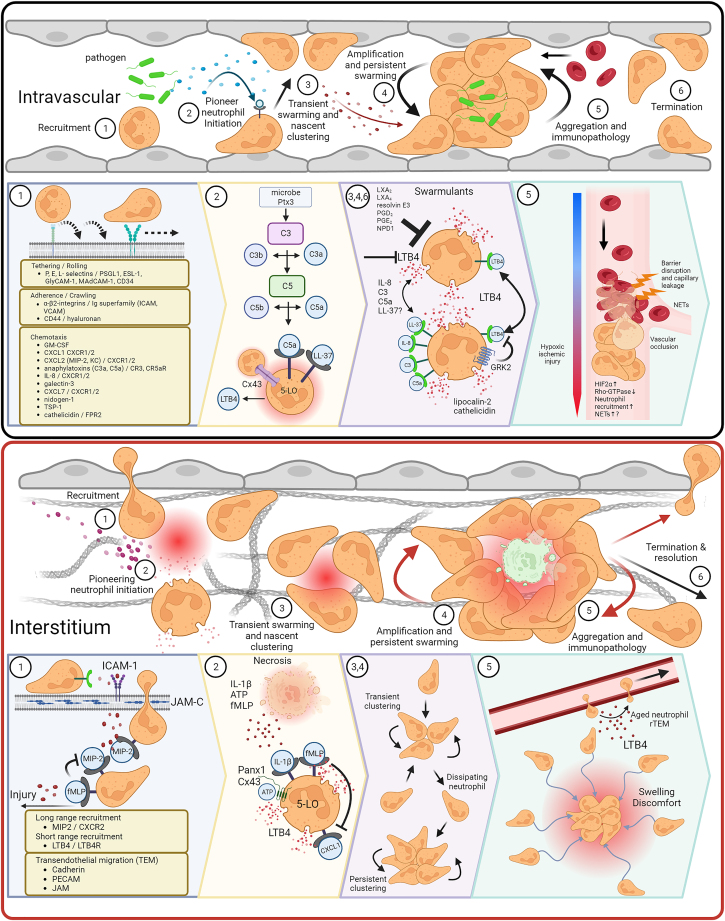
Intravascular and tissue neutrophil swarming cascades
Neutrophil swarming follows a stepwise cascade involving (1) recruitment, (2) initiation, (3) transient swarming and nascent clustering, (4) amplification and persistent swarming, (5) immunopathology, or (6) termination of the swarm. Neutrophil recruitment to the vasculature is facilitated by several chemoattractants. Here, neutrophils stay within the intravascular space and begin to swarm pathogens, a common cause of systemic intravascular swarming (See Table 1). Microbes and inflammatory Ptx3 trigger the complement cascade, resulting in C5a-induced 5-LO LTB4 production and release. In addition, Cx43 hemichannel-induced calcium fluxing and cathelicidin (LL-37) promote LTB4 production, leading to transient neutrophil swarms and nascent clustering. LTB4 feedforward loops sustain swarming and amplify LTB4 gradients, attracting more distal neutrophils to the inflamed site. In the absence of LTB4, other swarmulants including IL-8 (or MIP-2), complement C3 and C5a, and potentially cathelicidin, sustain neutrophil swarming. Swarming is eventually terminated via GRK2 self-limitation or various lipoxins, resolvins, and prostaglandins. This amplification leads to mass neutrophil accumulations and augments transient swarms to form persistent swarms. Typically, persistent swarming within the microvasculature results in lethal vascular occlusions that drive hypoxia and barrier dysfunction, which may result in cytoskeletal rearrangements leading to NET formation. Interstitial swarming follows many characteristics as intravascular swarming, including steps 2, 3, 4, and 6. However, the most common target of extravascular inflammatory insults is sterile injury. Therefore, inflammatory signatures differ significantly. Unlike intravascular migration, neutrophil recruitment to the extravascular space requires transendothelial migration through junctional proteins. Once in the interstitium, neutrophils follow local gradients of MIP-2 until sensing of necrotactic fMLP, which leads to desensitization of previous guidance cues leaving fMLP being the end-target cue. Furthermore, ATP release and contact with necrotic tissue induces calcium fluxing in pioneer and clustering neutrophils, which can then propagate the swarm by contacting newly arrived neutrophils. Transient swarms compete for incoming neutrophils and can dissipate to join larger, persistent clusters. Due to the expanse of extravascular tissue, such as the skin, large persistent neutrophil clusters can cause excessive inflammation. However, intact vasculature can continue to supply nutrients and oxygens to the tissue. Little data exists on the morbidity and mortality of interstitial infections compared to intravascular clustering. However, the effect of aged pioneer and clustered neutrophils undergoing reverse transendothelial migration may cause inflammation in distal organs. Created with BioRender.com.
In considering how to define swarming, it is worth discussing other complex neutrophil behaviors. In the vascular space, clusters of immune cells, including neutrophils have been described aggregated with platelets when forming immunothrombosis.52,53 These large intravascular clot-like cellular structures result from injury and bloodstream infections. Immunothrombosis has similarities and differences from swarming. For instance, immunothrombosis is an intravascular process involving aggregates of activated neutrophils and platelets, yet we found that intravascular neutrophil clustering which demonstrates swarming behaviors does not require platelet accumulation during candidemia.13 These anti-fungal intravascular swarms did require LTB4, an essential swarm initiator, but LTB4 is not known to drive immunothrombosis, a process requiring serine proteases.52,53 Thus, the relationship between swarming and immunothrombosis remains unclarified. Another complicating neutrophil behavior is the generation and release of neutrophil extracellular traps (NETs) which are pro-inflammatory mixtures of DNA and granular proteins.54,55 NETs are pro-inflammatory, but they also connect neutrophil host defense to coagulation via reciprocal activation of platelets leading to immunothrombosis which can ensnare vascular infections within pulmonary and hepatic circulations.56,57 Indeed, NETs directly initiate immunothrombosis.58,59,60,61 In the extravascular space, NET release by pioneer neutrophils initiates and coordinates swarming.10 Additionally, inhibition of NET components including gasdermin D, neutrophil elastase, and myeloperoxidase decreases swarm frequency.10 Therefore, several mechanisms link NETs, swarming, and immunothrombosis. However, these relationships are not straightforward and remain incompletely understood. The defining features distinguishing swarming, clustering, aggregations and immunothrombosis require further mechanistic investigations. Despite the complexities of untangling the nomenclature and biological processes, we aim to review the important discoveries made in the area of neutrophil swarming.
Swarming in anatomical context
The anatomical architecture of different microenvironments may play a major role in shaping when and how swarming occurs. It is known that neutrophils have the capacity to respond to different biophysical forces during inflammation.62 For instance, during hepatic sterile injury and infection, leading neutrophils enter vascular branches and alter hydraulic resistance and chemotactic gradients, causing trailing neutrophils to take alternate branches at capillary bifurcations. Furthermore, spatial contact of leading and trailing neutrophils influences trailing neutrophils to take alternative routes of high resistance to the inflamed site.62 As well, during sterile tissue damage of the liver and skin, a sizeable collagen-free zone with altered physical architecture is created that is disruptive to neutrophil migration.11,14,63
Contrasting extravascular tissue anatomy and physical forces is the blood vessel confined anatomical area where intravascular swarming occurs. Here neutrophils encounter oscillatory shear forces and continuous cell-cell interactions with red blood cells, platelets, and other circulating immune cells. Additionally, neutrophils are on average 40% larger than the pulmonary capillaries where swarming is observed, but during unstimulated conditions neutrophils can deform and flow through without being physically trapped or impeded. Activated neutrophils can crawl along the lung microvasculature in a Mac-1-dependent manner following either LPS or E. coli administration, without swarming and becoming physically trapped, or occluding blood vessels.25 However, during candidemia, zymosan (a structural component of fungi) stimulation, or Aspergillus pneumonia, neutrophils swarm within the capillaries and physically occlude the blood flow resulting in worsening lung function and hypoxemia.13,34 Interestingly, circulating B cells regulate neutrophil swarming and clustering via the production of pro-resolving lipoxins, such as LXA4, thus attenuating excessive neutrophilic pulmonary inflammation.34 Although swarming is a host resistance mechanism required for fungal containment it leads to tissue hypoxia resulting from vascular occlusion.13,64 The hypoxia response subsequently influences neutrophil motility in highly confined tissue microenvironments. During models of acute inflammation, hypoxia-inducible factor 2 (HIF2α) augments rapid neutrophil infiltration and results in excessive accumulation independent of chemotaxis signaling.65 Sustained HIF2α activation reduces Rho GTPase activity, mechanistically affecting neutrophil cytoskeleton dynamics. This leads to enhanced neutrophil nondirected migration in complex environments and aggravated damage in LPS-induced acute lung injury.65,66 Interestingly, HIF2α-Rho GTPase regulation of cytoskeletal F-actin rearrangements could result in excessive NET formations.67,68,69 Therefore, the confinement of large persistent swarms probably acts as a double-edged sword near fragile tissue environments and could be a gateway for NETosis, particularly within hypoxic organs carrying out vital functions. The additional features that contrast intravascular swarming from tissue swarming are the presence of the coagulation and complement cascades and the presence of platelets. How the microenvironmental differences between the intravascular and extravascular spaces alter swarming behavior continues to be an area of research interest.
Identifying the “swarmulants” involved
Several molecular molecules have been identified that drive swarming. During sterile tissue injury, adenosine triphosphate (ATP) released from necrotic cells initiates inflammasome formation with subsequent release of interleukin-1β (IL-1β) that upregulates ICAM-1 expression on endothelial cells within the vicinity of sterile injury, providing an anchor for neutrophil chemotaxis.14 Furthermore, inflammasome activation of endothelial cells enables vascular coating of MIP-2 chemokines, creating an intravascular gradient that guides neutrophils through the path of least resistance toward the foci of damage. Concerted waves of neutrophils then migrate at high speed (10–20 μm/min) from >300 μm away to the inflamed site. The neutrophils then swarm around the injured site to form a tight seal before infiltrating the final 150 μm collagen-free zone in a Talin-1 and β2-integrin-dependent manner.11 Inhibition of MIP2 prevents distal neutrophils from directionally migrating toward the foci of damage and leaves them in a non-directional “random walk”; yet proximal neutrophils continue to migrate toward the injured site, indicating that a compartmentalized hierarchy of chemoattractants exists within specific boundaries of tissue injury.14,70
LTB4 has been consistently reported as an essential swarm mediator. Pioneer neutrophils respond to zymosan and increase intracellular calcium levels that promote a positive-feedback loop of LTB4 production. In addition, complement activation and anaphylatoxin C5a release precipitates LTB4-mediated neutrophil intravascular swarming in both murine and human lung models of fungemia.13 Interestingly, LTB4-induced swarming is not limited to neutrophils and has been shown to amplify eosinophil and monocyte accumulation and swarming in response to parasitic infection and Staphylococcus aureus bioparticles, respectively.37,47,71 At the site of injury, pioneer neutrophils release LTB4, derived from the arachidonate 5-lipoxygenase (5-LO) pathway, which is essential for amplifying local death signals that augment the recruitment of distal neutrophils via BLT1.11 Indeed, transcellular LTB4 biosynthesis is a main determinant of neutrophil-neutrophil amplified swarming.72 During sterile injury in zebrafish, pioneer neutrophil release of LTB4 is sustained by calcium flux upon contact with necrotic tissue. ATP-gated calcium channel alarm signaling via contact-dependent connexin-43 (Cx43) hemichannels promotes 5-LO translocation into the nuclear envelope membrane and converts arachidonic acid into LTA4, which drives LTB4 biosynthesis. Upon contact with calcium fluxing pioneering and clustering neutrophils, newly recruited neutrophils begin calcium fluxing. Therefore, this feedforward loop initiates and propagates the swarm. Cx43 is critical for the initiation of the swarm and promotes wound defense from opportunistic bacterial invasion.29 In mice, inflammatory mediators upregulate Cx43, resulting in modulation of neutrophil recruitment and arrest within the lungs after LPS administration. Moreover, recent studies demonstrate that LPS-induced release of ATP through Cx43 activates P2X purinoreceptor 1 (P2X1), inducing calcium fluxing that activates myosin light chain kinase-dependent pathways which inhibit neutrophil chemotaxis at the site of infection.73 Although Cx43 has been considered a prominent cell surface protein that contributes to neutrophil migration, ATP is also exported via vesicular nucleotide transporter protein and ATP-gated pannexin hemichannels.74 Interestingly, ATP-release via Cx43 acts as a negative signal for neutrophil migration, whereas ATP-release via pannexin 1 (Panx1) has been described as a positive signal for neutrophil activation, migration, and effector functions during infection.73,75 Coincidentally, Panx1-dependent ATP release and P2X1 induce NET formation, an effector function closely associated with neutrophil swarming mentioned previously.76 Therefore, LPS-induced expression of Cx43 subsequent calcium fluxing may act as an arrest step or “stop” signal during neutrophil migration, leading to neutrophil accumulation. Here, increased intracellular calcium and mechanical stress of densely populated neutrophils environments activates Panx1, acting as a “go” signal.74 The balance of function and expression of Cx43/Panx1 could determine the positive and negative regulation of purinergic signaling during chemotaxis and swarming, which is consistent with recent evidence suggesting ATP is the main driver for neutrophil swarming.77,78,79 In concert, Cx43 and Panx1 may be responsible for transient clustering observed during neutrophil swarming. Furthermore, future investigations into other putative pathways of ATP release may reveal contributions to neutrophil migration during swarming.
In terms of regulation, neutrophils have adapted a self-limiting mechanism through G-protein-coupled receptor (GPCR) kinase 2 (GRK2) desensitization of LTB4 and CXCL2 that diminishes persistent neutrophil swarming.37 Aberration of GRK2 promotes excessive neutrophil and eosinophil migration without the formation of swarming aggregates. Instead, neutrophils and eosinophils continue to search for new pathogens rather than forming clusters around those that have previously been found. Self-limiting activity is essential for both neutrophils and eosinophils and helps them focus on preventing the escape of encircled bacteria and parasites within the skin.37
The study of human neutrophil swarming on microscale array of particle clusters has identified several other key molecules that are released during the early stages of neutrophil swarming. Here, IL-8, complement C3, and C5a can sustain neutrophil swarming, but only in the absence of LTB4. Pentraxin-3 (PTX3) is also released during initial swarming and is a potent activator of complement where downstream C3 and C5a release promotes LTB4-mediated neutrophil swarming in fungal infections.13,46,50 Interestingly, Ptx3-null mice have increased susceptibility to Aspergillus fumigatus lung infection, which is typically targeted by neutrophil swarms.80 In addition, neutrophil chemoattractants, galectin-3, CXCL7, nidogen-1 (or entactin), and TSP-1, are released during early swarming stages and are increased over time (See Figure 1).81,82,83,84 Furthermore, neutrophil swarms release exosomes and extracellular microvesicles containing galectin-3 that mediate neutrophil activation.48 Secondary granule antimicrobial peptides, lipocalin-2 and cathelicidin, are both released during initial swarming and serve to restrict bacterial growth by sequestering iron-loaded siderophores and by directly lysing open bacterial membranes, respectively.85,86
Interestingly, cathelicidin is increasingly becoming known for exhibiting both pro-inflammatory and anti-inflammatory immunomodulatory effects on neutrophils. However, cathelicidin is highly context-dependent and can have both beneficial and deleterious effects on disease and health.87 For example, cathelicidin promotes neutrophil recruitment and suppresses apoptotic signals, leading to enhanced accumulation at the site of inflammation.88,89,90 During anti-inflammatory wound healing, murine neutrophil-derived cathelin-related antimicrobial peptide (CRAMP) induces neutrophil swarming that protects from neointima-hyperplasia of the carotid artery.44 During pro-inflammatory insults, CRAMP-deficient mice have increased susceptibility to respiratory infections, displaying delayed neutrophil influx, impaired bacterial clearance and increased bacterial dissemination that reduces survival.91,92 Importantly, cathelicidin facilitates pro-inflammatory NET formation and prevents them from being proteolytically cleaved by bacterial nucleases.93,94 Conversely, both highly conserved human (LL-37) and murine (CRAMP) cathelicidin cause immunopathology by priming platelets, which mediate arterial neutrophil-platelet clustering.95,96,97 Although cathelicidin is a promiscuous peptide that can act through several receptors, it has been demonstrated to bind to formyl peptide receptor 2 (FPR2) and induce intracellular calcium mobilization in a dose-dependent manner. Activation of FPR2 leads to signal transduction via p38 MAP kinase and phosphorylation of phospholipase A2 (cPLA2), leading to LTB4 release. In turn, LTB4 binds to BLT1 which initiates neutrophil swarming. However, LTB4 release promotes the further release of neutrophil-derived cathelicidin in a pro-inflammatory feedforward loop and can be inhibited by lipoxin A4 and resolvin E1.97,98 Interestingly, microscale array techniques have revealed that pro-resolving LXA2, resolvin E3, prostaglandins D2 (PGD2) and E2 (PGE2), and neuroprotection D1/protectin D1 (NPD1/PD1) blunt neutrophil swarming. Therefore, initiating “swarmulants” that drive LTB4-induced swarming requires further examination. In addition, mechanisms other than GRK2 self-limiting have been found to bring neutrophil swarming to arrest, including tissue-resident macrophage cloaking of microlesions and their debris; thereby preventing further neutrophil activation and neutrophil-driven inflammation that causes excess tissue damage.99 Therefore, several molecules and mechanisms involved in swarming, beyond the classical LTB4 initiation and GRK2 self-limitation, must exist and warrant further investigation.
Swarming in human disease
Intravital microscopy has demonstrated neutrophil swarming in models of sterile inflammation and infectious diseases, but it remains challenging to observe neutrophil behavior in human disease states. Nonetheless, many important diseases have pathological evidence that is compatible with neutrophil swarming. Diseases that have intravascular pathology related to clotting and immunothrombosis warrant further consideration. Both cancer and autoimmune disorders can increase blood clotting risks and the involvement of neutrophils, NETs and immunothrombosis are predicted in lupus, vasculitis, and venous thromboembolic disorders.54,58,100,101 More recently, the roles of neutrophils, NETs, immunothrombosis and thus potentially swarming as an underlying phenomenon, has been highlighted in SARS-CoV-2 infection, and infection induced acute respiratory distress syndrome (ARDS) in general. Autopsy and lung pathological findings in patients that succumbed to COVID-19 demonstrated intravascular neutrophils and immunothrombosis in addition to NETs.102,103,104,105,106 Interestingly, recent studies have identified that diabetes activates the LTB4 pathway at baseline, with ALOX5 expression and plasma LTB4 being increased in diabetic patients with COVID-19 infection.107 A better mechanistic understanding of neutrophil swarming will allow us to define the role of this behavior more clearly during human disease states.
Investigating swarming: Challenges
To date, direct evidence investigating neutrophils swarming has required direct imaging via in vivo animals’ intravital microscopy or in vitro human microfluidic arrays.3,11,13,14,37 Currently, there are no specific serum surrogates or biomarkers of swarming. A significant challenge of imaging in vivo swarming is the requirement to perform a surgical procedure to exteriorize tissues and organs for visualization. Combined with this is the need for anesthetic drugs and fluorescent tags or reporters. Even methods which bypass invasive surgical procedures and image intact skin have still required isolating, processing and re-injecting neutrophils back into tissues, thus representing significant cellular manipulation.11,37 Importantly animal models, particularly mice, do not always accurately recapitulate human disease. For example, mice do not spontaneously develop asthma, a disease that can be related to leukotrienes such as LTB4 and neutrophilic inflammation, and no mouse model mimics the entire phenotype completely.108
Human neutrophils can be assessed for swarming mechanisms using bioengineered microfluidic imaging devices.64,72,109 Using sophisticated modeling systems, many important aspects of human neutrophil swarming can be studied under biophysical replicas of human tissues. The complicating features of these assays are the need to isolate neutrophils, human donor variability, loss of authentic and heterogeneous tissue parenchymal cells, and complex molecular milieu. Additionally, replicating disease states is challenging using in vitro models. Nonetheless, advances in both non-invasive animal imaging, improved in vivo modeling, advanced microfluidic chambers, and organoid technologies, will allow for further scientific discoveries.
Future directions
Male and female sex differences are being recognized as important determinants of immune responses. Yet the effect of biological sex in driving complex neutrophil behaviors such as swarming remains unknown. Alterations in neutrophil effector functions have been found to be higher in females during infection, despite higher levels of inflammatory mediators along with increased TLR4 receptor expression on neutrophils in males.110 As an example, increased androgens suppress gender-specific extracellular signal-related kinase activity and LTB4 formation, the key driver of swarming, in males which blunts pro-inflammatory responses in asthma.111 LTB4 is found in abundance within the sputum of asthmatic patients and is persistently increased in the plasma of children after acute episodes.112,113,114,115,116 This may explain the disparity between the increased prevalence of asthma in young males versus females. Coincidentally, as young males age, the severity of asthma is disproportionately decreased when compared to adult females.117 Potentially, inappropriate release of LTB4, within the lungs of young males and adult females may contribute toward type 2 and non-type 2 asthma severity due to increased eosinophil and neutrophil swarming, respectively. Furthermore, LTB4-mediated reverse transendothelial cell migration of neutrophils may drive secondary inflammation at distal sites within the lungs during asthma.37,118,119 Therefore, sex can determine both disease severity and levels of LTB4, thus implicating differences in neutrophil swarming, Evaluation of sex-based neutrophil migration and swarming behavior may significantly improve our understanding of common diseases and provide sex-based differential therapeutics.
Neutrophil swarming has been well demonstrated as a response to pathogens. Yet, neutrophil mediated host resistance and neutrophil mediated tissue immunopathology can occur simultaneously. Indeed, for syndromes such as sepsis, or ARDS, the major issue causing death is severe organ injury and failure due to an overwhelming or dysregulated immune response. A better understanding of how to manipulate complex behaviors such as neutrophil swarming will allow for targeted therapeutic approaches. For instance, we discovered that during candidemia, neutrophils demonstrated intravascular swarming as they captured and contained fungi. However, the intravascular swarms resulted in vascular occlusion, hypoxia, and death. By allowing the neutrophils to target the fungi via complement (C5a), but blocking the amplified LTB4 swarming response, mice were able to efficiently clear the pathogen without subsequent organ immunopathology. It remains to be tested if dissociating host resistance from immunopathology can be employed in humans, and in other disease contexts.
Investigations into complex cellular behaviors, such as swarming, will be benefited by improving our molecular definitions of the process. Currently, molecularly defining various aspects of the dynamic process remain somewhat subjective, including the concepts of pioneering neutrophils, swarming, clustering, aggregating, and immunothrombosis. Complicating this is the significant differences in anatomical locations where swarming has been reported. As well, integrating how other complex neutrophil processes, such as NETosis, impacts and integrates with swarming remains a fascinating, but challenging area of investigation. Further complicating the study of neutrophils is the appreciation of the heterogeneity of neutrophils themselves, with more evidence suggesting that these cells exist as functional subsets. Indeed, recent discoveries have uncovered neutrophil plasticity in infection, where virus and bacterial infections alter interferon and prostaglandin-programmed neutrophils.120 In addition, large populations of resting neutrophils have been observed not to partake in swarming during sterile skin injury, but what makes them different remains unknown, but they could represent different subsets of neutrophils.28 Whether neutrophil subsets have unique contributions to the swarming process remains unknown but does represent a fascinating area of study.
In sum, neutrophil swarming is a complex but essential immune process responsible for providing protection from pathogens, but it also for injuring the host via immunopathology. Despite significant advancements in understanding neutrophils, we still have a long journey ahead to better define and understand of their complexities and contributions to health and disease.
Acknowledgments
B.G.Y. is a Tier II Canada Research Chair in Pulmonary, Immunology, Inflammation and Host Defense. L.B. is an Eyes High Scholarship recipient.
Author contributions
Conceptualization, L.B. and B.G.Y.; Writing – Original Draft, L.B. and B.G.Y.; Writing – Review & Editing, L.B. and B.G.Y.; Supervision, B.G.Y.
Declaration of interests
The authors declare no conflicts of interests.
References
Articles from iScience are provided here courtesy of Elsevier
Citations & impact
Impact metrics
Article citations
Evidence of Neutrophils and Neutrophil Extracellular Traps in Human NMSC with Regard to Clinical Risk Factors, Ulceration and CD8<sup>+</sup> T Cell Infiltrate.
Int J Mol Sci, 25(19):10620, 02 Oct 2024
Cited by: 0 articles | PMID: 39408949 | PMCID: PMC11476888
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Neutrophil swarming: an essential process of the neutrophil tissue response.
Immunol Rev, 273(1):76-93, 01 Sep 2016
Cited by: 107 articles | PMID: 27558329
Review
In the eye of the neutrophil swarm-navigation signals that bring neutrophils together in inflamed and infected tissues.
J Leukoc Biol, 100(1):55-63, 28 Sep 2015
Cited by: 60 articles | PMID: 26416718
Review
Pioneer neutrophils release chromatin within in vivo swarms.
Elife, 10:e68755, 21 Jul 2021
Cited by: 29 articles | PMID: 34292151 | PMCID: PMC8298094
Neutrophil swarming toward Cryptococcus neoformans is mediated by complement and leukotriene B4.
Biochem Biophys Res Commun, 477(4):945-951, 09 Jul 2016
Cited by: 25 articles | PMID: 27402276 | PMCID: PMC4967020




