Abstract
Free full text

SARS‐CoV‐2 antibody responses in solid organ transplant recipients
Abstract
Antibody responses among immunocompromised solid organ transplant recipients (SOT) infected with Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) may be diminished compared to the general population and have not been fully characterized. We conducted a cohort study at our transplant center to investigate the rate of seroconversion for SARS‐CoV‐2 IgG antibodies among SOT recipients who were diagnosed with Coronavirus disease 2019 (COVID‐19) and underwent serum SARS‐CoV‐2 IgG enzyme‐linked immunosorbent assay (ELISA) testing. The 61 patients who were included in the final analysis underwent initial SARS‐CoV‐2 IgG testing at a median of 62 days (Interquartile range 55.0–75.0) from symptom onset. Note that, 51 of 61 patients (83.6%) had positive SARS‐CoV‐2 IgG results, whereas 10 (16.4%) had negative IgG results. Six (60%) out of 10 seronegative patients underwent serial IgG testing and remained seronegative up to 17 weeks post‐diagnosis. Use of belatacept in maintenance immunosuppression was significantly associated with negative IgG antibodies to SARS‐CoV‐2 both in univariate and multivariate analyses (Odds ratio 0.04, p = .01). In conclusion, the majority of organ transplant recipients with COVID‐19 in our study developed SARS‐CoV‐2 antibodies. Further longitudinal studies of the durability and immunologic role of these IgG responses and the factors associated with lack of seroconversion are needed.
Abbreviations
- COVID‐19
- Coronavirus disease 2019
- SARS‐CoV‐2
- Severe Acute Respiratory Syndrome Coronavirus‐2
- SOT
- solid organ transplant
1. INTRODUCTION
Early reports of Coronavirus disease 2019 (COVID‐19) among adult solid organ transplant (SOT) recipients suggest that the risk of mortality in transplanted adults with confirmed infection may exceed that reported for elderly but presumably immunocompetent individuals. 1 , 2 Mortality rates of 13% to over 30% have been reported among SOT recipients with COVID‐19 infection. 1 , 3 , 4 , 5 , 6
Recent studies from China indicate that the majority of patients who recover from COVID‐19 develop IgG and Immunoglobulin M antibodies to Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) within 6 weeks of the onset of illness. 7 , 8 , 9 Although there is significant ambiguity surrounding the role of antibody testing in transplanted and non‐transplanted individuals, understanding humoral and cell‐mediated immune responses following SARS‐CoV‐2 infection may inform risk of reinfection and the effective use of COVID‐19 vaccines. Fung et al. described seroconversion for SARS‐CoV‐2 IgG among seven hospitalized organ transplant recipients with confirmed COVID‐19. All patients in this group were seroconverted within 27 days of symptom onset. 10 A report from France followed 40 kidney transplant recipients hospitalized with COVID‐19 and among 35 survivors, all developed positive SARS‐CoV‐2 IgG and Immunoglobulin M responses. 11 Larger cohort studies are needed to further understand antibody responses in immunocompromised transplant recipients.
Several factors suggest the possibility of diminished immune responses in SOT recipients and the need for transplant‐specific data. SOT recipients may have baseline lymphopenia secondary to both induction and maintenance immunosuppression, and at least one study in non‐transplanted adults found that peripheral lymphocyte count was inversely correlated to SARS‐CoV‐2 neutralizing antibody titer. 12 SOT recipients are also at risk for hypogammaglobulinemia, presumably secondary to immunosuppressive agents, 13 , 14 , 15 and have exhibited markedly diminished humoral immune responses following natural infection with influenza 16 and cytomegalovirus. 17 Thus, several potential factors suggest that solid organ transplant recipients may manifest diminished antibody responses to SARS‐CoV‐2 infection compared to the general population. The objectives of this study were therefore to investigate the rate of seroconversion for SARS‐CoV‐2 IgG at a minimum of 2 weeks post‐diagnosis and to identify potential correlates of seroconversion.
2. METHODS
2.1. Study participants
We conducted a retrospective cohort study at the NYU Langone Transplant Institute in New York City to investigate the rate of seroconversion for SARS‐CoV‐2 IgG among adult solid organ transplant recipients (>18 years old) who were diagnosed with SARS‐CoV‐2 infection between March 1, 2020 and June 5, 2020, and who underwent serum SARS‐CoV‐2 IgG ELISA testing as per routine clinical care at our transplant center. COVID‐19 was confirmed in all patients by SARS‐CoV‐2 reverse transcriptase‐polymerase chain reaction (RT‐PCR) from nasopharyngeal swab when they had symptoms suggestive of COVID‐19, known contact with a person with COVID‐19 infection, or prior to ambulatory or inpatient elective procedures as per standard of care. After SARS‐CoV‐2 serological testing became available in our institution on May 15, 2020, our institutional practice guidelines recommended testing at least once for serum SARS‐CoV‐2 IgG at a minimum of 2 weeks after onset of COVID‐19 symptoms. For patients with initially negative antibody testing, our practice guidelines recommended repeat antibody testing at 2‐week intervals to assess for delayed seroconversion. Ambulatory and hospitalized patients who had SARS‐CoV‐2 Abbott IgG testing performed at NYU Langone Health at least once after COVID‐19 diagnosis were included in the final analysis. Patients who had received convalescent plasma prior to the SARS‐CoV‐2 IgG testing were excluded from the final analysis due to the possibility of passive immunity. The follow‐up period for this study ended July 17, 2020. This study was approved with a waiver of informed consent by the New York University Grossman School of Medicine Institutional Review Board.
In addition to serological testing, we reviewed electronic medical records of all cohort patients for the following information: age; gender; race; ethnicity; transplanted organ; date of transplantation; body mass index (defined as the patient's weight in kilograms divided by the square of height in meters); co‐morbidities including diabetes mellitus, hypertension, and hyperlipidemia; chronic kidney disease of the native or transplanted kidney; chronic obstructive pulmonary disease; asthma; cirrhosis of the native or transplanted liver; coronary artery disease; active or history of malignancy; smoking history; maintenance immunosuppression; a dose of prednisone if used for maintenance immunosuppression; and date of symptom onset. Laboratory data were reviewed, including absolute lymphocyte count and gamma globulin levels during symptomatic illness (defined as the first 2 weeks of symptoms). Gamma globulin levels were defined as low if <768 mg/dl which is the lower limit of normal as per our microbiology lab's reference. Charts were also reviewed to evaluate the need for mechanical ventilation, extracorporeal membrane oxygenation, and death.
2.2. Severe acute respiratory syndrome coronavirus‐2 IgG ELISA
SARS‐CoV‐2 IgG ELISA (Abbott Laboratories, Abbott Park, IL) was approved by the US Food and Drug Administration under an emergency use authorization and became available at our institution on May 15, 2020. The assay was performed in accordance with the manufacturer's instructions. This qualitative, chemiluminescent assay detects IgG antibodies against the SARS‐CoV‐2 nucleocapsid protein in human serum and plasma. The response is measured in relative light units and compared to a calibrator to determine the calculated index (specimen/calibrator), and a positive or negative result is reported. An index of 1.4 or greater correlates with a positive result as per manufacturer specification.
2.3. Statistical methods
The primary study outcome of interest was the percentage of SOT recipients that developed detectable IgG antibodies to SARS‐CoV‐2 after a positive SARS‐CoV‐2 RT‐PCR result from a nasopharyngeal swab. The secondary objective was to investigate potential associations between seroconversion and clinical variables including age ≥ 65, gender, presence of symptoms, kidney SOT, infection within a year from transplantation, nadir absolute lymphocyte count during symptomatic illness < 1,000cells/μl (defined as first 2 weeks of symptoms), use of antimetabolite, mammalian target of rapamycin inhibitor or belatacept as maintenance immunosuppression, and use of high dose steroids, defined as the equivalent or greater than prednisone 5 mg per day, either as part of the maintenance immunosuppression or during COVID‐19 illness.
All calculations were performed using the Stata v15.0 software package (Stata Corporation, College Station, TX). Categorical variables were compared by chi‐square or Fisher's exact test. Continuous variables were evaluated as medians with interquartile range (IQR) and were compared by student's t‐test. Covariates with p < .5 in univariate analyses were entered in the multivariate model. A p‐value of < .05 was considered statistically significant.
3. RESULTS
Eighty‐nine SOT recipients were diagnosed with SARS‐CoV‐2 infection between March 1 and June 5, 2020. Sixteen of 89 patients (18.0%) died during their COVID‐19 illness prior to antibody testing becoming available at our institution, 11 (12.4%) did not have antibody testing completed during the study interval and one (1.1%) had an infusion of convalescent plasma versus placebo prior to SARS‐CoV‐2 IgG antibody testing, leaving 61 patients who were included in the final analysis (Figure 1).
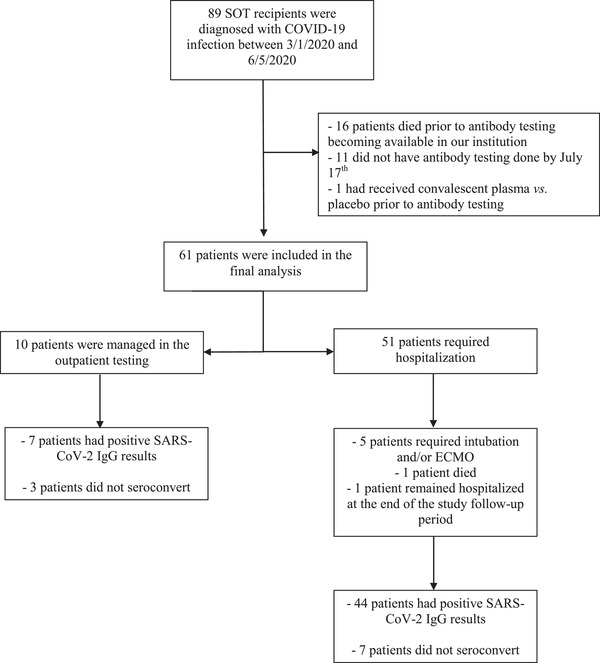
Patient sample and study inclusion
ECMO, extracorporeal membrane oxygenation; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus‐2; SOT, solid organ transplant
The median age of patients was 58 years (IQR 51–67) (Table 1). The majority of patients were kidney transplant recipients (43; 70.5%), followed by 7 heart (11.5%), 6 liver (9.8%), 3 lung (4.9%), and 2 combined kidney‐heart transplant recipients (3.3%). The median time from transplantation to COVID‐19 diagnosis was 493 days (IQR 295–756) with 21 of the 62 patients (34.4%) diagnosed with COVID‐19 within the first year after transplantation. All patients were receiving maintenance immunosuppression at the time of COVID‐19 diagnosis. The induction immunosuppressive regimen included basiliximab plus methylprednisolone in 27 patients (44.3%), antithymocyte globulin plus methylprednisolone in 20 patients (32.8%), mycophenolate and methylprednisolone in five patients (8.2%), and methylprednisolone alone in three patients (4.9%). One patient received one dose of antithymocyte globulin plus methylprednisolone and was then switched to basiliximab plus methylprednisolone due to concern for reaction to the globulin (1.6%) and information on induction immunosuppression was not available for five patients (8.2%). One patient received rituximab 7 days prior to COVID‐19 symptom onset for antibody‐mediated rejection. The maintenance immunosuppressive regimens included tacrolimus alone in two patients (3.3%), tacrolimus plus steroids in three patients (4.9%), tacrolimus and mycophenolate mofetil in six patients (9.8%), tacrolimus, mycophenolate mofetil, and prednisone in 39 patients (63.9%), and other in 10 patients (16.4%) including five on belatacept‐based regimens (Table 1). The maintenance immunosuppressive regimen was changed in 52 out of 61 patients (85.2%). In the majority of cases, 46 out of 52 patients, the antimetabolite was held or was administered in a reduced dose.
TABLE 1
Patient characteristics of the study population
| Demographics/Patient characteristics | No. of patients (%) or median (IQR) |
|---|---|
| Age, years | 58 (51–67) |
| Gender | |
| Female | 25 (41.0%) |
| Male | 36 (59.0%) |
| Race | |
| White | 11 (18.0%) |
| African American/Black | 22 (36.1%) |
| Asian | 3 (4.9%) |
| Other/ Unknown | 25 (41.0%) |
| Ethnicity | |
| Hispanic | 17 (27.9%) |
| Non‐Hispanic | 24 (39.3%) |
| Other/Unknown | 20 (32.8%) |
| Transplanted Organ | |
| Kidney | 43 (70.5%) |
| Heart | 7 (11.5%) |
| Liver | 6 (9.8%) |
| Lung | 3 (4.9%) |
| Combined heart and kidney | 2 (3.3%) |
| Days from transplantation (Median, IQR) | 493(295–756) |
| Comorbidities (No., %) | |
| Obesity | 20 (34.5%) |
| Diabetes Mellitus | 27 (44.3%) |
| Hypertension | 51 (83.6%) |
| Hyperlipidemia | 34 (55.7%) |
| CKD (of the native or transplanted kidney) | 18 (29.5%) |
| COPD | 4 (6.6%) |
| Asthma | 8 (13.1%) |
| Cirrhosis (of the native or transplanted liver) | 0 (0.0%) |
| CAD | 16 (26.2%) |
| Active or past malignancy | 12 (19.7%) |
| Tobacco use (current or former) | 21 (35.0%) |
| Immunosuppressive regimen (No., %) | |
| Calcineurin Inhibitor | 59 (96.7%) |
| Prednisone | 51 (83.6%) |
| Antimetabolite | 50 (82.0%) |
| Belatacept | 5 (8.2%) |
| mTOR inhibitor | 5 (8.2%) |
Abbreviations: COPD, Chronic Obstructive Pulmonary Disease; IQR, Interquartile Range; mTOR, mammalian target of rapamycin.
Fifty‐seven (93.4%) SOT recipients were symptomatic at the time of positive SARS‐CoV‐2 RT‐PCR testing. Four patients (6.6%) were asymptomatic at the time of diagnosis, including one patient with known SARS‐CoV‐2 exposure and three who were found to have positive SARS‐CoV‐2 RT‐PCR testing on routine pre‐procedure or pre‐admission screening. Duration of symptoms at the time of testing was available for 52 SOT recipients and was a median of 4 days (IQR 2–7).
Fifty‐one of the 61 patients (83.6%) required hospitalization for COVID‐19, and 10 were managed in the outpatient setting. Five patients (8.2%) required intubation and/or extracorporeal membrane oxygenation during their hospitalization. Maintenance immunosuppression was modulated in 52 of 61 (85.2%) patients in this study and in 90.2% of those who required hospitalization. One patient received convalescent plasma but had positive SARS‐CoV‐2 IgG from blood obtained immediately prior to plasma administration. The median nadir lymphocyte count among hospitalized patients was 400 10 × 3/ul(IQR 200–800 10 × 3/ul). Six patients (9.8%) had total serum IgG levels assessed during the first 2 weeks of symptomatic illness, including two with hypogammaglobulinemia (273 and 754 mg/dl). The remaining four patients had normal IgG levels (median 1037 mg/dl, range 922–1327 mg/dl). One patient who underwent SARS‐CoV‐2 antibody testing died (1.6%), while one patient remained hospitalized at the end of the study follow‐up period.
The patients included in the final analysis underwent initial SARS‐CoV‐2 IgG testing at a median of 62 days (IQR 55.0–75.0) from symptom onset. Among the four asymptomatic patients and the six for whom the day of symptom onset was not available the initial SARS‐CoV‐2 IgG testing was performed at a median of 42.5 days (IQR 41.0‐62.0). Fifty‐one of 61 patients (83.6%) had positive SARS‐CoV‐2 IgG results, whereas 10 (16.4%) did not seroconvert. The median number of days between SARS‐CoV‐2 RT‐PCR and IgG testing for those with positive IgG results was 55.0 days (IQR 45.0–66.0). Among the three patients who were tested within the first 4 weeks following COVID‐19 diagnosis, all (100%) were seropositive. Among the 13 patients who were tested within 6 weeks of COVID‐19 diagnosis, 11 (84.6%) were seropositive.
Three of 52 patients who were seropositive on initial testing underwent repeat SARS‐CoV‐2 IgG testing. Two continued to be seropositive at 15 and 28 days after their initial IgG testing, whereas one had negative antibodies on repeat testing at 12, 26, and 35 days following the initial IgG testing.
Among the 10 seronegative patients, the median time between positive SARS‐CoV‐2 RT‐PCR and SARS‐CoV‐2 IgG testing was 56.5 days (range 40–81 days). The characteristics of the patients who did not seroconvert are presented in Table 2. Six (60%) of 10 seronegative patients underwent serial antibody testing and remained seronegative up to 17 weeks post‐diagnosis. Of note, all of the patients who had negative SARS‐CoV‐2 IgG results had been symptomatic at the time of positive SARS‐CoV‐2 RT‐PCR, with a median duration of symptoms of 7.5 days (range 1–31 days) prior to SARS‐CoV‐2 RT‐PCR testing. Five of the 6 persistently seronegative patients (83.3%) required hospitalization, whereas one was managed in the outpatient setting. Among those who were hospitalized, two (33.3%) required ICU level of care and one (16.7%) was intubated. None had received induction immunosuppression within the prior 90 days but one had received methylprednisolone, thymoglobulin, and plasmapheresis within the prior 90 days for acute cellular rejection, and one was on eculizumab for chronic rejection. Among the 10 patients who did not seroconvert five had repeat nasopharyngeal swabs performed, out of whom three (60.0%) had positive SARS‐CoV‐2 PCR at a median of 32, 49 and 75 days from initial testing and two (40.0%) had negative results at 29 and 42 days.
TABLE 2
Characteristics of patients who did not develop Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) antibodies
| Demographics/Patient characteristics | No. of patients (%) or median (IQR) |
|---|---|
| Age, years | 56 (53–67) |
| Gender | |
| Female | 3 (30.0%) |
| Male | 7 (70.0%) |
| Race | |
| White | 1 (10.0%) |
| African American/Black | 4 (40.0%) |
| Asian | 3 (30.0%) |
| Other/ Unknown | 2 (20.0%) |
| Ethnicity | |
| Hispanic | 2 (20.0%) |
| Non‐Hispanic | 5 (50.0%) |
| Other/Unknown | 3 (30.0%) |
| Transplanted Organ | |
| Kidney | 6 (60.0%) |
| Heart | 1 (10.0%) |
| Liver | 2 (20.0%) |
| Lung | 1 (10.0%) |
| Days from transplantation (Median, IQR) | 468 (254–557) |
| Immunosuppressive regimen (No., %) | |
| Calcineurin inhibitor | 9 (90.0%) |
| Prednisone | 10 (100.0%) |
| Antimetabolite | 10 (100.0%) |
| Belatacept | 3 (30.0%) |
| mTOR inhibitor | 0 (0.0%) |
Abbreviations: IQR, Interquartile Range; mTOR, mammalian target of rapamycin.
Age >65 years, gender, presence of symptoms, kidney SOT, nadir absolute lymphocyte count <1000 cells/μl during first 2 weeks of symptoms, use of antimetabolite, mammalian target of rapamycin inhibitors, and high‐dose steroids as part of maintenance immunosuppression or for COVID‐19 treatment were not found to be significantly associated with the development of SARS‐CoV‐2 antibodies (Table 3). However, the use of belatacept as maintenance immunosuppression was significantly associated with a lack of seroconversion (p = .01). Three out of 10 patients (30%) with negative IgG testing were on belatacept compared to three of 52 (5.8%) with positive SARS‐CoV‐2 antibody results. In multivariate analysis, belatacept use remained significantly associated with negative SARS‐CoV‐2 antibody result (OR 0.04; p = .01).
TABLE 3
Univariate risk factors for Severe Acute Respiratory Syndrome Coronavirus‐2 (SARS‐CoV‐2) IgG seroconversion
| Patient characteristic | Percentage of patients who seroconverted (%) | p‐value |
|---|---|---|
| Age | ||
| <65 | 82.9 | ref |
| ≥65 | 85.0 | 0.84 |
| Gender | ||
| Female | 88.0 | ref |
| Male | 80.6 | 0.44 |
| Symptomatic | ||
| No | 75.0 | ref |
| Yes | 84.2 | 0.63 |
| Kidney SOT | ||
| No | 77.8 | ref |
| Yes | 86.0 | 0.43 |
| Within a Year from Transplant | ||
| No | 82.5 | ref |
| Yes | 85.7 | 0.75 |
| Nadir ALC (cells/μl) | ||
| <1000 | 84.2 | ref |
| ≥1000 | 83.3 | 0.93 |
| High‐dose steroids | ||
| No | 84.9 | ref |
| Yes | 75.0 | 0.48 |
| Antimetabolite | ||
| No | 100 | ref |
| Yes | 80.4 | 0.13 |
| mTOR Inhibitor | ||
| No | 82.1 | ref |
| Yes | 100 | 0.30 |
| Belatacept Use | ||
| No | 87.5 | ref |
| Yes | 40 | 0.01 |
Abbreviations: ALC, absolute lymphocyte count; mTOR, mammalian target of rapamycin; OR, odds ratio; ref, reference; SOT, solid organ transplant.
4. DISCUSSION
The majority of patients with confirmed SARS‐CoV‐2 infection in our cohort of immunosuppressed solid organ transplant recipients developed a detectable SARS‐CoV‐2 IgG antibody response. Seroconversion response rates in large general adult patient cohorts have varied between 80%–100%. 7 , 8 There are emerging data on SARS‐CoV‐2 antibody responses in immunocompromised patient populations. One small study from France 18 from a cohort of adults with confirmed COVID‐19 found that oncology patients were less likely to seroconvert relative to healthcare workers (31% vs. 70%) at 15 days post‐diagnosis. Nearly all seronegative patients in this study had received cytotoxic chemotherapy in the preceding 4 weeks. Similarly, Roeker et al. reported only 67% of patients with chronic lymphocytic leukemia at a large New York City cancer center seroconverted 19 ; importantly, nearly half of patients had hypogammaglobulinemia. In a cohort of multiple myeloma patients diagnosed with COVID‐19, more than 80% of the patients who were on active immunosuppressive treatment, 96% of survivors had positive SARS‐CoV‐2 antibody titers when tested at a median of 32 days after COVID‐19 diagnosis. 20 These limited data suggest that our transplant cohort may have exhibited a more robust antibody response compared to patients receiving myeloablative chemotherapy.
Data regarding antibody responses after COVID‐19 in organ transplant recipients are limited. A case series of organ transplant recipients with COVID‐19 found that all hospitalized patients had positive SARS‐CoV‐2 IgG response when tested within 4 weeks of symptom onset, however, the findings are limited by small sample size. 6 , 7 Benotmane et al. also found 100% seropositivity among 35 kidney transplant recipients with COVID‐19 who were tested after infection. By contrast, Burack et al. found that only 51% of SOT recipients tested at a median of >6 weeks following diagnosis had detectable antibodies. 21 Standardized quantitative IgG assays are lacking and as a result rates of antibody response observed in our cohort cannot be reliably compared in a quantitative manner to data from published studies to date. Nonetheless, the larger number of patients in our study cohort may have allowed detection of patients who may not seroconvert after COVID‐19 in the context of underlying immunosuppression. Importantly, our study includes a larger number of patients who were relatively early post‐transplant (within the first year of transplant) at the time of COVID‐19 diagnosis and potentially less likely to seroconvert due to induction immunosuppression therapy.
The timing of serologic testing after infection is also an important consideration. Prior studies have shown SARS‐CoV‐2 IgG responses as early as 10–14 days following COVID‐19 illness. The majority of patients included in our study were tested for antibody response more than 6 weeks after COVID‐19 illness due to the timing of availability of serologic testing at our institution in the early months of the pandemic. However, all of the patients tested within the first 4 weeks from COVID‐19 illness, and more than 80% of those tested within the first 6 weeks were seropositive. The long‐term durability of antibody response remains unclear, though Fung et al. reported persistently positive IgG testing up to 2 months after symptom onset. 10 Only three patients in our cohort who were seropositive had undergone repeat IgG testing, with two remaining seropositive 28 days later and one becoming seronegative on repeat testing at an interval of 43 days from diagnosis and 12 days from first positive IgG testing. We are therefore are unable to make meaningful conclusions regarding the durability of IgG from our study. Among 10 patients who had a negative IgG test at a median of 56.5 days after RT‐PCR diagnosis, six had serial repeat testing and remained seronegative up to 17 weeks after diagnosis.
In our study, belatacept use was the only risk factor significantly associated with the lack of IgG antibodies to SARS‐CoV‐2. Although there is ambiguity surrounding the role of antibodies to SARS‐CoV‐2 in COVID‐19, our study is the first to find a potential association between the use of belatacept and lack of seroconversion. Prior literature has described the impact of abatacept, a predecessor of belatacept, on post‐vaccination serologic responses and also the potential for belatacept to prevent antibody formation. 22 , 23 However, the low sample size of patients receiving belatacept limits the conclusions to be drawn from the association we found. These findings warrant further study in a larger cohort of transplanted patients and with serial testing beginning closer to the time of diagnosis of infection which again was limited in our study due to lack of availability of IgG assays during the first phase of the pandemic. Due to a small number of seronegative patients, our study may have been underpowered to reveal other differences in clinical and immunologic parameters between patients who did not develop IgG response and those who did. For example, lymphopenia has been described to correlate with more severe COVID‐19 illness in both immunocompetent and immunocompromised patient populations. 1 , 2 , 24 Lee et al. described lymphopenia to be predictive of IgG response to SARS‐CoV among hospitalized patients in Hong Kong. 25
Limitations of our relatively small study include that the fact most patients underwent IgG testing >4 weeks after symptom onset due to the lack of available COVID‐19 serologic assays earlier during the study period. Therefore we were unable to study the overall kinetics of serological conversion. It is possible, for example, that some seronegative patients may have had early but nondurable SARS‐CoV‐2 IgG responses. Studies of antibody responses after COVID‐19 in regions more recently affected by the pandemic, after widespread antibody testing became available, may be able to further evaluate this possibility. The majority of patients were on stable immunosuppression and few had received induction immunosuppression with in the prior year. Seroconversion rates may be lower in patients who are early post‐transplant. Also, patients who received intravenous immunoglobulin or blood products were not excluded for the purposes of this study, and the possibility of passive immunity in at least some patients cannot be excluded. In addition, we were unable to assess IgG responses in patients with severe COVID‐19 at our center who died prior to the availability of SARS‐CoV‐2 serology testing. Finally, there are no published data on the sensitivity and specificity of the SARS‐CoV‐2 IgG ELISA (Abbott Laboratories, Abbott Park, IL) test.
The majority of organ transplant recipients with COVID‐19 in our study developed SARS‐CoV‐2 antibody responses, albeit at a lower rate than what has been reported in immunocompetent adult patients. Further longitudinal studies with earlier and serial testing of antibody responses and whether IgG seropositivity confers immunity to SARS‐CoV‐2 reinfection are needed, particularly in immunocompromised patients.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
FUNDING INFORMATION
None.
AUTHOR CONTRIBUTIONS
Fainareti N. Zervou, Sapna A. Mehta, Rebecca Pellett Madan, and Henry J. Neumann contributed to the study design.
Fainareti N. Zervou, Nicole M. Ali, and Sapna A. Mehta contributed to data collection and interpretation of results.
Fainareti N. Zervou and Sapna A. Mehta drafted the manuscript.
Rebecca Pellett Madan, Nicole M. Ali, and Henry J. Neumann contributed to manuscript revision.
ACKNOWLEDGMENTS
We thank all NYU Langone Transplant Institute staff and NYU Langone Health laboratory technologists for their selfless devotion to patient care during the COVID‐19 pandemic.
Notes
Zervou FN, Ali NM, Neumann HJ, Madan RP, Mehta SA. SARS‐CoV‐2 antibody responses in solid organ transplant recipients. Transpl Infect Dis. 2021;23:e13728. 10.1111/tid.13728 [Europe PMC free article] [Abstract] [CrossRef] [Google Scholar]
Contributor Information
Fainareti N. Zervou, Email: [email protected].
Sapna A. Mehta, Email: [email protected].
REFERENCES
Full text links
Read article at publisher's site: https://doi.org/10.1111/tid.13728
Read article for free, from open access legal sources, via Unpaywall:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8646321
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/113269313
Smart citations by scite.ai
Explore citation contexts and check if this article has been
supported or disputed.
https://scite.ai/reports/10.1111/tid.13728
Article citations
Longitudinal evaluation of the impact of immunosuppressive regimen on immune responses to COVID-19 vaccination in kidney transplant recipients.
Front Med (Lausanne), 9:978764, 22 Aug 2022
Cited by: 1 article | PMID: 36072955 | PMCID: PMC9441691
COVID-19 and solid organ transplantation: Finding the right balance.
Transplant Rev (Orlando), 36(3):100710, 04 Jul 2022
Cited by: 9 articles | PMID: 35809422 | PMCID: PMC9251959
Review Free full text in Europe PMC
Seroconversion Rate After SARS-CoV-2 Infection and Two Doses of Either ChAdOx1-nCOV COVISHIELD™ or BBV-152 COVAXIN™ Vaccination in Renal Allograft Recipients: An Experience of Two Public and Private Tertiary Care Center.
Front Immunol, 13:911738, 30 Jun 2022
Cited by: 4 articles | PMID: 35844596 | PMCID: PMC9280041
COVID-19 and kidney disease: insights from epidemiology to inform clinical practice.
Nat Rev Nephrol, 18(8):485-498, 13 Apr 2022
Cited by: 30 articles | PMID: 35418695 | PMCID: PMC9006492
Review Free full text in Europe PMC
SARS-CoV-2 antibody responses in solid organ transplant recipients.
Transpl Infect Dis, 23(5):e13728, 22 Sep 2021
Cited by: 5 articles | PMID: 34505324 | PMCID: PMC8646321
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Prevalence and predictors of SARS-CoV-2 antibodies among solid organ transplant recipients with confirmed infection.
Am J Transplant, 21(6):2254-2261, 06 May 2021
Cited by: 34 articles | PMID: 33590675 | PMCID: PMC8014874
Seroprevalence of SARS-CoV-2 in Croatian solid-organ transplant recipients.
Biochem Med (Zagreb), 31(3):030901, 01 Oct 2021
Cited by: 1 article | PMID: 34658649 | PMCID: PMC8495621
SARS-CoV-2 Infection in Pediatric Solid Organ Transplant Recipients: A Single Center Observation.
J Pediatr Gastroenterol Nutr, 75(3):276-285, 09 Aug 2022
Cited by: 1 article | PMID: 35758426 | PMCID: PMC9365074
SARS-CoV-2 vaccination in solid-organ transplant recipients: What the clinician needs to know.
Transpl Int, 34(10):1776-1788, 20 Sep 2021
Cited by: 27 articles | PMID: 34450686 | PMCID: PMC8646251
Review Free full text in Europe PMC
 1
,
2
1
,
2




