Abstract
Background
Characterization of neutralization antibodies to SARS-CoV-2 infection or vaccination in children and young adults with inflammatory bowel disease (IBD) receiving biologic therapies is crucial.Methods
We performed a prospective longitudinal cohort study evaluating SARS-CoV-2 spike protein receptor binding domain (S-RBD) IgG positivity along with consistent clinical symptoms in patients with IBD receiving infliximab or vedolizumab. Serum was also obtained following immunization with approved vaccines. The IgG antibody to the spike protein binding domain of SARS-CoV-2 was assayed with a fluorescent bead-based immunoassay that takes advantage of the high dynamic range of fluorescent molecules using flow cytometry. A sensitive and high-throughput neutralization assay that incorporates SARS-CoV-2 spike protein onto a lentivirus and measures pseudoviral entry into ACE2-angiotensin converting enzyme 2 (ACE2) expressing human embryonic kidney 293 (HEK-293) cells was used.Results
There were 436 patients enrolled (mean age, 17 years, range 2-26 years; 58% male; 71% Crohn's disease, 29% ulcerative colitis, IBD-unspecified). Forty-four (10%) of enrolled subjects had SARS-CoV-2 S-RBD IgG antibodies. Compared to non-IBD adults (ambulatory) and hospitalized pediatric patients with PCR documented SARS-CoV-2 infection, S-RBD IgG antibody levels were significantly lower in the IBD cohort and by 6 months post infection most patients lacked neutralizing antibody. Following vaccination (n = 33), patients had a 15-fold higher S-RBD antibody response in comparison with natural infection, and all developed neutralizing antibodies to both wild type and variant SARS-CoV-2.Conclusions
The lower and less durable SARS-CoV-2 S-RBD IgG response to natural infection in IBD patients receiving biologics puts them at risk of reinfection. The robust response to immunization is likely protective.Free full text

Antibody Responses to SARS-CoV-2 After Infection or Vaccination in Children and Young Adults With Inflammatory Bowel Disease
Abstract
Background
Characterization of neutralization antibodies to SARS-CoV-2 infection or vaccination in children and young adults with inflammatory bowel disease (IBD) receiving biologic therapies is crucial.
Methods
We performed a prospective longitudinal cohort study evaluating SARS-CoV-2 spike protein receptor binding domain (S-RBD) IgG positivity along with consistent clinical symptoms in patients with IBD receiving infliximab or vedolizumab. Serum was also obtained following immunization with approved vaccines. The IgG antibody to the spike protein binding domain of SARS-CoV-2 was assayed with a fluorescent bead-based immunoassay that takes advantage of the high dynamic range of fluorescent molecules using flow cytometry. A sensitive and high-throughput neutralization assay that incorporates SARS-CoV-2 spike protein onto a lentivirus and measures pseudoviral entry into ACE2-angiotensin converting enzyme 2 (ACE2) expressing human embryonic kidney 293 (HEK-293) cells was used.
Results
There were 436 patients enrolled (mean age, 17 years, range 2–26 years; 58% male; 71% Crohn’s disease, 29% ulcerative colitis, IBD-unspecified). Forty-four (10%) of enrolled subjects had SARS-CoV-2 S-RBD IgG antibodies. Compared to non-IBD adults (ambulatory) and hospitalized pediatric patients with PCR documented SARS-CoV-2 infection, S-RBD IgG antibody levels were significantly lower in the IBD cohort and by 6 months post infection most patients lacked neutralizing antibody. Following vaccination (n = 33), patients had a 15-fold higher S-RBD antibody response in comparison with natural infection, and all developed neutralizing antibodies to both wild type and variant SARS-CoV-2.
Conclusions
The lower and less durable SARS-CoV-2 S-RBD IgG response to natural infection in IBD patients receiving biologics puts them at risk of reinfection. The robust response to immunization is likely protective.
Introduction
Individuals with inflammatory bowel disease (IBD) potentially have higher risk of symptomatic or severe SARS-CoV-2 disease because of the immunosuppressive therapies they receive. However, results from large multicenter international studies performed in adults and children with IBD to date show similar severity of illness and risk of hospitalization or death compared with age-matched individuals without IBD.1–3 Nonetheless, corticosteroid therapy, thiopurine therapy, or combination anti-TNF/thiopurine at the time of infection have been associated with more severe disease.1,4
The SARS-CoV-2 elicits a robust humoral and cellular immune response.5–12 A strong antibody response generated by effector B cells is critical for the eventual clearance of viruses, and the development of potent neutralizing antibodies is a major part of the memory response that prevents reinfection. Indeed, SARS-CoV-2 elicits virus-specific IgM, IgG and IgA, and neutralizing IgG antibodies (nAbs) following 7 to 14 days postinfection.9,10 The IgG levels to SARS-CoV-2 nucleocapsid (N) and spike proteins increase gradually following infection. Antibodies that bind to the receptor binding domain (RBD) of spike protein may neutralize viral entry into cells and play an important role in the protective immune response to SARS-CoV-2.11 A recent study from the United Kingdom of 7000 adult patients receiving either the biological therapies infliximab (anti-TNFα) or vedolizumab (anti-α4β7 integrin)13 revealed lower seroprevalence of antinucleocapsid SARS-CoV-2 in infliximab-treated patients compared with vedolizumab-treated patients (3.4% [161 of 4685] vs 6.0% [134 of 2250], P < .0001) though rates of symptomatic and polymerase chain reaction (PCR) proven SARS-CoV2 infection were the same in both groups. In patients with confirmed SARS-CoV-2 infection, seroconversion was observed in fewer infliximab-treated patients than vedolizumab-treated patients (48% [39 of 81] vs 83% [30 of 36], P = .00044), and the magnitude of anti-SARS-CoV-2 reactivity was lower.
In the late winter/spring 2020, Connecticut, USA, was a “hot spot” for SARS-CoV-2 infection, presenting a unique opportunity to perform longitudinal surveillance on a geographically defined population of children and young adults with IBD receiving biologics in our ambulatory infusion center. Here, we report IgG spike protein receptor binding domain (S-RBD) antibody response to SARS-CoV-2 infection and its durability over time, assess the neutralization capability of serum from our patient cohort to native and variant virus, and examine the relationship of this humoral response to clinical expression of COVID-19. The recent availability of SARS-CoV2 vaccine for young individuals further allowed us to assess SARS-CoV2 S-RBD IgG responses following vaccination.
Materials and Methods
Subjects
Between May 2020 and April 2021, we performed a single-center prospective longitudinal study evaluating seropositivity to SARS-CoV2 spike protein binding domain in an ambulatory infusion center in Farmington, Connecticut. Patient eligibility included: (1) diagnosis of IBD, (2) ≥3 infusions with either infliximab or vedolizumab prior to study entry, and (3) assent/consent. At each serum sampling (approximately every 8–16 weeks), participants/parents completed a paper questionnaire capturing clinical information suggestive of clinical signs or symptoms of COVID-19 in the study participant or household members and whether there was PCR documentation of SARS-CoV-2 infection dating back to January 2020 in the participant or a family member. Demographic characteristics, IBD activity, and concomitant medications were recorded. Data were entered into a Research Electronic Data Capture (REDCap) system.14
Serum for antibody titers was obtained at the time of the biologic infusion. Approximately 5 mL of blood was drawn in a serum separator collection tube. Tubes were spun and stored at 4°C for ≤2 days until transportation to Jackson Laboratories for Genomic Medicine (JAX-GM), where serum was extracted and stored at −80°C. Serum was obtained from non-IBD pediatric patients hospitalized with SARS-CoV-2 infection (n = 11). None required mechanical ventilation, though 3 were admitted to the Intensive Care Unit. Serum from adults without IBD (UConn Healthcare workers, n = 23) with mild to moderate SARS-CoV-2 infection who remained ambulatory were collected at the time of diagnosis (baseline) and at 2, 4, and 6 months. Consent and assent were obtained from the participant/family in all cases. This study was reviewed by the institutional review board at Connecticut Children’s and deemed minimal risk. The study is registered at Clinicaltrials.gov (NCT04838834).
SARS-CoV-2 Spike Protein Binding Domain IgG Antibody Responses
To measure SARS-CoV-2 S-RBD IgG antibodies, we used a fluorescent bead-based immunoassay developed as previously described.15 We screened patient serum samples for SARS-CoV-2 wild type (WT) S-RBD or K417N, E484K, N501 mutant (mt S-RBD) spike protein receptor binding domain specific IgG antibodies. Both WT S-RBD and mt S-RBD proteins were biotinylated and purchased from Acro Biosystems. Ten-fold serially diluted serum samples were assayed and then analyzed by flow cytometry using iQue Screener Plus (IntelliCyt, MI).15 Flow cytometry data were analyzed using FlowJo (BD Biosciences). Titration curves for each sample were used to normalize the area under the curve (AUC) values to quantitate the antibody levels. Statistical analyses were performed using GraphPad Prism 8.0 software (GraphPad Software).
Pseudotyped Virus Neutralization Assay
We developed a sensitive SARS-CoV-2 neutralization assay15 by incorporating WT SARS-CoV-2 spike protein or with N501Y mutation, representing the current prevalent B.1.1.7 (alpha variant) mutation in the United States, onto lentiviruses and measuring pseudoviral entry into angiotensin converting enzyme 2 (ACE2) overexpressing 293 cells (293-ACE2). To determine the half-maximal inhibitory concentration (NT50), 3-fold serially diluted serum samples from different patients were incubated with red fluorescent protein-encoding WT SARS-CoV-2 or alpha variant pseudotyped virus at 0.2 multiplicity of infection (MOI) for 1 hour at 37°C. The mixture was subsequently incubated with 293-ACE2 cells for 72 hours, after which cells were collected, washed with FACS buffer (1 × phosphate buffered saline + 2% fetal bovine serum) and analyzed by flow cytometry using BD FACSymphony A5 analyzer. Percent infection obtained was normalized for samples derived from cells infected with WT SARS-CoV-2 or alpha variant pseudotyped virus in the absence of serum. The NT50 was determined using 4-parameter nonlinear regression (GraphPad Prism 8.0).
Data Analysis and Statistical Methods
To determine patient characteristics and the humoral immune response to SARS-CoV-2, correlations between antibody AUC levels, 50% neutralization titer (NT50) values, and demographics of the study subjects were analyzed. All immunological data were entered into a common Excel-based database. These data were then merged with cohort information drawn from the Redcap database, using study identification numbers. The database was updated monthly as the study proceeded and the monthly updates integrated to provide a longitudinal database for analysis. To compare differences across groups, χ 2 and Fisher exact tests were used to compare count or categorical data. T-tests and ANOVA were used to compare mean differences, and exact P values are reported. In small sample settings, nonparametric rank-sum tests were used to compare continuous measures. Multiple comparison corrections for pairwise comparisons were used as appropriate. All statistical analyses were performed using GraphPad Prism V8 software. Numbers of repeats for each experiment and sample sizes are described in the figure legends.
2 and Fisher exact tests were used to compare count or categorical data. T-tests and ANOVA were used to compare mean differences, and exact P values are reported. In small sample settings, nonparametric rank-sum tests were used to compare continuous measures. Multiple comparison corrections for pairwise comparisons were used as appropriate. All statistical analyses were performed using GraphPad Prism V8 software. Numbers of repeats for each experiment and sample sizes are described in the figure legends.
Results
Demographic Characteristics and Clinical Presentation
Of 472 patients with IBD treated in our infusion center, 436 (92%) were enrolled (mean age 17 years, range 2–26 years, 58% male). During the period of study, 44 of 436 (10.1%) demonstrated IgG antibodies specific to RBD part of the SARS-CoV2 spike protein (S-RBD), which also contains the main neutralization regions. Demographic and clinical characteristics of the 436 participants, including the 392 who remained seronegative and the 44 who were positive, are shown in Table 1. The group with anti-SARS-CoV-2 positivity was significantly older than the group without S-RBD IgG antibodies (P = .02). Twenty-four of the 44 reported a positive PCR test, 22 had a household member who tested positive, and 25 reported a household member who experienced symptoms consistent with infection. Duration between most recent positive PCR test and serum sampling was 3.9 weeks (median 2.6, range 1.0–12.6) in seropositive IBD subjects who had documented PCR. For 3 of 9 asymptomatic patients for whom PCR+ date was known, the average duration between PCR+ and positive serum was 5 weeks. For the other 6, the time of infection was unknown but less than 12 weeks. All adult control patients were PCR+ (mean age 41 years), with a mean duration between + PCR and serum for study of approximately 6 weeks. All non-IBD pediatric controls (mean age 13 years) were PCR+ and had serum drawn during their initial hospital admission for moderate to severe COVID-19.
Table 1.
comparison of demographic and treatment factors by seropositivity in the study population.
| Variable | Total (436) | Seronegative (392) | Seropositive (44) | P |
|---|---|---|---|---|
| Age (yrs) | Mean 17.0 (range 2–26) | 16.9 (2–26) | 18.2 (11–26) | 0.024 |
| Sex, % male | 253, 58.0% | 222, 56.6% | 31, 70.5% | 0.059 |
| Crohn’s disease | 308, 70.6% | 274, 69.9% | 34, 77.3% | 0.273 |
| UC/IBD-U | 128, 29.4% | 118, 30.1% | 10, 22.7% | 0.273 |
| Infliximab | 358, 82.1% | 319, 81.4% | 39, 88.6% | 0.161 |
| Infliximab only | 305, 70.0% | 276, 70.4% | 29, 65.9% | 0.549 |
| Infliximab/ methotrexate | 53, 12.2% | 43, 11.0% | 10, 22.7% | 0.071 |
| Vedolizumab | 78, 17.9% | 73, 18.6% | 5, 11.4% | 0.301 |
| Vedolizumab only | 74, 17.0% | 69, 17.6% | 5, 11.4% | 0.398 |
| Vedolizumab/ methotrexate | 4, 0.9% | 4, 1.1% | 0, 0% | 1.0 |
| Corticosteroids | 15, 3.8% | 15, 3.8% | 0, 0% | 0.382 |
No seropositive participants in the IBD cohort were hospitalized. Nine of 44 (20%) participants were asymptomatic; 3 had a history of positive PCR, and 1 had a family member with a history of COVID. The other 5 were only known to have had SARS-CoV-2 infection by serology. Reported symptoms included headache (27 of 44, 61%), anosmia/ageusia (18 of 44, 41%), myalgias (16 of 44, 36%), ≥3 days of cough (14 of 44, 32%), anorexia (11 of 44, 25%), fever ≥101oF (10 of 44, 23%), sore throat (10 of 44, 23%), diarrhea (7 of 44, 16%), and vomiting (2 of 44, 5%). No patient had a flare of their stable IBD following infection with SARS-CoV-2 infection.
Thirty-nine of 358 (10.9%) infliximab-treated patients were seropositive compared with 5 of 78 (6.4%) vedolizumab patients (χ 2 , P = .164). A total of 53 patients were receiving infliximab and methotrexate combination therapy, and 10 of these (18.9%) were found to be antibody positive. No patients on vedolizumab plus methotrexate (n = 4) were found to be positive. No patient who was receiving corticosteroids at the time of serum sampling proved seropositive (n = 15). No patients were receiving thiopurines.
2 , P = .164). A total of 53 patients were receiving infliximab and methotrexate combination therapy, and 10 of these (18.9%) were found to be antibody positive. No patients on vedolizumab plus methotrexate (n = 4) were found to be positive. No patient who was receiving corticosteroids at the time of serum sampling proved seropositive (n = 15). No patients were receiving thiopurines.
Magnitude of Serological Response
We first compared anti-S-RBD IgG responses in our IBD study population to non-IBD adults and pediatric patients with COVID-19. Serum anti-S-RBD IgG was significantly lower than either comparator group—most notably (9.9x difference) between IBD patients and non-IBD adults (Figure 1A). There was no significant difference between the biologic treatment groups. We next assessed the neutralizing activity of the serum from each of the 3 study groups using a pseudovirus test to block cell entry, as described.15 Although the neutralization titer between adult and pediatric non-IBD groups were similar, both were significantly higher compared with the IBD group (Fig 1B). No neutralizing activity was observed in 5 of 44 of the IBD group, nor in 1 of 23 of the adult non-IBD subjects, nor in 1 of 11 of the pediatric non-IBD subjects. We did not find any significant differences between the biologic treatment groups. There was also significant correlation between NT50 and S-RBD IgG levels (Supplemental Figure 1A). There was no difference in the magnitude of S-RBD IgG response or NT50 response between the 9 asymptomatic IBD patients and the 35 who developed mild to moderate symptoms (data not shown).
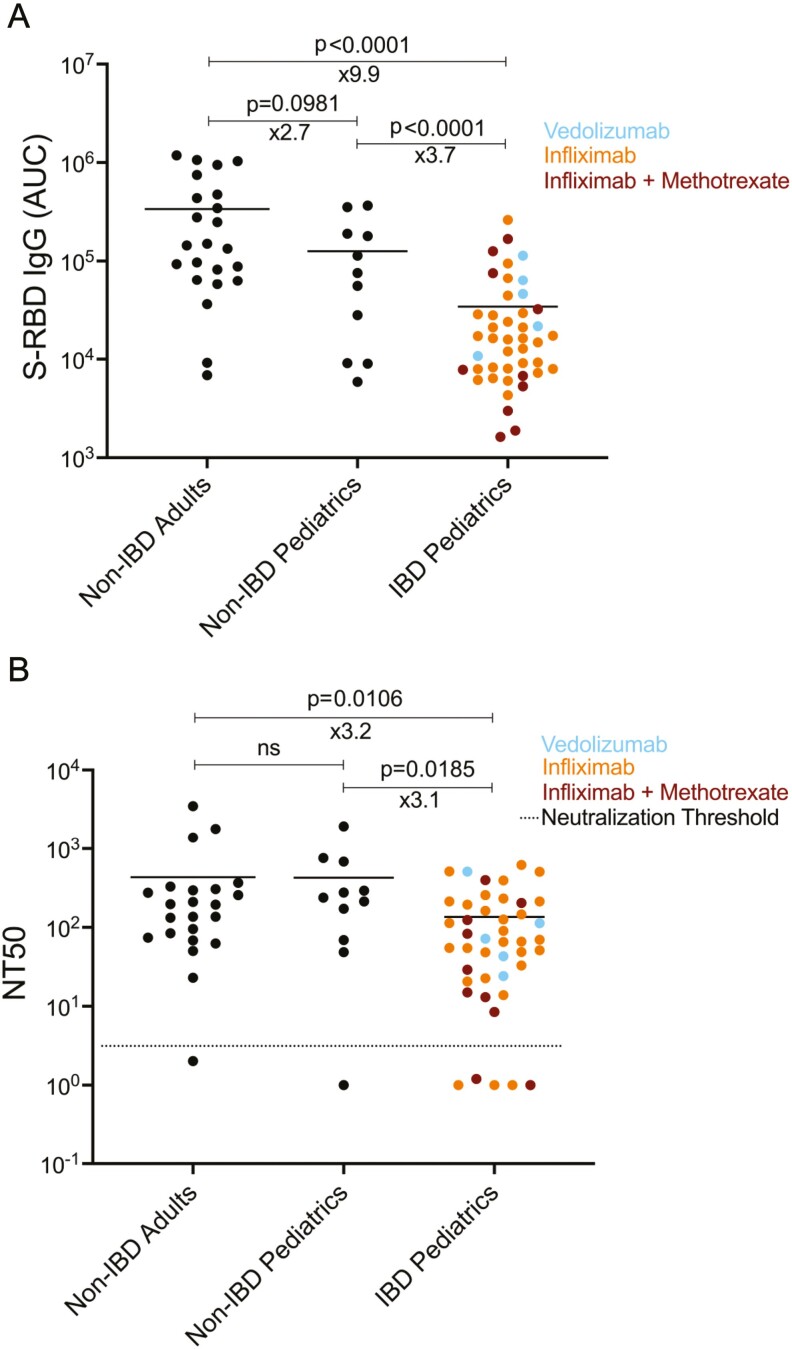
Comparison of the anti-spike protein IgG antibody levels and neutralization titers of adult non-IBD (inflammatory bowel disease), non-IBD pediatric, and IBD pediatric subjects receiving biologic therapy. A, SARS-CoV-2 spike protein receptor binding domain (S-RBD) specific IgG antibody levels measured in serum of non-IBD adult, non-IBD pediatric and IBD pediatric subjects. Area under the curve (AUC) values of serum antibodies were calculated from reciprocal dilution curves in antibody detection assay as described in the “Methods” (n = 23 for non-IBD adults, n = 11 for non-IBD pediatrics, and n = 44 for IBD pediatrics). Blue, orange, and red dots indicate the subjects with vedolizumab monotherapy, infliximab monotherapy, and infliximab + methotrexate cotherapy, respectively. Horizontal bars show the mean value; x values under the significance bars represent the fold changes between the mean values of groups. B, Half-maximal neutralization titer (NT50) values of adult non-IBD, pediatric non-IBD, and pediatric IBD subjects (n = 23 for non-IBD adults, n = 11 for non-IBD pediatrics, and n = 44 for IBD pediatrics). The NT50s of serum samples were measured via a neutralization assay using SARS-CoV-2 pseudotyped lentiviruses as described in the “Methods.” Dotted lines indicate the neutralization threshold which was NT50 of 5. Two-tailed Mann-Whitney U test was used to determine the statistical significances.
Durability of Antibody Response to SARS-CoV-2 Spike Protein
We next asked to what extent the SARS-CoV-2 S-RBD IgG response to infection in the adult non-IBD subjects compared with the IBD patients was sustained over a period of 6 months. Repeated sampling was performed in the adult subjects at approximately 2-month intervals and in the IBD subjects at approximately 3 and 6 months following initial positive sample. As expected, S-RBD specific IgG decreased over time in the adult cohort (Figure 2A) and in IBD subjects (Figure 2B); however, the magnitude of decrement between baseline and 6-months later appeared to be higher in IBD patients (3.7x decline). Interestingly, the ability to neutralize the virus was retained in almost all adult subjects after 2 months without significant further decrease (Figure 2C). However, neutralization capacity markedly decreased over time, and by 6 months, the majority of IBD patients had lost their neutralizing activity (10.6x decline, Figure 2D). Specific biologic regimen for the IBD patients did not show statistical significance.
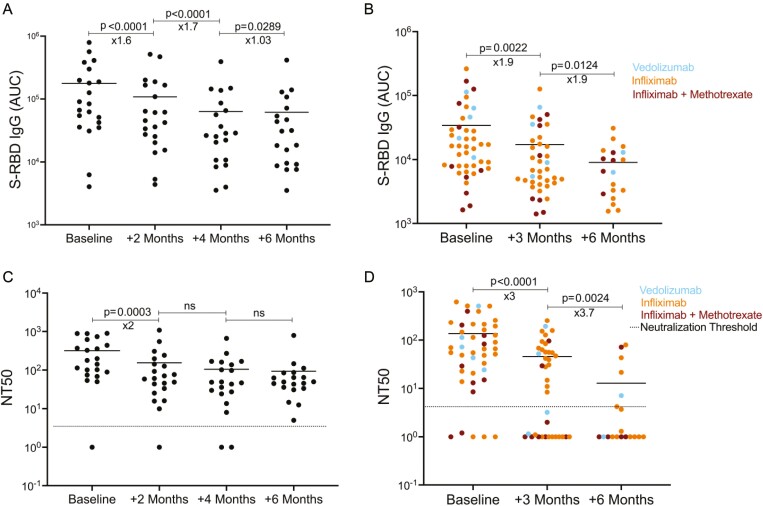
Antibody levels and neutralization titers of non-IBD adults and IBD pediatric subjects at different time points. A, S-RBD IgG levels of non-IBD adults at the time of diagnosis and 2, 4, and 6 months thereafter (n = 21 for baseline and + 2 months, n = 20 for + 4 months, n = 19 for + 6 months). B, S-RBD IgG levels of pediatric IBD subjects at the time of diagnosis and 3 and 6 months thereafter (n = 44 for baseline, n = 39 for + 3 months, n = 19 for + 6 months). C, Neutralization titer (NT50) of COVID-19 infected non-IBD adults and of D, IBD subjects over 6-month period. Horizontal bars show the mean value. Blue, orange, and red dots indicate the subjects with vedolizumab monotherapy, infliximab monotherapy, and infliximab + methotrexate cotherapy, respectively; x values under the significance bars represent the fold changes between the mean values of groups. Dotted lines indicate the neutralization threshold which was NT50 of 5. Two-tailed Mann-Whitney U test was used to determine the statistical significance.
SARS-CoV-2 Spike Specific Antibody Response Following Vaccination
We compared the anti-S-RBD antibody response to natural infection vs the response to immunization (n = 33 IBD subjects, mean age 21 years, range 16–27 years). Twenty-eight subjects received a mRNA vaccine (either Pfizer-BioNTech (n = 21) or Moderna (n = 7)) and 5 the adenovirus vector vaccine (AVV, Johnson & Johnson). Four out of 33 vaccinated IBD patients had documented previous SARS-CoV-2 infection. Serum sampling postsecond dose vaccination for the mRNA vaccine was obtained at a mean of 3.3 weeks (range 1–10 weeks). For the 5 subjects receiving the adenovirus vector vaccine, the duration between vaccination and serology had a mean of 3.1 weeks (range, 1.6–3.6 weeks). Spike protein receptor binding domain IgG responses were significantly higher (~15x) following vaccination in comparison with natural infection in the IBD subgroup (Figure 3A). Moreover, all IBD subjects had higher neutralizing IgG post vaccination compared with natural infection (~10x), including patients receiving both infliximab monotherapy and infliximab along with methotrexate (Figure 3B). Further, NT50 was highly correlated with S-RBD IgG levels in vaccinated individuals (Supplemental Figure 1B)
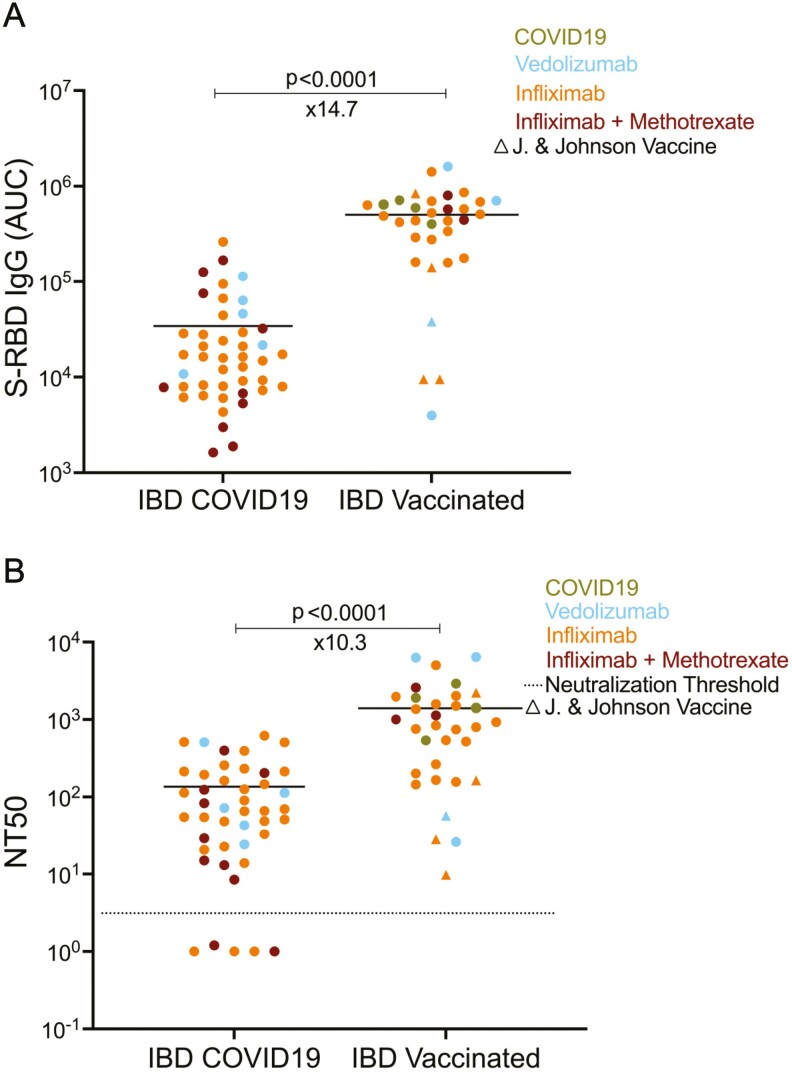
Comparing the antibody levels and neutralization titers of COVID-19-seropositive and vaccinated IBD pediatric subjects. A, S-RBD IgG levels of COVID-19-seropositive pediatric IBD subjects (n = 44) and vaccinated pediatric IBD subjects (n = 33), respectively. B, Neutralization titers (NT50) post-COVID19 and vaccination in IBD subjects. The average durations were 4.0 weeks after the first PCR+ test for IBD COVID-19 subjects and 3.2 weeks after the fully vaccination date for IBD vaccinated subjects. Blue, orange, and red dots indicate the subjects with vedolizumab monotherapy, infliximab monotherapy, and infliximab + methotrexate cotherapy, respectively. Green dots represent the COVID-19-seropositive subjects among the vaccinated group. Circles indicate RNA vaccines with 2 doses in the vaccinated group whereas triangles show Johnson & Johnson adenovirus vaccine, which was administered once. Horizontal bars show the mean value; x values under the significance bars represent the fold changes between the mean values of groups. Dotted lines indicate the neutralization threshold which was NT50 of 5. Two-tailed Mann-Whitney U test was used to determine the statistical significance.
We next compared anti-S-RBD IgG responses in our IBD cohort to wild-type and a mutated SARS-CoV-2 spike protein. In response to natural infection, mean S-RBD IgG levels were 3.7x lower than the mutant spike protein when compared with the wild-type spike protein (P < .0001, Figure 4A). We did not see a significant difference between WT and mutant strain spike protein S-RBD-IgG levels in response to vaccination (Figure 4B). Indeed, S-RBD IgG responses to the mutant spike protein were 34.3x lower in response to natural infection when compared with vaccine induced antibodies (Figure 4C). The neutralization of pseudovirus expressing wild-type spike protein vs that with N501Y mutation (main mutation in B.1.1.7 or alpha variant) was very similar postvaccination (Supplemental Figure 2A) and highly correlated with the S-RBD antibody levels (Supplemental Figure 2B). Taken together, these findings suggest that the vaccination of children and young adults with IBD elicits much higher antibody responses capable of overcoming immune-evading SARS-CoV-2 mutations.
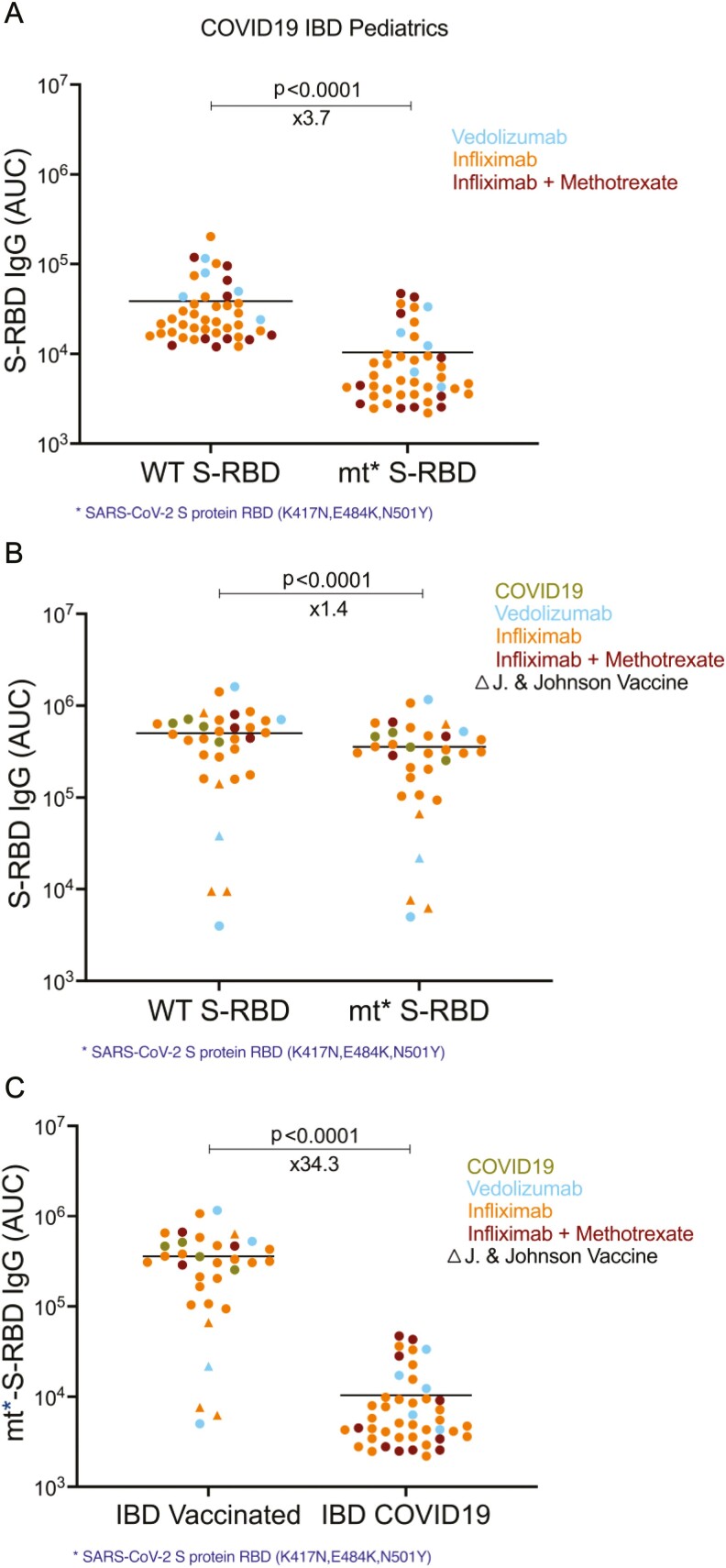
Specific antibody levels of wild-type S-RBD and mutant S-RBD in the serum of COVID-19-seropositive and vaccinated IBD pediatric subjects. A, Levels of anti-spike protein IgG antibodies to wild-type (WT S-RBD) and mutant S-RBD (mt* S-RBD) in COVID-19-seropositive (n = 44) IBD serum samples, and B, in vaccinated (n = 33) IBD subjects. respectively. Mutations in S-RBD protein were K417N, E484K, and N501Y. Blue, orange, and red dots indicate the subjects with vedolizumab monotherapy, infliximab monotherapy, and infliximab + methotrexate cotherapy, respectively. Green dots represent the COVID-19-seropositive subjects among the vaccinated group. Circles indicate RNA vaccines with 2 doses in the IBD vaccinated group, whereas triangles show Johnson & Johnson adenovirus vaccine, which was administered once. C, Comparison of the levels of mutant S-RBD specific IgG antibodies in vaccinated and COVID-19-seropositive IBD pediatrics serum samples. Horizontal bars show the mean value; x values under the significance bars represent the fold changes between the mean values of groups. Two-tailed Wilcoxon test was used to determine the statistical significance in (a-b) and Mann-Whitney U tests was used for the statistical calculation (c).
Tolerability of Vaccination
Following vaccination mild side effects were frequently noted. For the 28 mRNA vaccine recipients, the following were noted after the first dose and second dose, respectively: sore arm (10, 18); chills (1, 9); fever (1, 7); muscle aches (2, 6); headache (2, 10); and fatigue (3, 15). For the single-dose adenovirus vector vaccine, the following side effects were noted: sore arm (3), chills (1), fever (2), muscle aches (2), headache (2), fatigue (2). No patient had a flare of IBD symptoms following vaccination completion with a follow-up ranging from 2 to 6 months, and none have been diagnosed with COVID.
Discussion
Our data have significant and immediate implications for patients with IBD receiving biologic therapy and potentially for others with chronic inflammatory disease receiving similar therapies. The decreased S-RBD IgG response compared with adult non-IBD controls and the relatively rapid disappearance of neutralizing antibodies postinfection imply increased risk for reinfection. However, the strong response to vaccination in these subjects with the generation of S-RBD neutralizing antibody of higher titer than natural infection underscores the critical importance of immunization in this patient group.
During the period we performed our antibody screens, SARS-CoV-2 infection in the United States16 was around 10%—underscoring that our study population, which also had around 10% evidence of SARS-CoV-2 infection, was not at increased risk of infection. The clinical expression of disease was mild, as has been observed.1,2 Yet, our data differ from the study in the United Kingdom13 where less than half of PCR+ patients with IBD receiving infliximab were found to have antibodies to SARS-CoV-2. In our sampling, we found 44 of 436 (about 10%) to have S-RBD antibodies, including 24 IBD patients who had tested PCR+ and 23 of 24 who had S-RBD IgG antibodies and had detectable neutralizing antibody. Our overall prevalence of antibody positivity to SARS-CoV-2 was about twice that noted in the United Kingdom study, likely reflecting the epidemiology of infection in Connecticut compared with the United Kingdom. It is possible that different ethnic populations and differing SARS-CoV-2 strains between the United Kingdom and Connecticut populations may have contributed to different infection rates. Data are now emerging on the response to immunization with mRNA and other vaccines
A recent study demonstrated that infliximab was associated with attenuated immunogenicity to a single-dose of the BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines.17 However, vaccination after SARS-CoV-2 infection or a second dose of vaccine led to seroconversion in most patients. Our data, acquired after 2 doses of mRNA vaccine, showed robust seroconversion despite infliximab therapy, and all patients had neutralizing antibody. We did not sample patients after a single dose. Our observations strongly support the recent vaccination endorsement by the International Organization for the Study of IBD18 and lessen theoretical concerns about diminished response to immunization for influenza19,20 and hepatitis B,21 as has been reported in patients on anti-TNF therapy. Indeed, a recent systematic review and meta-analysis suggested that immunosuppressive therapy does not significantly reduce immunogenicity of vaccinations in children with IBD.22
The potential effect of antimetabolites on response to immunization remains unclear, and a recent report in solid organ transplant patients suggested a lessened response to mRNA vaccination.23 In adult patients with rheumatoid arthritis, a temporary discontinuation of methotrexate for 2 weeks prior to influenza vaccination improved immunogenicity.24 A recent report showed a blunted antibody response following the BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease in adults taking methotrexate.25 Methotrexate is often used either alone or in combination with anti-TNF agents in the treatment of IBD. In our cohort, the number of patients on combination infliximab and methotrexate who had also been vaccinated was too small to compare; although, all had similar S-RBD IgG levels. Future studies assessing larger numbers of IBD patients on combination infliximab and antimetabolites would be useful to determine if the addition of antimetabolite therapies lessen the antibody responses to either natural infection or immunization. Finally, 4 of our immunized patients were documented to have had SARS-CoV-2 infection prior to immunization, and all received 2 doses of a mRNA vaccine. It has been suggested that only 1 dose may be necessary in healthy subjects with prior infection,26 but we did not obtain samples after 1 dose of vaccine in our patient cohort, and thus the impact of the second dose remains to be determined.
Our study has several significant strengths. We performed longitudinal surveillance on a defined population, allowing us to identify both symptomatic and asymptomatic infection. In contrast to previous studies that just measured antibody levels, we determined neutralization activity of the anti-S-RBD IgG to both wild-type and variant virus. Thus, we were able to determine in an assay assumed to be a surrogate for protection from clinical infection that most of our patients generated titers of neutralizing antibody postinfection that rapidly declined over a 6-month period.
There were also several limitations. We did not have control data from IBD patients not receiving biologics, particularly those on methotrexate or thiopurine monotherapy. We also do not yet have longitudinal data on the durability of antibody response following vaccination. Finally, we did not test antibody neutralization ability from our patients against emerging SARS CoV-2 variants, which may reveal lower titers and a more rapid decline in protective levels. However, given an almost perfect correlation of S-RBD IgG levels with the NT50 (Supplemental Figure 1B) and a 34.3-times lower antibody response to mutant S-RBD in natural infection compared with vaccine (Figure 4C), we anticipate neutralization activity to decline as we observed for the S-RBD antibody level.
In conclusion, the humoral response to SARS-CoV-2 infection in children and young adults was less than in adult and pediatric non-IBD controls, and neutralizing antibody was absent in most infected IBD patients by 6 months. The robust response to immunization was reassuring and supports the utility of vaccinating these patients. Further data will be required to understand the durability of the response to the vaccine, whether previous infection will enhance the antibody response to the vaccine in this patient group lessening the need for a second dose, and whether chronic antimetabolite administration will have a mitigating influence over time.
Supplementary Material
izab207_suppl_Supplementary_Figure_1
izab207_suppl_Supplementary_Figure_2
izab207_suppl_Supplementary_Figure_Captions
Acknowledgments
The authors thank the patients and families cared for at the Connecticut Children’s Infusion Center for their participation, the nursing team at the Connecticut Children’s Infusion Center for their strong support of this study, Kristen Volz for logistical support, and the Mandell Braunstein IBD Research Chair Fund (J.H.), the David and Geri Epstein Foundation Fund (J.H.), the Ellen Oland Endowed Fund for Pediatric IBD Research (J.H.), the Achelis and Bodman Foundation (D.U.), and NIH grants U19 AI142733-01 (D.U.) and R61HD105613 (J.S.) for financial support.
Contributor Information
Joelynn Dailey, Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA. Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA.
Lina Kozhaya, Jackson Laboratory for Genomic Medicine, Farmington, CT, USA.
Mikail Dogan, Jackson Laboratory for Genomic Medicine, Farmington, CT, USA.
Dena Hopkins, Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA. Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA.
Blaine Lapin, Division of Rheumatology, Connecticut Children’s Medical Center, Hartford, CT, USA. Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA.
Katherine Herbst, Division of Research, Connecticut Children’s Medical Center, Hartford, CT, USA. Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA.
Michael Brimacombe, Division of Research, Connecticut Children’s Medical Center, Hartford, CT, USA. Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA.
Kristen Grandonico, Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA. Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA.
Fatih Karabacak, Jackson Laboratory for Genomic Medicine, Farmington, CT, USA.
John Schreiber, Division of Pediatric Infectious Diseases and Immunology, Connecticut Children’s Medical Center, Hartford, CT, USA. Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA.
Bruce Tsan-Liang Liang, Pat and Jim Calhoun Cardiology Center, University of Connecticut School of Medicine, Farmington, CT, USA.
Juan C Salazar, Division of Pediatric Infectious Diseases and Immunology, Connecticut Children’s Medical Center, Hartford, CT, USA. Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA.
Derya Unutmaz, Jackson Laboratory for Genomic Medicine, Farmington, CT, USA.
Jeffrey S Hyams, Department of Pediatrics, University of Connecticut School of Medicine, Farmington, CT, USA. Division of Digestive Diseases, Hepatology, and Nutrition, Connecticut Children’s Medical Center, Hartford, CT, USA.
Author Contributions
J.H. had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. Concept and design: J.H., J.D., L.K., M.D, D.H., M.B., J.S., J.C.S, and D.U. Acquisition, analysis, and interpretation of the data: J.H., J.D., D.H., L.K., M.K., B.T.L.L., B.L., K.H., K.G., M.B., J.S., J.C.S., and D.U. Drafting of the manuscript: J.H., J.D., D.H., L.K., M.K., M.B., J.C.S., and D.U. Critical revision of the manuscript for important intellectual content: J.H., J.D., D.H., L.K., M.K., B.L., B.T.L.L., K.H., M.B., K.G., J.S., J.C.S., and D.U. Supervision: J.H., J.C.S., and D.U.
Conflict of Interest
J.H. serves on advisory boards for Janssen (infliximab) and Takeda (vedolizumab), respectively. The other authors report no conflict of interest.
References
Articles from Inflammatory Bowel Diseases are provided here courtesy of Oxford University Press
Full text links
Read article at publisher's site: https://doi.org/10.1093/ibd/izab207
Read article for free, from open access legal sources, via Unpaywall:
https://academic.oup.com/ibdjournal/article-pdf/28/7/1019/44267964/izab207.pdf
Citations & impact
Impact metrics
Citations of article over time
Alternative metrics

Discover the attention surrounding your research
https://www.altmetric.com/details/113624812
Article citations
Impact of COVID-19 on Pediatric Inflammatory Bowel Diseases-From Expectations to Reality.
J Pers Med, 14(4):399, 09 Apr 2024
Cited by: 0 articles | PMID: 38673026 | PMCID: PMC11051136
Review Free full text in Europe PMC
COVID-19 Vaccination and Immunosuppressive Therapy in Immune-Mediated Inflammatory Diseases.
Vaccines (Basel), 11(12):1813, 04 Dec 2023
Cited by: 2 articles | PMID: 38140217 | PMCID: PMC10747214
Review Free full text in Europe PMC
Disease Flares Following COVID-19 Vaccination in Patients with Inflammatory Bowel Disease.
Intern Med, 62(24):3579-3584, 29 Sep 2023
Cited by: 2 articles | PMID: 37779068 | PMCID: PMC10781543
SARS-CoV-2 breakthrough infections after COVID-19 vaccination in patients with inflammatory bowel disease: a systematic review and meta-analysis.
Therap Adv Gastroenterol, 16:17562848231174295, 15 Jul 2023
Cited by: 2 articles | PMID: 37461739 | PMCID: PMC10350577
The safety of COVID-19 vaccination in immunocompromised children and young adults with immune-mediated inflammatory disease.
Acta Paediatr, 112(4):794-801, 06 Jan 2023
Cited by: 5 articles | PMID: 36583590 | PMCID: PMC9880735
Go to all (29) article citations
Data
Data behind the article
This data has been text mined from the article, or deposited into data resources.
BioStudies: supplemental material and supporting data
Clinical Trials
- (1 citation) ClinicalTrials.gov - NCT04838834
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
COVID-19 vaccine-induced antibody and T-cell responses in immunosuppressed patients with inflammatory bowel disease after the third vaccine dose (VIP): a multicentre, prospective, case-control study.
Lancet Gastroenterol Hepatol, 7(11):1005-1015, 09 Sep 2022
Cited by: 31 articles | PMID: 36088954 | PMCID: PMC9458592
Use of Tumor Necrosis Factor-α Antagonists Is Associated With Attenuated IgG Antibody Response Against SARS-CoV-2 in Vaccinated Patients With Inflammatory Bowel Disease.
Front Immunol, 13:920333, 05 Jul 2022
Cited by: 2 articles | PMID: 35865529 | PMCID: PMC9294156
Vaccine escape, increased breakthrough and reinfection in infliximab-treated patients with IBD during the Omicron wave of the SARS-CoV-2 pandemic.
Gut, 72(2):295-305, 28 Jul 2022
Cited by: 23 articles | PMID: 35902214
Funding
Funders who supported this work.
Achelis and Bodman Foundation
David and Geri Epstein Foundation Fund
Ellen Oland Endowed Fund for Pediatric IBD Research
Mandell Braunstein IBD Research Chair
NIAID NIH HHS (1)
Grant ID: U19 AI142733
NICHD NIH HHS (1)
Grant ID: R61 HD105613
National Institutes of Health (2)
Grant ID: U19 AI142733-01
Grant ID: R61HD105613





